User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Will a one-dose drug mean the end of sleeping sickness?
A single dose of oral acoziborole resulted in a greater than 95% cure or probable cure rate for human African trypanosomiasis (HAT), also known as sleeping sickness, according to results from a clinical trial testing a one-dose experimental drug.
The drug has “the potential to revolutionize treatment” for the disease, which remains endemic in sub-Saharan Africa, said Antoine Tarral, MD, head of the human African trypanosomiasis clinical program at the Drugs for Neglected Diseases initiative, based in Geneva, and senior author of the study, in a press release.
he told this news organization. “It’s the first drug we can use without hospitalization. ... All the previous medications needed hospitalization, and therefore we could not treat the population early before they started expressing symptoms.”
The World Health Organization “has been working for decades for such a possibility to implement a new strategy for this disease,” Dr. Tarral said.
Current (2019) WHO guidelines recommend oral fexinidazole as first-line treatment for any stage of the disease. The 10-day course often requires hospitalization and skilled staff. Previous recommendations required disease-staging with cerebrospinal fluid (CSF) sampling and 7 days of intramuscular pentamidine for early-stage disease or nifurtimox-eflornithine combination therapy (NECT) with hospitalization for late-stage disease.
By contrast, acoziborole, which was codeveloped by the DND initiative and Sanofi, “is administered in a single dose and is effective across every stage of the disease, thereby eliminating the many barriers currently in place for people most vulnerable to the diseases, such as invasive treatments and long travel distances to a hospital or clinic, and opening the door to screen-and-treat approaches at the village level,” Dr. Tarral said in the statement.
“Today, and in the future, we will have less and less support to do this long and costly diagnostic process and treatment in the hospital,” he said in an interview. “This development means we can go for a simple test and a simple treatment, which means we can meet the WHO 2030 goal for ending transmission of this disease.”
Results from the multicenter, prospective, open-label, single-arm, noncomparative, phase 2/3 study were published in The Lancet.
Pragmatic study design
Sleeping sickness is caused by Trypanosoma brucei gambiense (gambiense HAT). It is transmitted by the tsetse fly and mostly fatal when left untreated.
The study enrolled 208 adults and adolescents (167 with late-stage, and 41 with early-stage or intermediate-stage disease) from 10 hospitals in the Democratic Republic of the Congo and Guinea. All patients were treated with acoziborole 960 mg – an unusual study design.
“Due to the substantial decline in incidence, enrolling patients with gambiense HAT into clinical trials is challenging,” the authors wrote. “Following advice from the European Medicines Agency, this study was designed as an open-label, single-arm trial with no comparator or control group.”
After 18 months of follow-up, treatment success, defined as absence of trypanosomes and less than 20 white blood cells per mcL of CSF, occurred in 159 (95.2%) of the late-stage patients, and 100% of the early- and intermediate-stage patients, “which was similar to the estimated historical results for NECT,” the authors noted.
Serious treatment-emergent adverse events were reported in 21 (10%) of patients, “but none of these events were considered drug-related,” they added.
The DND initiative and the WHO are currently nearing completion of a much larger, double-blind, placebo-controlled trial of acoziborole to “increase the safety database,” Dr. Tarral explained.
“Purists will say that acoziborole has not been evaluated according to current standards, because the study was not a randomized trial, there was no control group, and the number of participants was small,” said Jacques Pépin, MD, from the University of Sherbrooke (Que.), in a linked commentary.
“But these were difficult challenges to overcome, considering the drastic reduction in the number of patients with HAT and dispersion over a vast territory, particularly in the Democratic Republic of the Congo. For these reasons, the authors took a pragmatic approach instead,” he wrote.
A potential new tool for eradication of sleeping sickness
“This is really an exciting development, which will be useful in the drive for eradication/interruption of transmission of this disease,” Dr. Pépin told this news organization.
Dr. Pépin treated around 1,000 trypanosomiasis patients during an outbreak in Zaire in the early 1980s. Because the asymptomatic incubation period for the disease can be several months or even years, “the core strategy for controlling the disease is active screening,” he said in an interview.
“You try to convince the whole population of endemic villages to show up on a given day, and then you have a mobile team of nurses who examine everybody, trying to find those with early trypanosomiasis. This includes physical examination for lymph nodes in the neck, but also a blood test whose results are available within minutes,” he said.
“Until now, these persons with a positive serology would undergo additional and labor-intensive examinations of blood to try to find trypanosomes and prove that they have the disease. So far those with a positive serology and negative parasitological assays (‘serological suspects’) were left untreated, because the treatments were toxic and cumbersome, and because a substantial but unknown proportion of these ‘suspects’ just have a false positive of their serological test, without having the disease,” Dr. Pépin said.
“Now with acoziborole, which seems to have little serious toxicity ... and can be given as a single-dose oral med, it might be reasonable to treat the ‘serological suspects,’ ” he said.
“Take it one step further, it might be possible to do the serological test only and treat all individuals with a positive serology without bothering to do parasitological assays. This is what they call ‘test-and-treat’ strategy. It would make sense, provided that we are sure that the drug is very well tolerated.”
Dr. Pépin added that he is “just slightly worried” about three patients described in the paper who had psychiatric adverse events 3 months after treatment. “If that happens to patients who indeed have trypanosomiasis, that’s a reasonable price to pay considering the toxicity of other drugs,” he said. “If that happens to serological suspects, many of whom don’t have any disease, this becomes a preoccupation.”
But Dr. Tarral said, “We have no indication that the drug can provoke psychiatric symptoms. In fact, the psychiatric symptoms did not emerge – they re-emerged after 3 months due to some patients’ refusal to be followed up.”
“We included patients in very advanced stages of the disease, and these symptoms are considered disease sequelae,” Dr. Tarral said. “The majority of patients who have such psychiatric symptoms need follow-up after treatment. If not, they can relapse very early. There were a lot of patients who had such symptoms and the investigators proposed they should be followed by a psychiatrist and some of them refused. And due to that only three of our patients had this relapse, and they were cured after psychiatric support.”
The study was funded through the DND initiative and was supported by grants from the Bill & Melinda Gates Foundation; UK Aid; the Federal Ministry of Education and Research through Kreditanstalt für Wiederaufbau, Germany; the Swiss Agency for Development and Cooperation; Médecins Sans Frontières; the Dutch Ministry of Foreign Affairs; the Norwegian Agency for Development Cooperation; the Stavros Niarchos Foundation; the Spanish Agency for International Development Cooperation; and the Banco Bilbao Vizcaya Argentaria Foundation.
A number of study investigators, including Dr. Tarral, report employment at the DND initiative. Other investigators report fees from the DND initiative for the statistical report, consulting fees from CEMAG, D&A Pharma, Inventiva, and OT4B Pharma. The Swiss Tropical and Public Health Institute acted as a service provider for the DND initiative by monitoring the study sites. Dr. Pépin declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A single dose of oral acoziborole resulted in a greater than 95% cure or probable cure rate for human African trypanosomiasis (HAT), also known as sleeping sickness, according to results from a clinical trial testing a one-dose experimental drug.
The drug has “the potential to revolutionize treatment” for the disease, which remains endemic in sub-Saharan Africa, said Antoine Tarral, MD, head of the human African trypanosomiasis clinical program at the Drugs for Neglected Diseases initiative, based in Geneva, and senior author of the study, in a press release.
he told this news organization. “It’s the first drug we can use without hospitalization. ... All the previous medications needed hospitalization, and therefore we could not treat the population early before they started expressing symptoms.”
The World Health Organization “has been working for decades for such a possibility to implement a new strategy for this disease,” Dr. Tarral said.
Current (2019) WHO guidelines recommend oral fexinidazole as first-line treatment for any stage of the disease. The 10-day course often requires hospitalization and skilled staff. Previous recommendations required disease-staging with cerebrospinal fluid (CSF) sampling and 7 days of intramuscular pentamidine for early-stage disease or nifurtimox-eflornithine combination therapy (NECT) with hospitalization for late-stage disease.
By contrast, acoziborole, which was codeveloped by the DND initiative and Sanofi, “is administered in a single dose and is effective across every stage of the disease, thereby eliminating the many barriers currently in place for people most vulnerable to the diseases, such as invasive treatments and long travel distances to a hospital or clinic, and opening the door to screen-and-treat approaches at the village level,” Dr. Tarral said in the statement.
“Today, and in the future, we will have less and less support to do this long and costly diagnostic process and treatment in the hospital,” he said in an interview. “This development means we can go for a simple test and a simple treatment, which means we can meet the WHO 2030 goal for ending transmission of this disease.”
Results from the multicenter, prospective, open-label, single-arm, noncomparative, phase 2/3 study were published in The Lancet.
Pragmatic study design
Sleeping sickness is caused by Trypanosoma brucei gambiense (gambiense HAT). It is transmitted by the tsetse fly and mostly fatal when left untreated.
The study enrolled 208 adults and adolescents (167 with late-stage, and 41 with early-stage or intermediate-stage disease) from 10 hospitals in the Democratic Republic of the Congo and Guinea. All patients were treated with acoziborole 960 mg – an unusual study design.
“Due to the substantial decline in incidence, enrolling patients with gambiense HAT into clinical trials is challenging,” the authors wrote. “Following advice from the European Medicines Agency, this study was designed as an open-label, single-arm trial with no comparator or control group.”
After 18 months of follow-up, treatment success, defined as absence of trypanosomes and less than 20 white blood cells per mcL of CSF, occurred in 159 (95.2%) of the late-stage patients, and 100% of the early- and intermediate-stage patients, “which was similar to the estimated historical results for NECT,” the authors noted.
Serious treatment-emergent adverse events were reported in 21 (10%) of patients, “but none of these events were considered drug-related,” they added.
The DND initiative and the WHO are currently nearing completion of a much larger, double-blind, placebo-controlled trial of acoziborole to “increase the safety database,” Dr. Tarral explained.
“Purists will say that acoziborole has not been evaluated according to current standards, because the study was not a randomized trial, there was no control group, and the number of participants was small,” said Jacques Pépin, MD, from the University of Sherbrooke (Que.), in a linked commentary.
“But these were difficult challenges to overcome, considering the drastic reduction in the number of patients with HAT and dispersion over a vast territory, particularly in the Democratic Republic of the Congo. For these reasons, the authors took a pragmatic approach instead,” he wrote.
A potential new tool for eradication of sleeping sickness
“This is really an exciting development, which will be useful in the drive for eradication/interruption of transmission of this disease,” Dr. Pépin told this news organization.
Dr. Pépin treated around 1,000 trypanosomiasis patients during an outbreak in Zaire in the early 1980s. Because the asymptomatic incubation period for the disease can be several months or even years, “the core strategy for controlling the disease is active screening,” he said in an interview.
“You try to convince the whole population of endemic villages to show up on a given day, and then you have a mobile team of nurses who examine everybody, trying to find those with early trypanosomiasis. This includes physical examination for lymph nodes in the neck, but also a blood test whose results are available within minutes,” he said.
“Until now, these persons with a positive serology would undergo additional and labor-intensive examinations of blood to try to find trypanosomes and prove that they have the disease. So far those with a positive serology and negative parasitological assays (‘serological suspects’) were left untreated, because the treatments were toxic and cumbersome, and because a substantial but unknown proportion of these ‘suspects’ just have a false positive of their serological test, without having the disease,” Dr. Pépin said.
“Now with acoziborole, which seems to have little serious toxicity ... and can be given as a single-dose oral med, it might be reasonable to treat the ‘serological suspects,’ ” he said.
“Take it one step further, it might be possible to do the serological test only and treat all individuals with a positive serology without bothering to do parasitological assays. This is what they call ‘test-and-treat’ strategy. It would make sense, provided that we are sure that the drug is very well tolerated.”
Dr. Pépin added that he is “just slightly worried” about three patients described in the paper who had psychiatric adverse events 3 months after treatment. “If that happens to patients who indeed have trypanosomiasis, that’s a reasonable price to pay considering the toxicity of other drugs,” he said. “If that happens to serological suspects, many of whom don’t have any disease, this becomes a preoccupation.”
But Dr. Tarral said, “We have no indication that the drug can provoke psychiatric symptoms. In fact, the psychiatric symptoms did not emerge – they re-emerged after 3 months due to some patients’ refusal to be followed up.”
“We included patients in very advanced stages of the disease, and these symptoms are considered disease sequelae,” Dr. Tarral said. “The majority of patients who have such psychiatric symptoms need follow-up after treatment. If not, they can relapse very early. There were a lot of patients who had such symptoms and the investigators proposed they should be followed by a psychiatrist and some of them refused. And due to that only three of our patients had this relapse, and they were cured after psychiatric support.”
The study was funded through the DND initiative and was supported by grants from the Bill & Melinda Gates Foundation; UK Aid; the Federal Ministry of Education and Research through Kreditanstalt für Wiederaufbau, Germany; the Swiss Agency for Development and Cooperation; Médecins Sans Frontières; the Dutch Ministry of Foreign Affairs; the Norwegian Agency for Development Cooperation; the Stavros Niarchos Foundation; the Spanish Agency for International Development Cooperation; and the Banco Bilbao Vizcaya Argentaria Foundation.
A number of study investigators, including Dr. Tarral, report employment at the DND initiative. Other investigators report fees from the DND initiative for the statistical report, consulting fees from CEMAG, D&A Pharma, Inventiva, and OT4B Pharma. The Swiss Tropical and Public Health Institute acted as a service provider for the DND initiative by monitoring the study sites. Dr. Pépin declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A single dose of oral acoziborole resulted in a greater than 95% cure or probable cure rate for human African trypanosomiasis (HAT), also known as sleeping sickness, according to results from a clinical trial testing a one-dose experimental drug.
The drug has “the potential to revolutionize treatment” for the disease, which remains endemic in sub-Saharan Africa, said Antoine Tarral, MD, head of the human African trypanosomiasis clinical program at the Drugs for Neglected Diseases initiative, based in Geneva, and senior author of the study, in a press release.
he told this news organization. “It’s the first drug we can use without hospitalization. ... All the previous medications needed hospitalization, and therefore we could not treat the population early before they started expressing symptoms.”
The World Health Organization “has been working for decades for such a possibility to implement a new strategy for this disease,” Dr. Tarral said.
Current (2019) WHO guidelines recommend oral fexinidazole as first-line treatment for any stage of the disease. The 10-day course often requires hospitalization and skilled staff. Previous recommendations required disease-staging with cerebrospinal fluid (CSF) sampling and 7 days of intramuscular pentamidine for early-stage disease or nifurtimox-eflornithine combination therapy (NECT) with hospitalization for late-stage disease.
By contrast, acoziborole, which was codeveloped by the DND initiative and Sanofi, “is administered in a single dose and is effective across every stage of the disease, thereby eliminating the many barriers currently in place for people most vulnerable to the diseases, such as invasive treatments and long travel distances to a hospital or clinic, and opening the door to screen-and-treat approaches at the village level,” Dr. Tarral said in the statement.
“Today, and in the future, we will have less and less support to do this long and costly diagnostic process and treatment in the hospital,” he said in an interview. “This development means we can go for a simple test and a simple treatment, which means we can meet the WHO 2030 goal for ending transmission of this disease.”
Results from the multicenter, prospective, open-label, single-arm, noncomparative, phase 2/3 study were published in The Lancet.
Pragmatic study design
Sleeping sickness is caused by Trypanosoma brucei gambiense (gambiense HAT). It is transmitted by the tsetse fly and mostly fatal when left untreated.
The study enrolled 208 adults and adolescents (167 with late-stage, and 41 with early-stage or intermediate-stage disease) from 10 hospitals in the Democratic Republic of the Congo and Guinea. All patients were treated with acoziborole 960 mg – an unusual study design.
“Due to the substantial decline in incidence, enrolling patients with gambiense HAT into clinical trials is challenging,” the authors wrote. “Following advice from the European Medicines Agency, this study was designed as an open-label, single-arm trial with no comparator or control group.”
After 18 months of follow-up, treatment success, defined as absence of trypanosomes and less than 20 white blood cells per mcL of CSF, occurred in 159 (95.2%) of the late-stage patients, and 100% of the early- and intermediate-stage patients, “which was similar to the estimated historical results for NECT,” the authors noted.
Serious treatment-emergent adverse events were reported in 21 (10%) of patients, “but none of these events were considered drug-related,” they added.
The DND initiative and the WHO are currently nearing completion of a much larger, double-blind, placebo-controlled trial of acoziborole to “increase the safety database,” Dr. Tarral explained.
“Purists will say that acoziborole has not been evaluated according to current standards, because the study was not a randomized trial, there was no control group, and the number of participants was small,” said Jacques Pépin, MD, from the University of Sherbrooke (Que.), in a linked commentary.
“But these were difficult challenges to overcome, considering the drastic reduction in the number of patients with HAT and dispersion over a vast territory, particularly in the Democratic Republic of the Congo. For these reasons, the authors took a pragmatic approach instead,” he wrote.
A potential new tool for eradication of sleeping sickness
“This is really an exciting development, which will be useful in the drive for eradication/interruption of transmission of this disease,” Dr. Pépin told this news organization.
Dr. Pépin treated around 1,000 trypanosomiasis patients during an outbreak in Zaire in the early 1980s. Because the asymptomatic incubation period for the disease can be several months or even years, “the core strategy for controlling the disease is active screening,” he said in an interview.
“You try to convince the whole population of endemic villages to show up on a given day, and then you have a mobile team of nurses who examine everybody, trying to find those with early trypanosomiasis. This includes physical examination for lymph nodes in the neck, but also a blood test whose results are available within minutes,” he said.
“Until now, these persons with a positive serology would undergo additional and labor-intensive examinations of blood to try to find trypanosomes and prove that they have the disease. So far those with a positive serology and negative parasitological assays (‘serological suspects’) were left untreated, because the treatments were toxic and cumbersome, and because a substantial but unknown proportion of these ‘suspects’ just have a false positive of their serological test, without having the disease,” Dr. Pépin said.
“Now with acoziborole, which seems to have little serious toxicity ... and can be given as a single-dose oral med, it might be reasonable to treat the ‘serological suspects,’ ” he said.
“Take it one step further, it might be possible to do the serological test only and treat all individuals with a positive serology without bothering to do parasitological assays. This is what they call ‘test-and-treat’ strategy. It would make sense, provided that we are sure that the drug is very well tolerated.”
Dr. Pépin added that he is “just slightly worried” about three patients described in the paper who had psychiatric adverse events 3 months after treatment. “If that happens to patients who indeed have trypanosomiasis, that’s a reasonable price to pay considering the toxicity of other drugs,” he said. “If that happens to serological suspects, many of whom don’t have any disease, this becomes a preoccupation.”
But Dr. Tarral said, “We have no indication that the drug can provoke psychiatric symptoms. In fact, the psychiatric symptoms did not emerge – they re-emerged after 3 months due to some patients’ refusal to be followed up.”
“We included patients in very advanced stages of the disease, and these symptoms are considered disease sequelae,” Dr. Tarral said. “The majority of patients who have such psychiatric symptoms need follow-up after treatment. If not, they can relapse very early. There were a lot of patients who had such symptoms and the investigators proposed they should be followed by a psychiatrist and some of them refused. And due to that only three of our patients had this relapse, and they were cured after psychiatric support.”
The study was funded through the DND initiative and was supported by grants from the Bill & Melinda Gates Foundation; UK Aid; the Federal Ministry of Education and Research through Kreditanstalt für Wiederaufbau, Germany; the Swiss Agency for Development and Cooperation; Médecins Sans Frontières; the Dutch Ministry of Foreign Affairs; the Norwegian Agency for Development Cooperation; the Stavros Niarchos Foundation; the Spanish Agency for International Development Cooperation; and the Banco Bilbao Vizcaya Argentaria Foundation.
A number of study investigators, including Dr. Tarral, report employment at the DND initiative. Other investigators report fees from the DND initiative for the statistical report, consulting fees from CEMAG, D&A Pharma, Inventiva, and OT4B Pharma. The Swiss Tropical and Public Health Institute acted as a service provider for the DND initiative by monitoring the study sites. Dr. Pépin declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What is the genetic influence on the severity of COVID-19?
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
A striking characteristic of COVID-19 is that the severity of clinical outcomes is remarkably variable. Establishing a prognosis for individuals infected with COVID-19 remains a challenge.
Since the start of the COVID-19 pandemic, the heterogeneity of individuals who progress toward severe disease or death, along with the fact that individuals directly exposed to the virus do not necessarily become sick, supports the hypothesis that genetic risk or protective factors are at play.
In an interview with this news organization, Mayana Zatz, PhD, head professor of genetics and coordinator of the Human Genome and Stem Cell Study Center at the University of São Paulo, explained: “The first case that caught my eye was the case of my neighbors, a couple. He presented COVID-19 symptoms, but his wife, who took care of him, had absolutely no symptoms. I thought that it was strange, but we received 3,000 emails from people saying, ‘This happened to me, too.’”
Reports in the media about seven pairs of monozygotic (MZ) twins who died from COVID-19 within days of one another in Brazil also stood out, said the researcher.
, as well as their pathology. Dr. Zatz’s team analyzed the case of a 31-year-old Brazilian MZ twin brother pair who presented simultaneously with severe COVID-19 and the need for oxygen support, despite their age and good health conditions. Curiously, they were admitted and intubated on the same day, but neither of the twins knew about the other’s situation; they found out only when they were extubated.
The study was carried out at the USP with the collaboration of the State University of São Paulo. The authors mapped the genetic profile (by sequencing the genome responsible for coding proteins, or whole-exome sequencing) and the immune cell profile to evaluate innate and adaptive immunity.
The MZ twin brothers shared the same two rare genetic mutations, which may be associated with their increased risk of developing severe COVID-19. However, since these variants were not studied at the protein or functional level, their pathogenicity has yet to be determined. The twins also had [human leukocyte antigen (HLA)] alleles associated with severe COVID-19, which are important candidates for the mechanisms of innate and adaptive immunity and susceptibility to COVID-19 infection and manifestation.
But one particular oddity stood out to the researchers: One of the brothers required longer hospitalization, and only he reported symptoms of long COVID.
In the authors’ eyes, even though the patients shared genetic mutations potentially associated with the risk of developing severe COVID-19, the differences in clinical progression emphasize that, beyond genetic risk factors, continuous exposure to pathogens over a lifetime and other environmental factors mean that each individual’s immune response is unique, even in twins.
“There is no doubt that genetics contribute to the severity of COVID-19, and environmental factors sometimes give us the opportunity to study the disease, too. Such [is the case with] MZ twins who have genetic similarities, even with changes that take place over a lifetime,” José Eduardo Krieger, MD, PhD, professor of molecular medicine at the University of São Paulo Medical School (FMUSP), told this news organization. “Examining MZ twins is a strategy that may help, but, with n = 2, luck really needs to be on your side to get straight to the problem. You need to combine [these findings] with other studies to solve this conundrum,” said Dr. Krieger, who did not take part in the research.
Large cohorts
Genomic and computer resources allow for the study of large sets of data from thousands of individuals. In each of those sets of data, the signal offered by thousands of markers distributed throughout the genome can be studied. This is the possibility offered by various genomic studies of large cohorts of patients with different clinical manifestations.
“Researchers examine thousands of genetic variants throughout the genome from a large sample of individuals and have the chance, for example, to identify genetic variants that are more prevalent in patients who have presented with severe disease than in those who presented with milder disease,” said Dr. Krieger. “These associations highlight a chromosome region in which one or more genes explain, at least in part, the differences observed.”
Genomewide association studies have identified some genetic variants that indicate severity of COVID-19, with potential impact on the virus entering the cell, the immune response, or the development of cytokine storms.
One of these studies, COVID-19 Host Genetics Initiative (COVID-19 HGI), is an international, open-science collaboration for sharing scientific methods and resources with research groups across the world, with the goal of robustly mapping the host genetic determinants of SARS-CoV-2 infection and the severity of the resulting COVID-19 disease. At the start of 2021, the COVID-19 HGI combined genetic data from 49,562 cases and 2 million controls from 46 studies in 19 countries. A total of 853 samples from the BRACOVID study were included in the meta-analysis. The endeavor enabled the identification of 13 genomewide significant loci that are associated with SARS-CoV-2 infection or severe manifestations of COVID-19.
The BRACOVID study, in which Dr. Krieger participates, aims to identify host genetic factors that determine the severity of COVID-19. It is currently the largest project of its kind in Latin America. An article provides the analysis of the first 5,233 participants in the BRACOVID study, who were recruited in São Paulo. Of these participants, 3,533 had been infected with COVID-19 and hospitalized at either the Heart Institute or the Central Institute of the FMUSP General Hospital. The remaining 1,700 made up the control group, which included health care professionals and members of the general population. The controls were recruited through serology assays or PCR tests for SARS-CoV-2.
The researchers discovered a region of chromosome 1 that could play a role in modulating immune response and that could lead to an increase in the likelihood of hospitalization across a wide range of COVID-19 risk factors. This region of chromosome 1 was observed only in Brazilians with a strong European ancestry; however, this finding had not been mentioned in previous studies, suggesting that it could harbor a risk allele specific to the Brazilian population.
The study also confirmed most, but not all, of the regions recorded in the literature, which may be significant in identifying factors determining severity that are specific to a given population.
Including information from the BRACOVID study, other studies have enhanced the knowledge on affected organs. Combined data from 14,000 patients from nine countries evaluated a region of a single chromosome and found that carriers of a certain allele had a higher probability of experiencing various COVID-19 complications, such as severe respiratory failure, venous thromboembolism, and liver damage. The risk was even higher for individuals aged 60 years and over.
Discordant couples
Smaller sample sizes of underrepresented populations also provide relevant data for genomic studies. Dr. Zatz’s team carried out genomic studies on smaller groups, comparing serodiscordant couples (where one was infected and symptomatic while the partner remained asymptomatic and seronegative despite sharing the same bedroom during the infection). Their research found genetic variants related to immune response that were associated with susceptibility to infection and progression to severe COVID-19.
The team also went on to study a group of patients older than 90 years who recovered from COVID-19 with mild symptoms or who remained asymptomatic following a positive test for SARS-CoV-2. They compared these patients with a sample of elderly patients from the same city (São Paulo), sampled before the current pandemic. The researchers identified a genetic variant related to mucin production. “In individuals with mild COVID-19, the degradation of these mucins would be more efficient,” said Dr. Zatz. It is possible for this variant to interfere not only with the production of mucus, but also in its composition, as there is an exchange of amino acids in the protein.
“We continued the study by comparing the extremes, i.e., those in their 90s with mild COVID-19 and younger patients with severe COVID-19, including several who died,” said Dr. Zatz.
More personalized medicine
The specialists agreed that a genetic test to predict COVID-19 severity is still a long way away. The genetic component is too little understood to enable the evaluation of individual risk. It has been possible to identify several important areas but, as Dr. Krieger pointed out, a variant identified in a certain chromosome interval may not be just one gene. There may be various candidate genes, or there may be a regulatory sequence for a distant gene. Furthermore, there are regions with genes that make sense as moderators of COVID-19 severity, because they regulate an inflammatory or immunologic reaction, but evidence is still lacking.
Reaching the molecular mechanism would, in future, allow a medicine to be chosen for a given patient, as already happens with other diseases. It also could enable the discovery of new medicines following as-yet-unexplored lines of research. Dr. Zatz also considers the possibility of genetic therapy.
Even with the knowledge of human genetics, one part of the equation is missing: viral genetics. “Many of the individuals who were resistant to the Delta variant were later affected by Omicron,” she pointed out.
Significance of Brazil
“We have an infinite amount of genomic data worldwide, but the vast majority originates from White Americans of European origin,” said Dr. Krieger. Moreover, genomic associations of COVID-19 severity discovered in the Chinese population were not significant in the European population. Besides underscoring the importance of collaborating with international studies, this situation supports scientists’ interest in carrying out genetic studies within Brazil, he added.
“In the genomic study of the Brazilian population, we found 2 million variants that were not present in the European populations,” said Dr. Zatz.
Dr. Krieger mentioned a technical advantage that Brazil has. “Having been colonized by different ethnic groups and mixed many generations ago, Brazil has a population with a unique genetic structure; the recombinations are different. When preparing the samples, the regions break differently.” This factor could help to separate, in a candidate region, the gene that is significant from those that might not be.
In general, severe COVID-19 would be a complex phenomenon involving several genes and interactions with environmental factors. The Brazilian studies tried to find a factor that was unique to Brazil, but the significance of the differences remained unclear. “We found some signs that were specific to our population,” concluded Dr. Krieger. “But the reason that more people in Brazil died as a result of COVID-19 was not genetic,” he added.
Dr. Zatz and Dr. Krieger reported no conflicts of interest. This article was translated from the Medscape Portuguese edition.
A version of this article first appeared on Medscape.com.
The surprising failure of vitamin D in deficient kids
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
My basic gripe is that you’ve got all these observational studies linking lower levels of vitamin D to everything from dementia to falls to cancer to COVID infection, and then you do a big randomized trial of supplementation and don’t see an effect.
And the explanation is that vitamin D is not necessarily the thing causing these bad outcomes; it’s a bystander – a canary in the coal mine. Your vitamin D level is a marker of your lifestyle; it’s higher in people who eat healthier foods, who exercise, and who spend more time out in the sun.
And yet ... if you were to ask me whether supplementing vitamin D in children with vitamin D deficiency would help them grow better and be healthier, I probably would have been on board for the idea.
And, it looks like, I would have been wrong.
Yes, it’s another negative randomized trial of vitamin D supplementation to add to the seemingly ever-growing body of literature suggesting that your money is better spent on a day at the park rather than buying D3 from your local GNC.
We are talking about this study, appearing in JAMA Pediatrics.
Briefly, 8,851 children from around Ulaanbaatar, Mongolia, were randomized to receive 14,000 international units of vitamin D3 or placebo every week for 3 years.
Before we get into the results of the study, I need to point out that this part of Mongolia has a high rate of vitamin D deficiency. Beyond that, a prior observational study by these authors had shown that lower vitamin D levels were linked to the risk of acquiring latent tuberculosis infection in this area. Other studies have linked vitamin D deficiency with poorer growth metrics in children. Given the global scourge that is TB (around 2 million deaths a year) and childhood malnutrition (around 10% of children around the world), vitamin D supplementation is incredibly attractive as a public health intervention. It is relatively low on side effects and, importantly, it is cheap – and thus scalable.
Back to the study. These kids had pretty poor vitamin D levels at baseline; 95% of them were deficient, based on the accepted standard of levels less than 20 ng/mL. Over 30% were severely deficient, with levels less than 10 ng/mL.
The initial purpose of this study was to see if supplementation would prevent TB, but that analysis, which was published a few months ago, was negative. Vitamin D levels went up dramatically in the intervention group – they were taking their pills – but there was no difference in the rate of latent TB infection, active TB, other respiratory infections, or even serum interferon gamma levels.
Nothing.
But to be fair, the TB seroconversion rate was lower than expected, potentially leading to an underpowered study.
Which brings us to the just-published analysis which moves away from infectious disease to something where vitamin D should have some stronger footing: growth.
Would the kids who were randomized to vitamin D, those same kids who got their vitamin D levels into the normal range over 3 years of supplementation, grow more or grow better than the kids who didn’t?
And, unfortunately, the answer is still no.
At the end of follow-up, height z scores were not different between the groups. BMI z scores were not different between the groups. Pubertal development was not different between the groups. This was true not only overall, but across various subgroups, including analyses of those kids who had vitamin D levels less than 10 ng/mL to start with.
So, what’s going on? There are two very broad possibilities we can endorse. First, there’s the idea that vitamin D supplementation simply doesn’t do much for health. This is supported, now, by a long string of large clinical trials that show no effect across a variety of disease states and predisease states. In other words, the observational data linking low vitamin D to bad outcomes is correlation, not causation.
Or we can take the tack of some vitamin D apologists and decide that this trial just got it wrong. Perhaps the dose wasn’t given correctly, or 3 years isn’t long enough to see a real difference, or the growth metrics were wrong, or vitamin D needs to be given alongside something else to really work and so on. This is fine; no study is perfect and there is always something to criticize, believe me. But we need to be careful not to fall into the baby-and-bathwater fallacy. Just because we think a study could have done something better, or differently, doesn’t mean we can ignore all the results. And as each new randomized trial of vitamin D supplementation comes out, it’s getting harder and harder to believe that these trialists keep getting their methods wrong. Maybe they are just testing something that doesn’t work.
What to do? Well, it should be obvious. If low vitamin D levels are linked to TB rates and poor growth but supplementation doesn’t fix the problem, then we have to fix what is upstream of the problem. We need to boost vitamin D levels not through supplements, but through nutrition, exercise, activity, and getting outside. That’s a randomized trial you can sign me up for any day.
Dr. Wilson is associate professor, department of medicine, Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this video transcript first appeared on Medscape.com.
U.S. flu activity already at mid-season levels
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.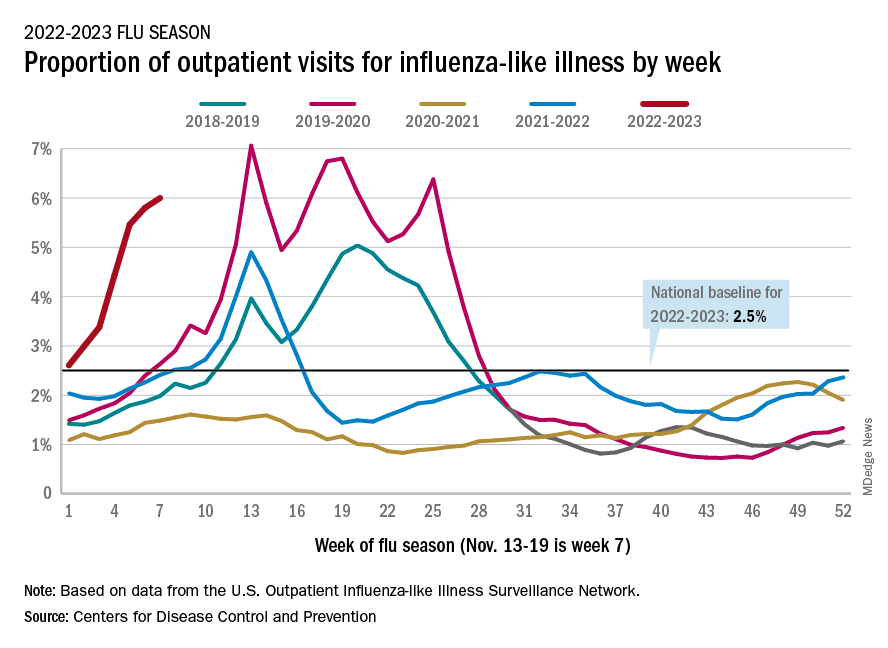
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
Is it long COVID, or dementia, or both?
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
More work needed to optimize STI screening in primary care settings
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study affirms shorter regimens for drug-resistant tuberculosis
Two short-course bedaquiline-containing treatment regimens for rifampicin-resistant tuberculosis showed “robust evidence” for superior efficacy and less ototoxicity compared to a 9-month injectable control regimen, researchers report.
The findings validate the World Health Organization’s current recommendation of a 9-month, bedaquiline-based oral regimen, “which was based only on observational data,” noted lead author Ruth Goodall, PhD, from the Medical Research Council Clinical Trials Unit at University College London, and colleagues.
The study was published in The Lancet.
The Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients With MDR-TB (STREAM) stage 2 study was a randomized, phase 3, noninferiority trial conducted at 13 hospital clinics in seven countries that had prespecified tests for superiority if noninferiority was shown. The study enrolled individuals aged 15 years or older who had rifampicin-resistant TB without fluoroquinolone or aminoglycoside resistance.
The study’s first stage, STREAM stage 1, showed that The 9-month regimen was recommended by the WHO in 2016. That recommendation was superceded in 2020 when concerns of hearing loss associated with aminoglycosides prompted the WHO to endorse a 9-month bedaquiline-containing, injectable-free alternative, the authors write.
Seeking shorter treatment for better outcomes
STREAM stage 2 used a 9-month injectable regimen as its control. The investigators measured it against a fully oral 9-month bedaquiline-based treatment (primary comparison), as well as a 6-month oral bedaquiline regimen that included 8 weeks of a second-line injectable (secondary comparison).
The 9-month fully oral treatment included levofloxacin, clofazimine, ethambutol, and pyrazinamide for 40 weeks; bedaquiline, high-dose isoniazid, and prothionamide were given for the 16-week intensive phase.
The 6-month regimen included bedaquiline, clofazimine, pyrazinamide, and levofloxacin for 28 weeks, supplemented by high-dose isoniazid with kanamycin for an 8-week intensive phase.
For both comparisons, the primary outcome was favorable status at 76 weeks, defined as cultures that were negative for Mycobacterium tuberculosis without a preceding unfavorable outcome (defined as any death, bacteriologic failure or recurrence, or major treatment change).
Among 517 participants in the modified intention-to-treat population across the study groups, 62% were men, and 38% were women (median age, 32.5 years).
For the primary comparison, 71% of the control group and 83% of the oral regimen group had a favorable outcome.
In the secondary comparison, 69% had a favorable outcome in the control group, compared with 91% of those receiving the 6-month regimen.
Although the rate of grade 3 or 4 adverse events was similar in all three groups, there was significantly less ototoxicity among patients who received the oral regimen, compared with control patients (2% vs. 9%); 4% of those taking the 6-month regimen had hearing loss, compared with 8% of control patients.
Exploratory analyses comparing both bedaquiline-containing regimens revealed a significantly higher proportion of favorable outcomes among participants receiving the 6-month regimen (91%), compared with patients taking the fully oral 9-month regimen (79%). There were no significant differences in the rate of grade 3 or 4 adverse events.
The trial’s main limitation was its open-label design, which might have influenced decisions about treatment change, note the investigators.
“STREAM stage 2 has shown that two short-course, bedaquiline-containing regimens are not only non-inferior but superior to a 9-month injectable-containing regimen,” they conclude.
“The STREAM stage 2 fully oral regimen avoided the toxicity of aminoglycosides, and the 6-month regimen was highly effective, with reduced levels of ototoxicity. These two regimens offer promising treatment options for patients with MDR or rifampicin-resistant tuberculosis,” the authors write.
Dr. Goodall added, “Although both STREAM regimens were very effective, participants experienced relatively high levels of adverse events during the trial (though many of these were likely due to the close laboratory monitoring of the trial).
“While hearing loss was reduced on the 6-month regimen, it was not entirely eliminated,” she said. “Other new regimens in the field containing the medicine linezolid report side effects such as anemia and peripheral neuropathy. So more work needs to be done to ensure the treatment regimens are as safe and tolerable for patients as possible. In addition, even 6 months’ treatment is long for patients to tolerate, and further regimen shortening would be a welcome development for patients and health systems.”
‘A revolution in MDR tuberculosis’
“The authors must be commended on completing this challenging high-quality, phase 3, non-inferiority, randomized controlled trial involving 13 health care facilities across Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, and Uganda ... despite the COVID-19 pandemic,” noted Keertan Dheda, MD, PhD, and Christoph Lange, MD, PhD, in an accompanying comment titled, “A Revolution in the Management of Multidrug-Resistant Tuberculosis”.
Although the WHO recently approved an all-oral 6-month bedaquiline, pretomanid, and linezolid plus moxifloxacin (BPaLM) regimen, results from the alternate 6-month regimen examined in STREAM stage 2 “do provide confidence in using 2 months of an injectable as part of a salvage regimen in patients for whom MDR tuberculosis treatment is not successful” or in those with extensively drug-resistant (XDR) or pre-XDR TB, “for whom therapeutic options are few,” noted Dr. Dheda, from the University of Cape Town (South Africa) and the London School of Hygiene and Tropical Medicine, and Dr. Lange, from the University of Lübeck (Germany), Baylor College of Medicine, and Texas Children’s Hospital, both in Houston.
The study authors and the commentators stress that safer and simpler treatments are still needed for MDR TB. “The search is now on for regimens that could further reduce duration, toxicity, and pill burden,” note Dr. Dheda and Dr. Lange.
However, they also note that “substantial resistance” to bedaquiline is already emerging. “Therefore, if we are to protect key drugs from becoming functionally redundant, drug-susceptibility testing capacity will need to be rapidly improved to minimize resistance amplification and onward disease transmission.”
The study was funded by USAID and Janssen Research and Development. Dr. Goodall has disclosed no relevant financial relationships. Dr. Dheda has received funding from the EU and the South African Medical Research Council for studies related to the diagnosis or management of drug-resistant tuberculosis. Dr. Lange is supported by the German Center for Infection Research and has received funding from the European Commission for studies on the development of novel antituberculosis medicines and for studies related to novel diagnostics of tuberculosis; consulting fees from INSMED; speaker’s fees from INSMED, GILEAD, and Janssen; and is a member of the data safety board of trials from Medicines sans Frontiers, all of which are unrelated to the current study.
A version of this article first appeared on Medscape.com.
Two short-course bedaquiline-containing treatment regimens for rifampicin-resistant tuberculosis showed “robust evidence” for superior efficacy and less ototoxicity compared to a 9-month injectable control regimen, researchers report.
The findings validate the World Health Organization’s current recommendation of a 9-month, bedaquiline-based oral regimen, “which was based only on observational data,” noted lead author Ruth Goodall, PhD, from the Medical Research Council Clinical Trials Unit at University College London, and colleagues.
The study was published in The Lancet.
The Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients With MDR-TB (STREAM) stage 2 study was a randomized, phase 3, noninferiority trial conducted at 13 hospital clinics in seven countries that had prespecified tests for superiority if noninferiority was shown. The study enrolled individuals aged 15 years or older who had rifampicin-resistant TB without fluoroquinolone or aminoglycoside resistance.
The study’s first stage, STREAM stage 1, showed that The 9-month regimen was recommended by the WHO in 2016. That recommendation was superceded in 2020 when concerns of hearing loss associated with aminoglycosides prompted the WHO to endorse a 9-month bedaquiline-containing, injectable-free alternative, the authors write.
Seeking shorter treatment for better outcomes
STREAM stage 2 used a 9-month injectable regimen as its control. The investigators measured it against a fully oral 9-month bedaquiline-based treatment (primary comparison), as well as a 6-month oral bedaquiline regimen that included 8 weeks of a second-line injectable (secondary comparison).
The 9-month fully oral treatment included levofloxacin, clofazimine, ethambutol, and pyrazinamide for 40 weeks; bedaquiline, high-dose isoniazid, and prothionamide were given for the 16-week intensive phase.
The 6-month regimen included bedaquiline, clofazimine, pyrazinamide, and levofloxacin for 28 weeks, supplemented by high-dose isoniazid with kanamycin for an 8-week intensive phase.
For both comparisons, the primary outcome was favorable status at 76 weeks, defined as cultures that were negative for Mycobacterium tuberculosis without a preceding unfavorable outcome (defined as any death, bacteriologic failure or recurrence, or major treatment change).
Among 517 participants in the modified intention-to-treat population across the study groups, 62% were men, and 38% were women (median age, 32.5 years).
For the primary comparison, 71% of the control group and 83% of the oral regimen group had a favorable outcome.
In the secondary comparison, 69% had a favorable outcome in the control group, compared with 91% of those receiving the 6-month regimen.
Although the rate of grade 3 or 4 adverse events was similar in all three groups, there was significantly less ototoxicity among patients who received the oral regimen, compared with control patients (2% vs. 9%); 4% of those taking the 6-month regimen had hearing loss, compared with 8% of control patients.
Exploratory analyses comparing both bedaquiline-containing regimens revealed a significantly higher proportion of favorable outcomes among participants receiving the 6-month regimen (91%), compared with patients taking the fully oral 9-month regimen (79%). There were no significant differences in the rate of grade 3 or 4 adverse events.
The trial’s main limitation was its open-label design, which might have influenced decisions about treatment change, note the investigators.
“STREAM stage 2 has shown that two short-course, bedaquiline-containing regimens are not only non-inferior but superior to a 9-month injectable-containing regimen,” they conclude.
“The STREAM stage 2 fully oral regimen avoided the toxicity of aminoglycosides, and the 6-month regimen was highly effective, with reduced levels of ototoxicity. These two regimens offer promising treatment options for patients with MDR or rifampicin-resistant tuberculosis,” the authors write.
Dr. Goodall added, “Although both STREAM regimens were very effective, participants experienced relatively high levels of adverse events during the trial (though many of these were likely due to the close laboratory monitoring of the trial).
“While hearing loss was reduced on the 6-month regimen, it was not entirely eliminated,” she said. “Other new regimens in the field containing the medicine linezolid report side effects such as anemia and peripheral neuropathy. So more work needs to be done to ensure the treatment regimens are as safe and tolerable for patients as possible. In addition, even 6 months’ treatment is long for patients to tolerate, and further regimen shortening would be a welcome development for patients and health systems.”
‘A revolution in MDR tuberculosis’
“The authors must be commended on completing this challenging high-quality, phase 3, non-inferiority, randomized controlled trial involving 13 health care facilities across Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, and Uganda ... despite the COVID-19 pandemic,” noted Keertan Dheda, MD, PhD, and Christoph Lange, MD, PhD, in an accompanying comment titled, “A Revolution in the Management of Multidrug-Resistant Tuberculosis”.
Although the WHO recently approved an all-oral 6-month bedaquiline, pretomanid, and linezolid plus moxifloxacin (BPaLM) regimen, results from the alternate 6-month regimen examined in STREAM stage 2 “do provide confidence in using 2 months of an injectable as part of a salvage regimen in patients for whom MDR tuberculosis treatment is not successful” or in those with extensively drug-resistant (XDR) or pre-XDR TB, “for whom therapeutic options are few,” noted Dr. Dheda, from the University of Cape Town (South Africa) and the London School of Hygiene and Tropical Medicine, and Dr. Lange, from the University of Lübeck (Germany), Baylor College of Medicine, and Texas Children’s Hospital, both in Houston.
The study authors and the commentators stress that safer and simpler treatments are still needed for MDR TB. “The search is now on for regimens that could further reduce duration, toxicity, and pill burden,” note Dr. Dheda and Dr. Lange.
However, they also note that “substantial resistance” to bedaquiline is already emerging. “Therefore, if we are to protect key drugs from becoming functionally redundant, drug-susceptibility testing capacity will need to be rapidly improved to minimize resistance amplification and onward disease transmission.”
The study was funded by USAID and Janssen Research and Development. Dr. Goodall has disclosed no relevant financial relationships. Dr. Dheda has received funding from the EU and the South African Medical Research Council for studies related to the diagnosis or management of drug-resistant tuberculosis. Dr. Lange is supported by the German Center for Infection Research and has received funding from the European Commission for studies on the development of novel antituberculosis medicines and for studies related to novel diagnostics of tuberculosis; consulting fees from INSMED; speaker’s fees from INSMED, GILEAD, and Janssen; and is a member of the data safety board of trials from Medicines sans Frontiers, all of which are unrelated to the current study.
A version of this article first appeared on Medscape.com.
Two short-course bedaquiline-containing treatment regimens for rifampicin-resistant tuberculosis showed “robust evidence” for superior efficacy and less ototoxicity compared to a 9-month injectable control regimen, researchers report.
The findings validate the World Health Organization’s current recommendation of a 9-month, bedaquiline-based oral regimen, “which was based only on observational data,” noted lead author Ruth Goodall, PhD, from the Medical Research Council Clinical Trials Unit at University College London, and colleagues.
The study was published in The Lancet.
The Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients With MDR-TB (STREAM) stage 2 study was a randomized, phase 3, noninferiority trial conducted at 13 hospital clinics in seven countries that had prespecified tests for superiority if noninferiority was shown. The study enrolled individuals aged 15 years or older who had rifampicin-resistant TB without fluoroquinolone or aminoglycoside resistance.
The study’s first stage, STREAM stage 1, showed that The 9-month regimen was recommended by the WHO in 2016. That recommendation was superceded in 2020 when concerns of hearing loss associated with aminoglycosides prompted the WHO to endorse a 9-month bedaquiline-containing, injectable-free alternative, the authors write.
Seeking shorter treatment for better outcomes
STREAM stage 2 used a 9-month injectable regimen as its control. The investigators measured it against a fully oral 9-month bedaquiline-based treatment (primary comparison), as well as a 6-month oral bedaquiline regimen that included 8 weeks of a second-line injectable (secondary comparison).
The 9-month fully oral treatment included levofloxacin, clofazimine, ethambutol, and pyrazinamide for 40 weeks; bedaquiline, high-dose isoniazid, and prothionamide were given for the 16-week intensive phase.
The 6-month regimen included bedaquiline, clofazimine, pyrazinamide, and levofloxacin for 28 weeks, supplemented by high-dose isoniazid with kanamycin for an 8-week intensive phase.
For both comparisons, the primary outcome was favorable status at 76 weeks, defined as cultures that were negative for Mycobacterium tuberculosis without a preceding unfavorable outcome (defined as any death, bacteriologic failure or recurrence, or major treatment change).
Among 517 participants in the modified intention-to-treat population across the study groups, 62% were men, and 38% were women (median age, 32.5 years).
For the primary comparison, 71% of the control group and 83% of the oral regimen group had a favorable outcome.
In the secondary comparison, 69% had a favorable outcome in the control group, compared with 91% of those receiving the 6-month regimen.
Although the rate of grade 3 or 4 adverse events was similar in all three groups, there was significantly less ototoxicity among patients who received the oral regimen, compared with control patients (2% vs. 9%); 4% of those taking the 6-month regimen had hearing loss, compared with 8% of control patients.
Exploratory analyses comparing both bedaquiline-containing regimens revealed a significantly higher proportion of favorable outcomes among participants receiving the 6-month regimen (91%), compared with patients taking the fully oral 9-month regimen (79%). There were no significant differences in the rate of grade 3 or 4 adverse events.
The trial’s main limitation was its open-label design, which might have influenced decisions about treatment change, note the investigators.
“STREAM stage 2 has shown that two short-course, bedaquiline-containing regimens are not only non-inferior but superior to a 9-month injectable-containing regimen,” they conclude.
“The STREAM stage 2 fully oral regimen avoided the toxicity of aminoglycosides, and the 6-month regimen was highly effective, with reduced levels of ototoxicity. These two regimens offer promising treatment options for patients with MDR or rifampicin-resistant tuberculosis,” the authors write.
Dr. Goodall added, “Although both STREAM regimens were very effective, participants experienced relatively high levels of adverse events during the trial (though many of these were likely due to the close laboratory monitoring of the trial).
“While hearing loss was reduced on the 6-month regimen, it was not entirely eliminated,” she said. “Other new regimens in the field containing the medicine linezolid report side effects such as anemia and peripheral neuropathy. So more work needs to be done to ensure the treatment regimens are as safe and tolerable for patients as possible. In addition, even 6 months’ treatment is long for patients to tolerate, and further regimen shortening would be a welcome development for patients and health systems.”
‘A revolution in MDR tuberculosis’
“The authors must be commended on completing this challenging high-quality, phase 3, non-inferiority, randomized controlled trial involving 13 health care facilities across Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, and Uganda ... despite the COVID-19 pandemic,” noted Keertan Dheda, MD, PhD, and Christoph Lange, MD, PhD, in an accompanying comment titled, “A Revolution in the Management of Multidrug-Resistant Tuberculosis”.
Although the WHO recently approved an all-oral 6-month bedaquiline, pretomanid, and linezolid plus moxifloxacin (BPaLM) regimen, results from the alternate 6-month regimen examined in STREAM stage 2 “do provide confidence in using 2 months of an injectable as part of a salvage regimen in patients for whom MDR tuberculosis treatment is not successful” or in those with extensively drug-resistant (XDR) or pre-XDR TB, “for whom therapeutic options are few,” noted Dr. Dheda, from the University of Cape Town (South Africa) and the London School of Hygiene and Tropical Medicine, and Dr. Lange, from the University of Lübeck (Germany), Baylor College of Medicine, and Texas Children’s Hospital, both in Houston.
The study authors and the commentators stress that safer and simpler treatments are still needed for MDR TB. “The search is now on for regimens that could further reduce duration, toxicity, and pill burden,” note Dr. Dheda and Dr. Lange.
However, they also note that “substantial resistance” to bedaquiline is already emerging. “Therefore, if we are to protect key drugs from becoming functionally redundant, drug-susceptibility testing capacity will need to be rapidly improved to minimize resistance amplification and onward disease transmission.”
The study was funded by USAID and Janssen Research and Development. Dr. Goodall has disclosed no relevant financial relationships. Dr. Dheda has received funding from the EU and the South African Medical Research Council for studies related to the diagnosis or management of drug-resistant tuberculosis. Dr. Lange is supported by the German Center for Infection Research and has received funding from the European Commission for studies on the development of novel antituberculosis medicines and for studies related to novel diagnostics of tuberculosis; consulting fees from INSMED; speaker’s fees from INSMED, GILEAD, and Janssen; and is a member of the data safety board of trials from Medicines sans Frontiers, all of which are unrelated to the current study.
A version of this article first appeared on Medscape.com.
Future HIV PrEP innovations aim to address adherence, women’s health, and combination treatments
TAMPA – Pre-exposure prophylaxis (PrEP) has shown to be effective in many clinical and real-world studies, but concerns remain, according to research presented at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Only about 20% of people who could benefit from PrEP use the preventative medication, for example. Another concern is adherence, as regular use generally drops off over time, rarely lasting more than a few months for most people.
Furthermore, most studies to date evaluated safety and effectiveness of PrEP options among men who have sex with men. Now the focus is increasing on other populations, including women at risk of HIV exposure.
Researchers working on new forms and formulations of PrEP are looking for ways to address those challenges.
said Craig W. Hendrix, MD, professor and director of the Division of Clinical Pharmacology at Johns Hopkins University School of Medicine, Baltimore.
“What I hear a lot of folks say [is] there are two or three options for PrEP, so why do we need more? We need choices that fit into a broader range of lifestyles,” Dr. Hendrix said.
For example, a medically fortified douche containing PrEP might be more likely to be used by people who use a douche before or after sex on a regular basis. This is called a “behaviorally congruent” strategy, Dr. Hendrix said.
In addition to a medical douche, formulations designed to continuously deliver PrEP, such as a subdermal implant, are in the works as well.
Another option for women, the dapivirine vaginal ring, is available internationally but not in the United States. “It was withdrawn from [Food and Drug Administration] consideration by the sponsor. I think it’s a huge loss not to have that,” Dr. Hendrix said.
During development, “frequent expulsions forced reformulation to a less stiff ring,” Dr. Hendrix said. “I don’t imagine that’s terrific, but it shows how important it is to have something that fits the anatomy and the lifestyle.”
“Currently, we have in the U.S. three licensed, really terrific options for PrEP, and they’re all for men that have sex with men and transgender women,” Dr. Hendrix said.
Three current options
The three current PrEP regimens in the United States often go by their abbreviations: F/TDF, F/TAF, and CAB-IM.
- F/TDF is emtricitabine (F) 200 mg in combination with tenofovir disoproxil fumarate (TDF) 300 mg (Truvada, Gilead or generics)
- F/TAF is emtricitabine (F) 200 mg in combination with tenofovir alafenamide (TAF) 25 mg (Descovy, Gilead)
- CAB-IM is cabotegravir (CAB) 600 mg injection (Apretude, GlaxoSmithKline)
There is an important distinction: Daily oral PrEP with F/TDF is recommended to prevent HIV infection among all people at risk through sex or injection drug use. Daily oral PrEP with F/TAF is recommended to prevent HIV infection among people at risk through sex, excluding people at risk through receptive vaginal sex, the CDC notes.
The cost-effectiveness of the injection remains a potential issue, Dr. Hendrix said. On the other hand, “cost-effectiveness goes out the window if there is no adherence.”
An active pipeline
There are 24 new PrEP products in development, as well as 24 other multipurpose prevention technologies (MPTs), which are combination products containing PrEP and one or two other medications.
These 48 products include 28 unique antiviral and contraceptive drugs and 12 delivery methods or formulations. “Why so many?” Dr. Hendrix asked. “Many will not make it through development.”
Pills that include HIV PrEP and contraception or PrEP and sexually transmitted infection (STI) treatment are being evaluated, for example. “HIV risk, pregnancy risk, and other viral STIs overlap. Ideally, you can have one target for all three. That would increase efficiency of dosing and adherence,” Dr. Hendrix said.
Dual prevention pills (DPPs) hypothetically provide HIV PrEP and contraception better than either product alone, Dr. Hendrix said. Plans are to market them as family planning or women’s health products to avoid any stigma or distrust associated with HIV PrEP. An initial rollout is planned in 2024 in sub-Saharan Africa where the unmet need is highest, he added.
“Imagine how effective this could be in women in the United States,” Dr. Hendrix said. “My hope is fourth-quarter 2024” availability in the United States.
A way to prevent STIs and HIV in an all-in-one product “would be terrific,” Dr. Hendrix said.
“I think we’re going to see a lot more innovation going in that direction. The pill is close. The other things are going to be further off because the regulatory pathway is a little more complicated.”
Longer lasting protection?
All of the innovations have gone one of two directions, Dr. Hendrix said. One direction is to make PrEP even longer acting, “so that you have even less to worry [about] in terms of adherence.”
Going forward, “most of the focus has all been on continuously acting or long-active PrEP. It’s getting longer and longer: We’ve got 2 months, and they’re looking at a 6-month subcutaneous injection,” Dr. Hendrix said. The investigational agent lenacapavir is in development as PrEP, as well as for HIV treatment.
“This could get us from 2 to 6 months,” Dr. Hendrix said.
Some of the subcutaneous implants look as if they could provide PrEP for up to 12 months, he added. “An implant could also avoid peaks and troughs with bi-monthly injections.”
On-demand PrEP
The other direction is on-demand. “This is for the folks that don’t want drug in their body all the time. They only want it when they need it. And a twist on that ... is actually using products that are already used with sex now but medicating them.”
On-demand rectal options include a medicated douche and a fast-dissolving insert or suppository.
Fast-dissolving vaginal inserts are also in development. “These inserts are small, easy to store, inexpensive, and possibly inapparent to a partner,” Dr. Hendrix said.
Phase 2 studies will need to determine if these products “fit into folks’ active sex lives,” he said. “There’s still a need for human-friendly, human-designed products.”
A rectal microbicide that got as far as Phase 2 research provides a cautionary tale. The concentrations and the biology worked fine, Dr. Hendrix said. “It was a gel with an applicator, and it just was not liked by the folks in the study.” He added, “Your adherence is going to be in the tank if you’ve got a product that people don’t like to use.”
‘Extremely excited’
Asked for her perspective on Dr. Hendrix’s presentation, session moderator Rasheeta D. Chandler, PhD, RN, an associate professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said: “I am extremely excited, because I work with cisgender women, particularly with underserved women and women of color, and there’s a tendency to focus on men who have sex with men.”
“I understand, because they are the population that is most affected, but Black women are also extremely affected by this disease,” Dr. Chandler told this news organization.
Dr. Chandler applauded Dr. Hendrix for addressing women’s health needs as well and not treating PrEP in women “as an afterthought.”
“Finally, our voices are being heard that [PrEP] should be equitable across all different types of individuals who identify differently in a sexual context,” Dr. Chandler said.
More work is warranted to evaluate PrEP in other populations, including transgender men and individuals who inject drugs, Dr. Hendrix said.
For more information and updates on HIV PrEP and MPTs, visit the website of the nonprofit AIDS Vaccine Advocacy Coalition.
Dr. Hendrix has disclosed receiving research grants from Gilead and Merck. Dr. Chandler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Pre-exposure prophylaxis (PrEP) has shown to be effective in many clinical and real-world studies, but concerns remain, according to research presented at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Only about 20% of people who could benefit from PrEP use the preventative medication, for example. Another concern is adherence, as regular use generally drops off over time, rarely lasting more than a few months for most people.
Furthermore, most studies to date evaluated safety and effectiveness of PrEP options among men who have sex with men. Now the focus is increasing on other populations, including women at risk of HIV exposure.
Researchers working on new forms and formulations of PrEP are looking for ways to address those challenges.
said Craig W. Hendrix, MD, professor and director of the Division of Clinical Pharmacology at Johns Hopkins University School of Medicine, Baltimore.
“What I hear a lot of folks say [is] there are two or three options for PrEP, so why do we need more? We need choices that fit into a broader range of lifestyles,” Dr. Hendrix said.
For example, a medically fortified douche containing PrEP might be more likely to be used by people who use a douche before or after sex on a regular basis. This is called a “behaviorally congruent” strategy, Dr. Hendrix said.
In addition to a medical douche, formulations designed to continuously deliver PrEP, such as a subdermal implant, are in the works as well.
Another option for women, the dapivirine vaginal ring, is available internationally but not in the United States. “It was withdrawn from [Food and Drug Administration] consideration by the sponsor. I think it’s a huge loss not to have that,” Dr. Hendrix said.
During development, “frequent expulsions forced reformulation to a less stiff ring,” Dr. Hendrix said. “I don’t imagine that’s terrific, but it shows how important it is to have something that fits the anatomy and the lifestyle.”
“Currently, we have in the U.S. three licensed, really terrific options for PrEP, and they’re all for men that have sex with men and transgender women,” Dr. Hendrix said.
Three current options
The three current PrEP regimens in the United States often go by their abbreviations: F/TDF, F/TAF, and CAB-IM.
- F/TDF is emtricitabine (F) 200 mg in combination with tenofovir disoproxil fumarate (TDF) 300 mg (Truvada, Gilead or generics)
- F/TAF is emtricitabine (F) 200 mg in combination with tenofovir alafenamide (TAF) 25 mg (Descovy, Gilead)
- CAB-IM is cabotegravir (CAB) 600 mg injection (Apretude, GlaxoSmithKline)
There is an important distinction: Daily oral PrEP with F/TDF is recommended to prevent HIV infection among all people at risk through sex or injection drug use. Daily oral PrEP with F/TAF is recommended to prevent HIV infection among people at risk through sex, excluding people at risk through receptive vaginal sex, the CDC notes.
The cost-effectiveness of the injection remains a potential issue, Dr. Hendrix said. On the other hand, “cost-effectiveness goes out the window if there is no adherence.”
An active pipeline
There are 24 new PrEP products in development, as well as 24 other multipurpose prevention technologies (MPTs), which are combination products containing PrEP and one or two other medications.
These 48 products include 28 unique antiviral and contraceptive drugs and 12 delivery methods or formulations. “Why so many?” Dr. Hendrix asked. “Many will not make it through development.”
Pills that include HIV PrEP and contraception or PrEP and sexually transmitted infection (STI) treatment are being evaluated, for example. “HIV risk, pregnancy risk, and other viral STIs overlap. Ideally, you can have one target for all three. That would increase efficiency of dosing and adherence,” Dr. Hendrix said.
Dual prevention pills (DPPs) hypothetically provide HIV PrEP and contraception better than either product alone, Dr. Hendrix said. Plans are to market them as family planning or women’s health products to avoid any stigma or distrust associated with HIV PrEP. An initial rollout is planned in 2024 in sub-Saharan Africa where the unmet need is highest, he added.
“Imagine how effective this could be in women in the United States,” Dr. Hendrix said. “My hope is fourth-quarter 2024” availability in the United States.
A way to prevent STIs and HIV in an all-in-one product “would be terrific,” Dr. Hendrix said.
“I think we’re going to see a lot more innovation going in that direction. The pill is close. The other things are going to be further off because the regulatory pathway is a little more complicated.”
Longer lasting protection?
All of the innovations have gone one of two directions, Dr. Hendrix said. One direction is to make PrEP even longer acting, “so that you have even less to worry [about] in terms of adherence.”
Going forward, “most of the focus has all been on continuously acting or long-active PrEP. It’s getting longer and longer: We’ve got 2 months, and they’re looking at a 6-month subcutaneous injection,” Dr. Hendrix said. The investigational agent lenacapavir is in development as PrEP, as well as for HIV treatment.
“This could get us from 2 to 6 months,” Dr. Hendrix said.
Some of the subcutaneous implants look as if they could provide PrEP for up to 12 months, he added. “An implant could also avoid peaks and troughs with bi-monthly injections.”
On-demand PrEP
The other direction is on-demand. “This is for the folks that don’t want drug in their body all the time. They only want it when they need it. And a twist on that ... is actually using products that are already used with sex now but medicating them.”
On-demand rectal options include a medicated douche and a fast-dissolving insert or suppository.
Fast-dissolving vaginal inserts are also in development. “These inserts are small, easy to store, inexpensive, and possibly inapparent to a partner,” Dr. Hendrix said.
Phase 2 studies will need to determine if these products “fit into folks’ active sex lives,” he said. “There’s still a need for human-friendly, human-designed products.”
A rectal microbicide that got as far as Phase 2 research provides a cautionary tale. The concentrations and the biology worked fine, Dr. Hendrix said. “It was a gel with an applicator, and it just was not liked by the folks in the study.” He added, “Your adherence is going to be in the tank if you’ve got a product that people don’t like to use.”
‘Extremely excited’
Asked for her perspective on Dr. Hendrix’s presentation, session moderator Rasheeta D. Chandler, PhD, RN, an associate professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said: “I am extremely excited, because I work with cisgender women, particularly with underserved women and women of color, and there’s a tendency to focus on men who have sex with men.”
“I understand, because they are the population that is most affected, but Black women are also extremely affected by this disease,” Dr. Chandler told this news organization.
Dr. Chandler applauded Dr. Hendrix for addressing women’s health needs as well and not treating PrEP in women “as an afterthought.”
“Finally, our voices are being heard that [PrEP] should be equitable across all different types of individuals who identify differently in a sexual context,” Dr. Chandler said.
More work is warranted to evaluate PrEP in other populations, including transgender men and individuals who inject drugs, Dr. Hendrix said.
For more information and updates on HIV PrEP and MPTs, visit the website of the nonprofit AIDS Vaccine Advocacy Coalition.
Dr. Hendrix has disclosed receiving research grants from Gilead and Merck. Dr. Chandler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Pre-exposure prophylaxis (PrEP) has shown to be effective in many clinical and real-world studies, but concerns remain, according to research presented at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Only about 20% of people who could benefit from PrEP use the preventative medication, for example. Another concern is adherence, as regular use generally drops off over time, rarely lasting more than a few months for most people.
Furthermore, most studies to date evaluated safety and effectiveness of PrEP options among men who have sex with men. Now the focus is increasing on other populations, including women at risk of HIV exposure.
Researchers working on new forms and formulations of PrEP are looking for ways to address those challenges.
said Craig W. Hendrix, MD, professor and director of the Division of Clinical Pharmacology at Johns Hopkins University School of Medicine, Baltimore.
“What I hear a lot of folks say [is] there are two or three options for PrEP, so why do we need more? We need choices that fit into a broader range of lifestyles,” Dr. Hendrix said.
For example, a medically fortified douche containing PrEP might be more likely to be used by people who use a douche before or after sex on a regular basis. This is called a “behaviorally congruent” strategy, Dr. Hendrix said.
In addition to a medical douche, formulations designed to continuously deliver PrEP, such as a subdermal implant, are in the works as well.
Another option for women, the dapivirine vaginal ring, is available internationally but not in the United States. “It was withdrawn from [Food and Drug Administration] consideration by the sponsor. I think it’s a huge loss not to have that,” Dr. Hendrix said.
During development, “frequent expulsions forced reformulation to a less stiff ring,” Dr. Hendrix said. “I don’t imagine that’s terrific, but it shows how important it is to have something that fits the anatomy and the lifestyle.”
“Currently, we have in the U.S. three licensed, really terrific options for PrEP, and they’re all for men that have sex with men and transgender women,” Dr. Hendrix said.
Three current options
The three current PrEP regimens in the United States often go by their abbreviations: F/TDF, F/TAF, and CAB-IM.
- F/TDF is emtricitabine (F) 200 mg in combination with tenofovir disoproxil fumarate (TDF) 300 mg (Truvada, Gilead or generics)
- F/TAF is emtricitabine (F) 200 mg in combination with tenofovir alafenamide (TAF) 25 mg (Descovy, Gilead)
- CAB-IM is cabotegravir (CAB) 600 mg injection (Apretude, GlaxoSmithKline)
There is an important distinction: Daily oral PrEP with F/TDF is recommended to prevent HIV infection among all people at risk through sex or injection drug use. Daily oral PrEP with F/TAF is recommended to prevent HIV infection among people at risk through sex, excluding people at risk through receptive vaginal sex, the CDC notes.
The cost-effectiveness of the injection remains a potential issue, Dr. Hendrix said. On the other hand, “cost-effectiveness goes out the window if there is no adherence.”
An active pipeline
There are 24 new PrEP products in development, as well as 24 other multipurpose prevention technologies (MPTs), which are combination products containing PrEP and one or two other medications.
These 48 products include 28 unique antiviral and contraceptive drugs and 12 delivery methods or formulations. “Why so many?” Dr. Hendrix asked. “Many will not make it through development.”
Pills that include HIV PrEP and contraception or PrEP and sexually transmitted infection (STI) treatment are being evaluated, for example. “HIV risk, pregnancy risk, and other viral STIs overlap. Ideally, you can have one target for all three. That would increase efficiency of dosing and adherence,” Dr. Hendrix said.
Dual prevention pills (DPPs) hypothetically provide HIV PrEP and contraception better than either product alone, Dr. Hendrix said. Plans are to market them as family planning or women’s health products to avoid any stigma or distrust associated with HIV PrEP. An initial rollout is planned in 2024 in sub-Saharan Africa where the unmet need is highest, he added.
“Imagine how effective this could be in women in the United States,” Dr. Hendrix said. “My hope is fourth-quarter 2024” availability in the United States.
A way to prevent STIs and HIV in an all-in-one product “would be terrific,” Dr. Hendrix said.
“I think we’re going to see a lot more innovation going in that direction. The pill is close. The other things are going to be further off because the regulatory pathway is a little more complicated.”
Longer lasting protection?
All of the innovations have gone one of two directions, Dr. Hendrix said. One direction is to make PrEP even longer acting, “so that you have even less to worry [about] in terms of adherence.”
Going forward, “most of the focus has all been on continuously acting or long-active PrEP. It’s getting longer and longer: We’ve got 2 months, and they’re looking at a 6-month subcutaneous injection,” Dr. Hendrix said. The investigational agent lenacapavir is in development as PrEP, as well as for HIV treatment.
“This could get us from 2 to 6 months,” Dr. Hendrix said.
Some of the subcutaneous implants look as if they could provide PrEP for up to 12 months, he added. “An implant could also avoid peaks and troughs with bi-monthly injections.”
On-demand PrEP
The other direction is on-demand. “This is for the folks that don’t want drug in their body all the time. They only want it when they need it. And a twist on that ... is actually using products that are already used with sex now but medicating them.”
On-demand rectal options include a medicated douche and a fast-dissolving insert or suppository.
Fast-dissolving vaginal inserts are also in development. “These inserts are small, easy to store, inexpensive, and possibly inapparent to a partner,” Dr. Hendrix said.
Phase 2 studies will need to determine if these products “fit into folks’ active sex lives,” he said. “There’s still a need for human-friendly, human-designed products.”
A rectal microbicide that got as far as Phase 2 research provides a cautionary tale. The concentrations and the biology worked fine, Dr. Hendrix said. “It was a gel with an applicator, and it just was not liked by the folks in the study.” He added, “Your adherence is going to be in the tank if you’ve got a product that people don’t like to use.”
‘Extremely excited’
Asked for her perspective on Dr. Hendrix’s presentation, session moderator Rasheeta D. Chandler, PhD, RN, an associate professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said: “I am extremely excited, because I work with cisgender women, particularly with underserved women and women of color, and there’s a tendency to focus on men who have sex with men.”
“I understand, because they are the population that is most affected, but Black women are also extremely affected by this disease,” Dr. Chandler told this news organization.
Dr. Chandler applauded Dr. Hendrix for addressing women’s health needs as well and not treating PrEP in women “as an afterthought.”
“Finally, our voices are being heard that [PrEP] should be equitable across all different types of individuals who identify differently in a sexual context,” Dr. Chandler said.
More work is warranted to evaluate PrEP in other populations, including transgender men and individuals who inject drugs, Dr. Hendrix said.
For more information and updates on HIV PrEP and MPTs, visit the website of the nonprofit AIDS Vaccine Advocacy Coalition.
Dr. Hendrix has disclosed receiving research grants from Gilead and Merck. Dr. Chandler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANAC 2022
People living with HIV are a model population for vaccination
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
TAMPA – People living with HIV (PLWH) were more likely than other populations to get vaccinated for flu and COVID-19, to seek reputable sources of information, and to be connected through essential community organizations that share essential health and wellness information, according to the results of a large survey.
PLWH, therefore, would have been an ideal model population for creating and disseminating effective messaging around COVID-19 immunizations earlier in the pandemic, said Kathleen Gallagher, MPH, an epidemiologist, researcher, and health services administrator at the Patient Advocate Foundation.
The PLWH community can still offer valuable insights into effective ways to reach out to people, to disseminate correct information, and to link people with resources, Ms. Gallagher said during a poster presentation at the annual meeting of the Association of Nurses in AIDS Care (ANAC).
Local, community-based organizations “are the people that these individuals trust, they are people entrenched in their community, and they have existing relationships with them in terms of getting vaccinated and listening to their concerns,” Ms. Gallagher said.
“It’s a missed opportunity.”
A highly compliant group
The July 2021 survey of 271 PLWH was part of a larger, longitudinal survey of 1,400 people with any chronic illness asked about attitudes and barriers to vaccination. The PLWH population was important to focus on, the researchers note, because they could be potentially high risk for more serious COVID-19 outcomes.
The PLWH group was 81% White and 90% male, and 83% were age 56 or older. In addition, 86% had an annual household income below $48,000.
Ninety-three percent of the PLWH group had had flu vaccination in the prior 3 years and received at least one COVID-19 vaccination.
Unable vs. unwilling to vaccinate
Ms. Gallagher and colleagues found 12 people (4%) in the PLWH group did not get vaccinated against COVID-19. It’s a small number, “so you have to take this with a grain of salt,” she said. “But we asked them why they were hesitant. They either were unable or unwilling – and the unable part is not surprising.”
Those who were unable to get vaccinated were either homebound or had concerns about being in a clinic where they could be exposed to COVID while waiting to get the vaccine.
“And then there were some who were just not willing” to get vaccinated, Ms. Gallagher said. She added most cited vaccine safety concerns and “a lot of the misinformation or confusing information around efficacy.”
Trusted information sources
Although people reported getting COVID-19 vaccine information from multiple sources, including online and from television, 64% or nearly two-thirds sought information from their doctors or health care teams.
In fact, doctors emerged as the most trusted source, as indicated by 72% of PLWH.
“I was a little surprised that doctors scored so highly because, sometimes in other cohorts that we looked at, it wasn’t the case,” Ms. Gallagher said. However, she added, a lot of PLWH “have a very strong trust bond with their provider because this is a very personal, very sensitive diagnosis.”
How did social media score? “A whopping 1%,” she said. “So at least this was a savvy group, and they realized that that was not the place to go for vaccination information.”
Overcoming barriers
A lack of vaccine availability at the time of their appointment was the number one barrier to immunization. Also, a small number of people said knowing someone who had an adverse reaction to COVID-19 vaccination was a barrier for them. Ms. Gallagher explained that, by definition in the survey, an adverse reaction to vaccination had to be serious enough to drive people to seek medical care.
When asked to comment on the poster, Andrew Komensky, RN, told this news organization that he found the results “interesting, because I’m an infection preventionist, in addition to being an HIV nurse.” He is director of infection prevention and control at CharterCARE Health Partners, Providence, R.I.
Mr. Komensky said he was surprised that a high proportion of PLWH cited their doctor – and not their nurse – as the most trusted source of information. “In my experience in COVID care ... it was a nursing staff who had most of the contact with patients, who did most of the education, and provided most of the information surrounding vaccination and potential side effects.”
It made sense to Mr. Komensky that the PLWH population would be compliant with vaccinations. “People who are living with HIV do everything they possibly can just to stay healthy.”
A version of this article first appeared on Medscape.com.
FROM ANAC 2022
Study finds chronic jet lag–like body clocks in people with HIV
, according to findings that suggest both a possible mechanism for increased comorbidities in PLWH and potential solutions.
“It is very well known that sleep problems are common in people living with HIV, and many different reasons for this have been proposed,” coauthor Malcolm von Schantz, PhD, professor of chronobiology at Northumbria University in Newcastle upon Tyne, England, said in an interview. “But the novelty of our findings is the observation of delayed circadian rhythms.”
The mistimed circadian phase in PLWH is linked to later sleep onset and earlier waking and has “important potential implications” for the health and well-being of PLWH, wrote senior author Karine Scheuermaier, MD, from the University of the Witwatersrand, in Johannesburg, South Africa, and coauthors.
Until now, research on sleep in HIV has focused primarily on its homeostatic components, such as sleep duration and staging, rather than on circadian-related aspects, they noted.
“If the lifestyle‐independent circadian misalignment observed in the current study is confirmed to be a constant feature of chronic HIV infection, then it may be a mediator both of poorer sleep health and of poorer physical health in PLWH, which could potentially be alleviated through light therapy or chronobiotic medication or supplements,” they suggested.
HIV endemic in study population
The study analyzed a random sample of 187 participants (36 with HIV and 151 without) in the HAALSI (Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study, which is part of the Agincourt Health and Socio-demographic Surveillance System.
The study population ranged in age from 45 to 93 years, with an average age of 60.6 years in the HIV-positive group and 68.2 years in the HIV-negative group. Demographic data, Pittsburgh Sleep Quality Index score, and valid actigraphy (measured with an accelerometer for 14 consecutive days) were available for 172 participants (18% with HIV). A subgroup of 51 participants (22% with HIV) also had valid dim light melatonin onset (DLMO) data, a sensitive measure of the internal circadian clock. DLMO was measured for a minimum of 5 consecutive days with hourly saliva sampling between 5 p.m. and 11 p.m. while sitting in a dimly lit room.
In 36 participants (16% with HIV) with both valid actigraphy and DLMO data, circadian phase angle of entrainment was calculated by subtracting DLMO time from habitual sleep-onset time obtained from actigraphy.
After adjustment for age and sex, the study found a slightly later sleep onset (adjusted average delay of 10 minutes), earlier awakening (adjusted average advance of 10 minutes), and shorter sleep duration in PLWH compared with HIV-negative participants.
At the same time, melatonin production in PLWH started more than an hour later on average than in HIV-negative participants, “with half of the HIV+ group having an earlier habitual sleep onset than DLMO time” the authors wrote. In a subgroup of 36 participants with both valid actigraphy and DLMO data, the median circadian phase angle of entrainment was smaller in PLWH (–6 minutes vs. +1 hour 25 minutes in the HIV-negative group).
“Collectively, our data suggest that the sleep phase occurred earlier than what would be biologically optimal among the HIV+ participants,” they added.
Asynchrony between bedtime and circadian time
“Ideally, with this delayed timing of circadian phase, they should have delayed their sleep phase (sleep timing) by an equal amount to be sleeping at their optimal biological time,” Dr. Scheuermaier explained. “Their sleep onset was delayed by 12 minutes (statistically significant but biologically not that much) while their circadian phase was delayed by more than an hour.”
Possible consequences of a smaller phase angle of entrainment include difficulty in initiating and maintaining sleep, the authors wrote. “The shorter, potentially mistimed sleep relative to the endogenous circadian cycle observed in this study provides objectively measured evidence supporting the abundant previous subjective reports of poor sleep quality and insomnia in PLWH.”
They noted that a strength of their study is that participants were recruited from rural South Africa, where HIV prevalence is not confined to the so-called “high-risk” groups of gay men, other men who have sex with men, people who inject drugs, and sex workers.
“Behavioral factors associated with belonging to one or more of these groups would be strong potential confounders for studies of sleep and circadian phase,” they explained. “By contrast, in rural southern Africa, the epidemic has been less demographically discriminating ... There are no notable differences in lifestyle between the HIV– and HIV+ individuals in this study. The members of this aging population are mostly beyond retirement age, living quiet, rural lives supported by government remittances and subsistence farming.”
Direct evidence warrants further study
The study is “unique” in that it provides “the first direct evidence for potential circadian disturbances in PWLH,” agreed Peng Li, PhD, who was not involved in the study.
“The assessment of dim light melatonin onset in PLWH is a strength of the study; together with actigraphy-based sleep onset assessment, it provides a measure for the phase angle of entrainment,” said Dr. Li, who is research director of the medical biodynamics program, division of sleep and circadian disorders, Brigham and Women’s Hospital, Boston.
But actigraphy has limitations that affect the interpretation of the results, he told this news organization.
“Without the help of sleep diaries, low specificity in assessing sleep using actigraphy has been consistently reported,” he said. “The low specificity means a significant overestimation of sleep. This lowers the value of the reported sleep readouts and limits the validity of sleep onset estimation, especially considering that differences in sleep measures between the two groups are relatively small, compromising the clinical meaning.”
Additionally, he explained that it’s not clear whether sleep onset in the study participants was spontaneous or was “forced” to accommodate routines. “This is a limitation in field study as compared with in-lab studies,” he said.
Dr. Li also pointed to the small sample size and younger age of PLWH, suggesting the study might have benefited from a matched design. Finally, he said the study did not examine gender differences.
“In the general population, it is known that females usually have advanced circadian phase compared to males. ... More rigorous design and analyses based on sex/gender especially in this often-marginalized population are warranted to better inform HIV-specific or general clinical guidelines.”
The study was supported by the Academy of Medical Sciences. The authors did not mention any competing interests. Dr. Li reported grant support from the BrightFocus Foundation. The study is not directly related to this paper. He also receives grant support from the NIH through a Departmental Award, Harvard University Center for AIDS Research and a Pilot Project, HIV and Aging Research Consortium. The projects are on circadian disturbances and cognitive performance in PLWH.
A version of this article first appeared on Medscape.com.
, according to findings that suggest both a possible mechanism for increased comorbidities in PLWH and potential solutions.
“It is very well known that sleep problems are common in people living with HIV, and many different reasons for this have been proposed,” coauthor Malcolm von Schantz, PhD, professor of chronobiology at Northumbria University in Newcastle upon Tyne, England, said in an interview. “But the novelty of our findings is the observation of delayed circadian rhythms.”
The mistimed circadian phase in PLWH is linked to later sleep onset and earlier waking and has “important potential implications” for the health and well-being of PLWH, wrote senior author Karine Scheuermaier, MD, from the University of the Witwatersrand, in Johannesburg, South Africa, and coauthors.
Until now, research on sleep in HIV has focused primarily on its homeostatic components, such as sleep duration and staging, rather than on circadian-related aspects, they noted.
“If the lifestyle‐independent circadian misalignment observed in the current study is confirmed to be a constant feature of chronic HIV infection, then it may be a mediator both of poorer sleep health and of poorer physical health in PLWH, which could potentially be alleviated through light therapy or chronobiotic medication or supplements,” they suggested.
HIV endemic in study population
The study analyzed a random sample of 187 participants (36 with HIV and 151 without) in the HAALSI (Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study, which is part of the Agincourt Health and Socio-demographic Surveillance System.
The study population ranged in age from 45 to 93 years, with an average age of 60.6 years in the HIV-positive group and 68.2 years in the HIV-negative group. Demographic data, Pittsburgh Sleep Quality Index score, and valid actigraphy (measured with an accelerometer for 14 consecutive days) were available for 172 participants (18% with HIV). A subgroup of 51 participants (22% with HIV) also had valid dim light melatonin onset (DLMO) data, a sensitive measure of the internal circadian clock. DLMO was measured for a minimum of 5 consecutive days with hourly saliva sampling between 5 p.m. and 11 p.m. while sitting in a dimly lit room.
In 36 participants (16% with HIV) with both valid actigraphy and DLMO data, circadian phase angle of entrainment was calculated by subtracting DLMO time from habitual sleep-onset time obtained from actigraphy.
After adjustment for age and sex, the study found a slightly later sleep onset (adjusted average delay of 10 minutes), earlier awakening (adjusted average advance of 10 minutes), and shorter sleep duration in PLWH compared with HIV-negative participants.
At the same time, melatonin production in PLWH started more than an hour later on average than in HIV-negative participants, “with half of the HIV+ group having an earlier habitual sleep onset than DLMO time” the authors wrote. In a subgroup of 36 participants with both valid actigraphy and DLMO data, the median circadian phase angle of entrainment was smaller in PLWH (–6 minutes vs. +1 hour 25 minutes in the HIV-negative group).
“Collectively, our data suggest that the sleep phase occurred earlier than what would be biologically optimal among the HIV+ participants,” they added.
Asynchrony between bedtime and circadian time
“Ideally, with this delayed timing of circadian phase, they should have delayed their sleep phase (sleep timing) by an equal amount to be sleeping at their optimal biological time,” Dr. Scheuermaier explained. “Their sleep onset was delayed by 12 minutes (statistically significant but biologically not that much) while their circadian phase was delayed by more than an hour.”
Possible consequences of a smaller phase angle of entrainment include difficulty in initiating and maintaining sleep, the authors wrote. “The shorter, potentially mistimed sleep relative to the endogenous circadian cycle observed in this study provides objectively measured evidence supporting the abundant previous subjective reports of poor sleep quality and insomnia in PLWH.”
They noted that a strength of their study is that participants were recruited from rural South Africa, where HIV prevalence is not confined to the so-called “high-risk” groups of gay men, other men who have sex with men, people who inject drugs, and sex workers.
“Behavioral factors associated with belonging to one or more of these groups would be strong potential confounders for studies of sleep and circadian phase,” they explained. “By contrast, in rural southern Africa, the epidemic has been less demographically discriminating ... There are no notable differences in lifestyle between the HIV– and HIV+ individuals in this study. The members of this aging population are mostly beyond retirement age, living quiet, rural lives supported by government remittances and subsistence farming.”
Direct evidence warrants further study
The study is “unique” in that it provides “the first direct evidence for potential circadian disturbances in PWLH,” agreed Peng Li, PhD, who was not involved in the study.
“The assessment of dim light melatonin onset in PLWH is a strength of the study; together with actigraphy-based sleep onset assessment, it provides a measure for the phase angle of entrainment,” said Dr. Li, who is research director of the medical biodynamics program, division of sleep and circadian disorders, Brigham and Women’s Hospital, Boston.
But actigraphy has limitations that affect the interpretation of the results, he told this news organization.
“Without the help of sleep diaries, low specificity in assessing sleep using actigraphy has been consistently reported,” he said. “The low specificity means a significant overestimation of sleep. This lowers the value of the reported sleep readouts and limits the validity of sleep onset estimation, especially considering that differences in sleep measures between the two groups are relatively small, compromising the clinical meaning.”
Additionally, he explained that it’s not clear whether sleep onset in the study participants was spontaneous or was “forced” to accommodate routines. “This is a limitation in field study as compared with in-lab studies,” he said.
Dr. Li also pointed to the small sample size and younger age of PLWH, suggesting the study might have benefited from a matched design. Finally, he said the study did not examine gender differences.
“In the general population, it is known that females usually have advanced circadian phase compared to males. ... More rigorous design and analyses based on sex/gender especially in this often-marginalized population are warranted to better inform HIV-specific or general clinical guidelines.”
The study was supported by the Academy of Medical Sciences. The authors did not mention any competing interests. Dr. Li reported grant support from the BrightFocus Foundation. The study is not directly related to this paper. He also receives grant support from the NIH through a Departmental Award, Harvard University Center for AIDS Research and a Pilot Project, HIV and Aging Research Consortium. The projects are on circadian disturbances and cognitive performance in PLWH.
A version of this article first appeared on Medscape.com.
, according to findings that suggest both a possible mechanism for increased comorbidities in PLWH and potential solutions.
“It is very well known that sleep problems are common in people living with HIV, and many different reasons for this have been proposed,” coauthor Malcolm von Schantz, PhD, professor of chronobiology at Northumbria University in Newcastle upon Tyne, England, said in an interview. “But the novelty of our findings is the observation of delayed circadian rhythms.”
The mistimed circadian phase in PLWH is linked to later sleep onset and earlier waking and has “important potential implications” for the health and well-being of PLWH, wrote senior author Karine Scheuermaier, MD, from the University of the Witwatersrand, in Johannesburg, South Africa, and coauthors.
Until now, research on sleep in HIV has focused primarily on its homeostatic components, such as sleep duration and staging, rather than on circadian-related aspects, they noted.
“If the lifestyle‐independent circadian misalignment observed in the current study is confirmed to be a constant feature of chronic HIV infection, then it may be a mediator both of poorer sleep health and of poorer physical health in PLWH, which could potentially be alleviated through light therapy or chronobiotic medication or supplements,” they suggested.
HIV endemic in study population
The study analyzed a random sample of 187 participants (36 with HIV and 151 without) in the HAALSI (Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study, which is part of the Agincourt Health and Socio-demographic Surveillance System.
The study population ranged in age from 45 to 93 years, with an average age of 60.6 years in the HIV-positive group and 68.2 years in the HIV-negative group. Demographic data, Pittsburgh Sleep Quality Index score, and valid actigraphy (measured with an accelerometer for 14 consecutive days) were available for 172 participants (18% with HIV). A subgroup of 51 participants (22% with HIV) also had valid dim light melatonin onset (DLMO) data, a sensitive measure of the internal circadian clock. DLMO was measured for a minimum of 5 consecutive days with hourly saliva sampling between 5 p.m. and 11 p.m. while sitting in a dimly lit room.
In 36 participants (16% with HIV) with both valid actigraphy and DLMO data, circadian phase angle of entrainment was calculated by subtracting DLMO time from habitual sleep-onset time obtained from actigraphy.
After adjustment for age and sex, the study found a slightly later sleep onset (adjusted average delay of 10 minutes), earlier awakening (adjusted average advance of 10 minutes), and shorter sleep duration in PLWH compared with HIV-negative participants.
At the same time, melatonin production in PLWH started more than an hour later on average than in HIV-negative participants, “with half of the HIV+ group having an earlier habitual sleep onset than DLMO time” the authors wrote. In a subgroup of 36 participants with both valid actigraphy and DLMO data, the median circadian phase angle of entrainment was smaller in PLWH (–6 minutes vs. +1 hour 25 minutes in the HIV-negative group).
“Collectively, our data suggest that the sleep phase occurred earlier than what would be biologically optimal among the HIV+ participants,” they added.
Asynchrony between bedtime and circadian time
“Ideally, with this delayed timing of circadian phase, they should have delayed their sleep phase (sleep timing) by an equal amount to be sleeping at their optimal biological time,” Dr. Scheuermaier explained. “Their sleep onset was delayed by 12 minutes (statistically significant but biologically not that much) while their circadian phase was delayed by more than an hour.”
Possible consequences of a smaller phase angle of entrainment include difficulty in initiating and maintaining sleep, the authors wrote. “The shorter, potentially mistimed sleep relative to the endogenous circadian cycle observed in this study provides objectively measured evidence supporting the abundant previous subjective reports of poor sleep quality and insomnia in PLWH.”
They noted that a strength of their study is that participants were recruited from rural South Africa, where HIV prevalence is not confined to the so-called “high-risk” groups of gay men, other men who have sex with men, people who inject drugs, and sex workers.
“Behavioral factors associated with belonging to one or more of these groups would be strong potential confounders for studies of sleep and circadian phase,” they explained. “By contrast, in rural southern Africa, the epidemic has been less demographically discriminating ... There are no notable differences in lifestyle between the HIV– and HIV+ individuals in this study. The members of this aging population are mostly beyond retirement age, living quiet, rural lives supported by government remittances and subsistence farming.”
Direct evidence warrants further study
The study is “unique” in that it provides “the first direct evidence for potential circadian disturbances in PWLH,” agreed Peng Li, PhD, who was not involved in the study.
“The assessment of dim light melatonin onset in PLWH is a strength of the study; together with actigraphy-based sleep onset assessment, it provides a measure for the phase angle of entrainment,” said Dr. Li, who is research director of the medical biodynamics program, division of sleep and circadian disorders, Brigham and Women’s Hospital, Boston.
But actigraphy has limitations that affect the interpretation of the results, he told this news organization.
“Without the help of sleep diaries, low specificity in assessing sleep using actigraphy has been consistently reported,” he said. “The low specificity means a significant overestimation of sleep. This lowers the value of the reported sleep readouts and limits the validity of sleep onset estimation, especially considering that differences in sleep measures between the two groups are relatively small, compromising the clinical meaning.”
Additionally, he explained that it’s not clear whether sleep onset in the study participants was spontaneous or was “forced” to accommodate routines. “This is a limitation in field study as compared with in-lab studies,” he said.
Dr. Li also pointed to the small sample size and younger age of PLWH, suggesting the study might have benefited from a matched design. Finally, he said the study did not examine gender differences.
“In the general population, it is known that females usually have advanced circadian phase compared to males. ... More rigorous design and analyses based on sex/gender especially in this often-marginalized population are warranted to better inform HIV-specific or general clinical guidelines.”
The study was supported by the Academy of Medical Sciences. The authors did not mention any competing interests. Dr. Li reported grant support from the BrightFocus Foundation. The study is not directly related to this paper. He also receives grant support from the NIH through a Departmental Award, Harvard University Center for AIDS Research and a Pilot Project, HIV and Aging Research Consortium. The projects are on circadian disturbances and cognitive performance in PLWH.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PINEAL RESEARCH