User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Early FMT shows promise for preventing recurrent C. difficile
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY
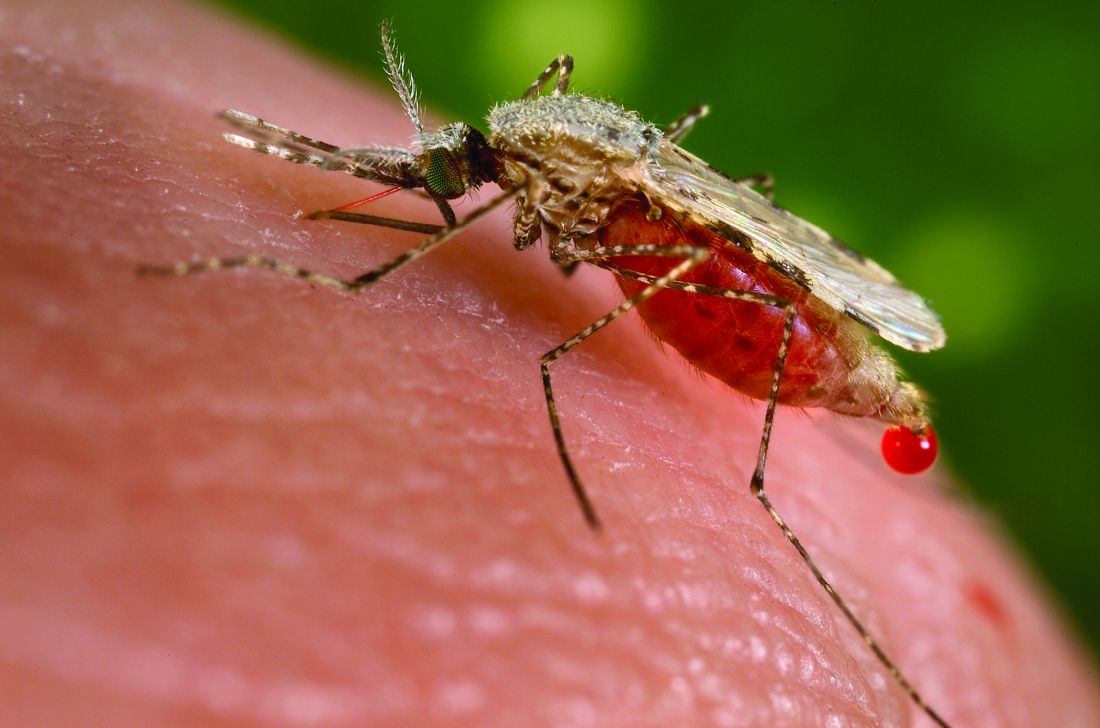
Malaria vaccine gets special delivery by tiny health personnel
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Children and COVID: Weekly cases dropped by 57% in September
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.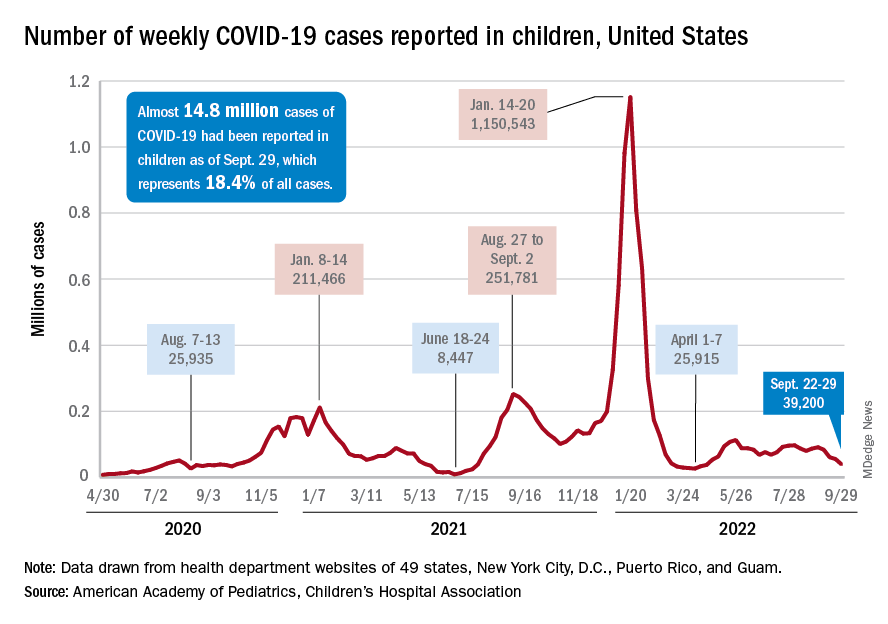
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
Sore throat becoming dominant COVID symptom: Reports
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.
Gardasil 9 HPV vaccine advised for MSM living with HIV
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROMES
FDA approves HIV-1 treatment ibalizumab for 30-second IV push
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
COVID attacks DNA in heart, unlike flu, study says
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
FROM IMMUNOLOGY
Strong link found between enterovirus and type 1 diabetes
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
AT EASD 2022
CDC: Masking no longer required in health care settings
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.
It’s a “major departure” from the CDC’s previous recommendation of universal masking to fight the COVID-19 pandemic, The Hill says.
“Updates were made to reflect the high levels of vaccine-and infection-induced immunity and the availability of effective treatments and prevention tools,” the CDC’s new guidance says.
The agency now says that facilities in areas without high transmission can decide for themselves whether to require everyone – doctors, patients, and visitors – to wear masks.
Community transmission “is the metric currently recommended to guide select practices in healthcare settings to allow for earlier intervention, before there is strain on the health care system and to better protect the individuals seeking care in these settings,” the CDC said.
About 73% of the country is having “high” rates of transmission, The Hill said.
“Community transmission” is different from the “community level” metric that’s used for non–health care settings.
Community transmission refers to measures of the presence and spread of SARS-CoV-2, the CDC said. “Community levels place an emphasis on measures of the impact of COVID-19 in terms of hospitalizations and health care system strain, while accounting for transmission in the community.”
Just 7% of counties are considered high risk, while nearly 62 percent are low.
The new guidance applies wherever health care is delivered, including nursing homes and home health, the CDC said.
A version of this article first appeared on WebMD.com.