User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Fever after a tropical trip: A guide to differential diagnosis
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.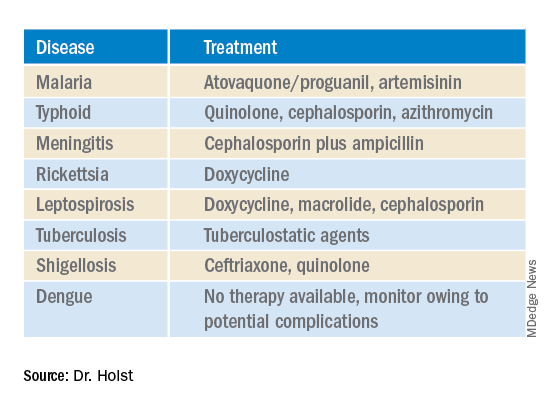
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
Doxycycline bests azithromycin for anorectal chlamydia in women
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
OTC meds, supplements, and other drugs may interact with HIV antiretrovirals
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Over-the-counter medications, food supplements, and other drugs may interact with antiretroviral therapy (ART) in people living with HIV and be harmful, an industry-sponsored clinical survey from Denmark reports.
“Our study confirms that polypharmacy and being on a protease inhibitor–based regimen increase the risk of potential drug-drug interactions [PDDIs] considerably and highlights the importance of questioning people living with HIV [PLWH] about dietary supplement intake,” the authors, led by Michaela Tinggaard, MD, Copenhagen University Hospital, wrote in HIV Medicine.
“Potential drug-drug interactions were common among our study population. Although the clinical significance of the majority of the identified PDDIs may be low, most of them were avoidable through a change or discontinuation of the comedication, a change in ART or by spacing drugs,” they added.
Senior author Thomas Benfield, MD, DTMH, DMSc, a professor of infectious diseases at the University of Copenhagen, and colleagues collected information on prescription medication, over-the-counter medication, and dietary supplements from adults living with HIV who received ART from two outpatient clinics.
The researchers estimated the prevalence of non-HIV comedications, and they used the University of Liverpool HIV Drug Interactions database to identify potential drug-drug interactions. They evaluated PDDIs and used logistic regression models to investigate links between PDDIs and relevant variables.
The study included 337 people living with HIV receiving ART. The median age was 53 years, 77% of them were male, and 96% were virally suppressed, with HIV-RNA viral load less than 50 copies/mL.
Overall, 26% of participants received five or more comedications, and 56% took dietary supplements.
In the medication lists of 52% of patients, the authors identified coadministration of drugs that required dose adjustment or monitoring; 4.5% of patients were taking drugs that should not be coadministered.
The researchers detected several factors that independently predicted PDDIs:
- Male sex (odds ratio, 1.9; 95% confidence interval, 1.0-3.4)
- Being on a protease inhibitor (OR, 4.3; 95% CI, 1.9-9.7)
- Receiving five or more comedications (OR, 3.3; 95% CI, 1.5-7.2)
- Taking over-the-counter medications (OR, 1.9; 95% CI, 1.1-3.3)
- Taking dietary supplements (OR, 2.0; 95% CI, 1.2-3.3)
Comorbidities and OTC medications increase in aging people with HIV
Indira Brar, MD, an infectious diseases senior staff physician and the medical director of HIV services at Henry Ford Health in Detroit, called the study and important resource for educating providers and patients about over-the-counter drugs.
“The main strength of the study is that it includes a decent number of aging patients living with HIV, the age group in which we worry about drug interactions,” she said in an interview.
“As patients get older, they have increased comorbidities. As comorbidities increase, the number of medications increases. As the number of medications increases, the drug interactions increase,” said Dr. Brar, who was not involved in the study. “Also, as patients get older, they tend to take more over-the-counter drugs.”
Dr. Brar explained how drug-drug interactions can harm patients.
“Drugs added to a patient who is already on ART could decrease the level of the ART and cause the patient to develop a drug-resistant HIV infection,” she said. “Or the ART the patient is on can increase the levels of the new drugs that have been added, and that could have potential toxicity and side effects.
“Food supplements, including multivitamins, calcium, and magnesium, are often overlooked because we think they’re benign. But these drugs can bind our new antiretrovirals, the integrase inhibitors. They can decrease their levels in the patient and cause drug-resistant HIV infection.
“In our clinic, we always tell our patients to please call us before they take any medication, so we can make sure there is no drug interaction,” Dr. Brar said.
Nan Wang, PharmD, a clinical pharmacy specialist at University Hospitals Cleveland Medical Center, noted in an email that drug-drug interactions with ARTs are common.
“Understanding the prevalence of antiretroviral drug interactions in a patient population can help identify certain medications that require enhanced vigilance and can guide our clinical interventions,” said Dr. Wang, who was not associated with the research.
Joseph Alvarnas, MD, a hematologist and oncologist at City of Hope Comprehensive Cancer Center in Duarte, Calif., said that this is “a methodologically sound and well-designed study that’s a timely, important reminder that providers need to think carefully and comprehensively when caring for their patients living with HIV.”
Dr. Alvarnas, who was not involved in the study, said that, with the widespread availability of ART, HIV has become a chronic, manageable condition in an aging population.
“ART agents, particularly the ritonavir-boosted protease inhibitors, increase the likelihood of patients having a potentially significant drug-drug interaction with one of their chronic care medications,” he added. “Even seemingly low-risk supplements such as multivitamins may result in a negative impact upon effective ART treatment of PLWH.”
“The essential next step is that these findings are integrated carefully into decision-support systems, electronic health record prescribing systems, and pharmacy safety-check systems to ensure that we reduce the risk of patient harm,” Dr. Alvarnas advised.
Dr. Benfield and several study coauthors reported financial relationships with GlaxoSmithKline and other pharmaceutical companies. Other coauthors, as well as Dr. Alvarnas, Dr. Brar, and Dr. Wang, reported no relevant financial relationships. The study was supported by GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
Bacterial cocktail, spores counter recurrent C. diff
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
AT DDW 2022
COVID-19 burnout? Turn off your mind, relax, and float downstream
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATS 2022
Rabies: CDC updates and simplifies preexposure prophylaxis vaccination recommendations
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
RSV kills 100,000 kids under age 5 a year worldwide
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Respiratory syncytial virus (RSV) caused more than 100,000 deaths in children under age 5 years globally in 2019, according to an analysis published online in The Lancet.
Researchers, led by You Li, PhD, of Nanjing (China) Medical University, found that nearly half of those (more than 45,000) occurred in children younger than 6 months old.
They estimated that RSV causes 1 in 50 deaths among children under 5 years old, and 1 in 28 deaths in children under 6 months old.
Additionally, RSV is responsible for an estimated 3.6 million hospital admissions globally each year, according to the report.
This analysis is the first to sift RSV disease burden into narrow age brackets, the authors said.
The numbers highlight that almost all of the deaths (97%) were in low- and middle-income countries.
Messages for prevention
Tina Hartert, MD, MPH, a professor in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., who was not part of the study, wrote in an invited commentary that these findings will be important in RSV prevention.
Among the most notable findings, she wrote, is the heavy mortality in the 0- to 6-month age group, which she notes is “the age group targeted by vaccination during pregnancy and birth-dose immunoprophylaxis.”
Dr. Hartert, who coauthored the commentary with Justin R. Ortiz, MD, MS, with the Center for Vaccine Development and Global Health, University of Maryland, Baltimore, told this news organization, “RSV is a respiratory virus that infects nearly every child by the time they are 2-3 years of age, with severe infection and death most common in the youngest infants. Vaccines that prevent the most severe infections in these young infants will likely be one of the best ways to prevent these severe infections and death.”
Though the authors found most deaths occur in low- and middle-income countries, RSV is one of the most common reasons for infant hospitalization in the US and affects 1% to 3% of infants, half of whom are full-term and otherwise healthy, Dr. Hartert said.
It is also one of the most common causes of infant lower respiratory tract infection in young children in the United States, she said, and it causes the most severe disease at the age extremes, with older adults experiencing significant morbidity with RSV.
Dr. Li said in an interview that although the team did not focus on reporting country-specific estimates in this work, their previous work, resulted in estimates of 98,000-155,000 RSV-related hospitalizations in children under 5 years old in the United States in 2019. Between 65,000 and 86,000 were in infants less than 1 year old.
Currently, he said, the only available RSV prophylaxis is palivizumab (Synagis), which is expensive and given only to high-risk infants in high-income countries, including the United States.
“There have been a number of promising RSV prophylactic products including maternal vaccine and monoclonal antibodies that have the potential for targeting the general infant population – not just high-risk infants – in late-phase clinical trials,” he said. “Our estimates of RSV-related disease burden will help anticipate the impact of future RSV immunization programs.”
Pandemic changed patterns
This research was completed before the COVID-19 pandemic, and it is not yet known how that could affect RSV disease burden long term.
However, Dr. Hartert said, RSV circulation has been significantly changed during the pandemic, both in intensity and timing, likely because of a combination of COVID and the public health preventive measures.
“As people return to normal activities and the public health measures put in place to stop the spread of COVID are eased, we are likely to see increases in circulation of RSV and return to its circulation during the winter months – typically similar to circulation of flu – from November through March in temperate climates in the northern hemisphere,” she said.
A coauthor of the paper, Harish Nair, PhD, with the Centre for Global Health, Usher Institute, University of Edinburgh, said in a press release that their findings have particular significance as COVID restrictions ease around the globe.
“The majority of the young children born in the last 2 years have never been exposed to RSV (and therefore have no immunity against this virus),” Nair wrote.
Most deaths occurring outside hospitals
A challenge in reducing the deaths in those 5 years old and younger is that most (76%) of deaths are happening in the community outside hospitals.
The authors wrote: “For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community.”
The percentage dying outside hospitals is even larger (81%) in low- to middle-income countries.
This work built on a previous review by the team that analyzed 317 studies. They updated their search with 113 new eligible studies and unpublished data from 51 papers published between Jan. 1, 2017, and Dec. 31, 2020.
The authors acknowledged some limitations, including variations in study settings and in definitions for acute lower respiratory infection, healthcare access, and eligibility for RSV testing.
The study was funded by EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe. Dr. Li reported grants from Wellcome Trust and the World Health Organization outside the submitted work. Dr. Hartert, Dr. Ortiz, and Dr. Nair disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
CDC signs off on COVID boosters in children ages 5-11
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Pancreatic involvement in COVID-19: What do we know?
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.