User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
A bold national plan to eliminate HCV by 2050
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
AT THE LIVER MEETING
Hospital financial decisions play a role in the critical shortage of pediatric beds for RSV patients
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The dire shortage of pediatric hospital beds plaguing the nation in the fall of 2022 is a byproduct of financial decisions made by hospitals over the past decade, as they shuttered children’s wards, which often operate in the red, and expanded the number of beds available for more profitable endeavors like joint replacements and cancer care.
To cope with the flood of young patients sickened by a sweeping convergence of nasty bugs – especially respiratory syncytial virus, influenza, and coronavirus – medical centers nationwide have deployed triage tents, delayed elective surgeries, and transferred critically ill children out of state.
A major factor in the bed shortage is a years-long trend among hospitals of eliminating pediatric units, which tend to be less profitable than adult units, said Mark Wietecha, MS, MBA, CEO of the Children’s Hospital Association. Hospitals optimize revenue by striving to keep their beds 100% full – and filled with patients whose conditions command generous insurance reimbursements.
“It really has to do with dollars,” said Scott Krugman, MD, MS, vice chair of pediatrics at the Herman and Walter Samuelson Children’s Hospital at Sinai in Baltimore. “Hospitals rely on high-volume, high-reimbursement procedures from good payers to make money. There’s no incentive for hospitals to provide money-losing services.”
The number of pediatric inpatient units in hospitals fell 19% from 2008 to 2018, according to a study published in 2021 in the journal Pediatrics. Just this year, hospitals have closed pediatric units in Boston and Springfield, Mass.; Richmond, Va.; and Tulsa, Okla.
The current surge in dangerous respiratory illnesses among children is yet another example of how COVID-19 has upended the health care system. The lockdowns and isolation that marked the first years of the pandemic left kids largely unexposed – and still vulnerable – to viruses other than COVID for two winters, and doctors are now essentially treating multiple years’ worth of respiratory ailments.
The pandemic also accelerated changes in the health care industry that have left many communities with fewer hospital beds available for children who are acutely ill, along with fewer doctors and nurses to care for them.
When intensive care units were flooded with older COVID patients in 2020, some hospitals began using children’s beds to treat adults. Many of those pediatric beds haven’t been restored, said Daniel Rauch, MD, chair of the American Academy of Pediatrics’ committee on hospital care.
In addition, the relentless pace of the pandemic has spurred more than 230,000 health care providers – including doctors, nurses, and physician assistants – to quit. Before the pandemic, about 10% of nurses left their jobs every year; the rate has risen to about 20%, Dr. Wietecha said. He estimates that pediatric hospitals are unable to maintain as many as 10% of their beds because of staffing shortages.
“There is just not enough space for all the kids who need beds,” said Megan Ranney, MD, MPH, who works in several emergency departments in Providence, R.I., including Hasbro Children’s Hospital. The number of children seeking emergency care in recent weeks was 25% higher than the hospital’s previous record.
“We have doctors who are cleaning beds so we can get children into them faster,” said Dr. Ranney, a deputy dean at Brown University’s School of Public Health.
There’s not great money in treating kids. About 40% of U.S. children are covered by Medicaid, a joint federal-state program for low-income patients and people with disabilities. Base Medicaid rates are typically more than 20% below those paid by Medicare, the government insurance program for older adults, and are even lower when compared with private insurance. While specialty care for a range of common adult procedures, from knee and hip replacements to heart surgeries and cancer treatments, generates major profits for medical centers, hospitals complain they typically lose money on inpatient pediatric care.
When Tufts Children’s Hospital closed 41 pediatric beds this summer, hospital officials assured residents that young patients could receive care at nearby Boston Children’s Hospital. Now, Boston Children’s is delaying some elective surgeries to make room for kids who are acutely ill.
Dr. Rauch noted that children’s hospitals, which specialize in treating rare and serious conditions such as pediatric cancer, cystic fibrosis, and heart defects, simply aren’t designed to handle this season’s crush of kids acutely ill with respiratory bugs.
Even before the autumn’s viral trifecta, pediatric units were straining to absorb rising numbers of young people in acute mental distress. Stories abound of children in mental crises being marooned for weeks in emergency departments while awaiting transfer to a pediatric psychiatric unit. On a good day, Dr. Ranney said, 20% of pediatric emergency room beds at Hasbro Children’s Hospital are occupied by children experiencing mental health issues.
In hopes of adding pediatric capacity, the American Academy of Pediatrics joined the Children’s Hospital Association last month in calling on the White House to declare a national emergency due to child respiratory infections and provide additional resources to help cover the costs of care. The Biden administration has said that the flexibility hospital systems and providers have been given during the pandemic to sidestep certain staffing requirements also applies to RSV and flu.
Doernbecher Children’s Hospital at Oregon Health & Science University has shifted to “crisis standards of care,” enabling intensive care nurses to treat more patients than they’re usually assigned. Hospitals in Atlanta, Pittsburgh, and Aurora, Colorado, meanwhile, have resorted to treating young patients in overflow tents in parking lots.
Alex Kon, MD, a pediatric critical care physician at Community Medical Center in Missoula, Mont., said providers there have made plans to care for older kids in the adult intensive care unit, and to divert ambulances to other facilities when necessary. With only three pediatric ICUs in the state, that means young patients may be flown as far as Seattle or Spokane, Wash., or Idaho.
Hollis Lillard took her 1-year-old son, Calder, to an Army hospital in Northern Virginia last month after he experienced several days of fever, coughing, and labored breathing. They spent 7 anguished hours in the emergency room before the hospital found an open bed and transferred them by ambulance to Walter Reed National Military Medical Center in Maryland.
With proper therapy and instructions for home care, Calder’s virus was readily treatable: He recovered after he was given oxygen and treated with steroids, which fight inflammation, and albuterol, which counteracts bronchospasms. He was discharged the next day.
Although hospitalizations for RSV are falling, rates remain well above the norm for this time of year. And hospitals may not get much relief.
People can be infected with RSV more than once a year, and Dr. Krugman worries about a resurgence in the months to come. Because of the coronavirus, which competes with other viruses, “the usual seasonal pattern of viruses has gone out the window,” he said.
Like RSV, influenza arrived early this season. Both viruses usually peak around January. Three strains of flu are circulating and have caused an estimated 8.7 million illnesses, 78,000 hospitalizations, and 4,500 deaths, according to the Centers for Disease Control and Prevention.
Dr. Krugman doubts the health care industry will learn any quick lessons from the current crisis. “Unless there is a radical change in how we pay for pediatric hospital care,” Dr. Krugman said, “the bed shortage is only going to get worse.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Why doctors are losing trust in patients; what should be done?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
No, you can’t see a different doctor: We need zero tolerance of patient bias
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
States cracking down harder on docs who sexually abuse patients
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections treatable with newer antibiotics, but guidance is needed
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CURRENT OPINION IN INFECTIOUS DISEASES
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.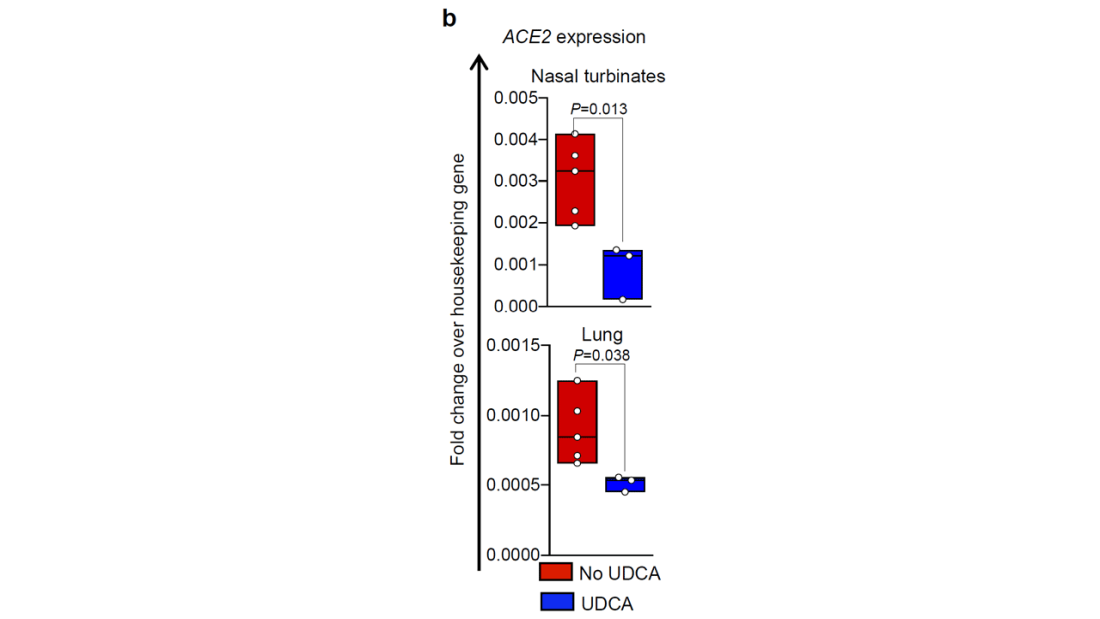
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Everyone wins when losers get paid
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.