User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Limiting antibiotic overprescription in pandemics: New guidelines
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Hep C, HIV coinfection tied to higher MI risk with age
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
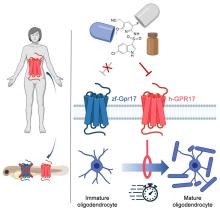
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
People of color bearing brunt of long COVID, doctors say
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.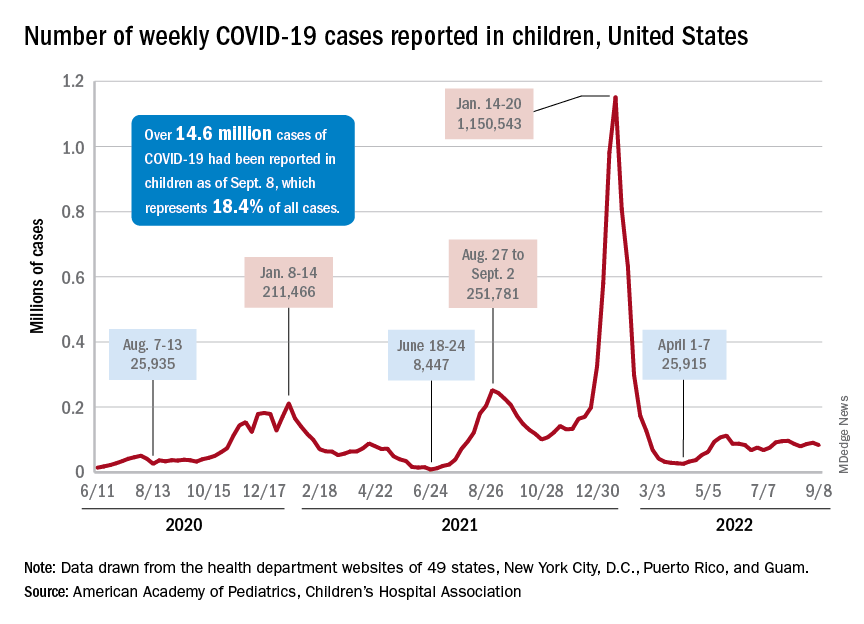
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
Integrase inhibitors and gestational weight gain: Should women worry?
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.