User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lawmakers argue for changes in prior authorization processes
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Murder of physician raises the stress level for all clinicians
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As if it weren’t enough that doctors work in a profession where it’s almost more a question of when they’ll be sued than if they’ll be sued – where COVID, staff shortages, long hours, and patients frustrated over canceled procedures have caused unrelenting fatigue and stress – they now have to worry that an unhappy patient is going to buy a gun, walk into their office, and kill them.
That’s exactly what happened in Tulsa, Okla., where a patient complaining of pain after back surgery murdered his doctor and several others who happened to be in the wrong place at the wrong time.
The temptation in the aftermath of such tragedies is to think about preventive measures: Make medical facilities “hardened” targets, like schools have become, with armed guards, metal detectors, automatically locking doors, physical barriers within, security cameras, and buzzers for entry – although hardening a large medical center where members of the community routinely come and go would be challenging.
What about the enormous stress on doctors, nurses, and others in the medical workplace? Physicians who have been sued for malpractice often describe how it changes the way they interact with patients: They now size patients up and make judgments about their potential litigiousness. Will the physicians now look over their patients’ shoulders at the video feed from a security camera when they’re taking a history? Will medical professionals be forced to make snap judgments about patients’ psychological state before deciding whether to treat them?
Remember, there was a time when school shootings were unimaginable. Once one person crosses that line, others inevitably follow.
It could be a drug-seeking patient complaining of ongoing pain, angry because he can’t get a new prescription. It could be a patient whose unpaid bill was turned over to a collection agency, angry because he’s now getting calls from collectors. It could be someone who blames a physician for the loss of a loved one. It could be someone who would otherwise have filed a lawsuit, who now thinks he has a more effective option for exacting retribution.
Most of us would find it unbearable to live and work under the kind of stress faced by medical professionals today. And unfortunately, there is no short-term, systemic relief on the horizon. But there are methods of relieving at least some of the psychological burden being carried by these dedicated individuals.
For starters, the government should provide funds to improve safety and security at medical facilities. It’s sad but it’s a fact of life. The physical structure of schools, along with emergency procedures, have been changed since Columbine and Sandy Hook, and our children and their teachers undergo active shooter drills. Health care facilities will need to adopt similar strategies.
But if we don’t also support the individuals who work in health care, we’ll no longer have even partially staffed health care facilities. Hospitals and medical groups need to be conscious of the effects stress may have on them. Medical staff and administrators need to recognize changes in their colleagues’ behavior and refer those cohorts to professional stress coaches who can get them back on track.
Medical personnel should be picking up on warning signs, like irritability, depression, sudden weight gain or loss, lack of motivation and job satisfaction, obsessiveness, unusual levels of fatigue, alcohol or drug use, and, of course, avoidable medical errors.
In addition, colleagues in the medical workplace need to know each other well. They are usually the first ones to notice if something is off and may be in the best position to refer coworkers for help. Also, medical malpractice insurance carriers should consider encouraging and covering coaching sessions, because helping physicians cope with this heightened stress will prevent medical errors and the lawsuits that inevitably accompany mistakes.
This needn’t be a long-term process like ongoing psychotherapy; a few sessions with a well-trained coach may help psychologically challenged peers restore their focus and perspective. It won’t eliminate the threat any more than litigation stress coaching eliminates the threat of being sued, but it can prevent that stress from leading to avoidable errors. It also can prevent physicians’ personal lives and relationships from going off the rails and driving them out of the medical profession.
None of us can afford to ignore the impacts that these new stressors are having and simply act as if it’s business as usual. The people in the trenches need our help.
Ms. Fiore is President of Winning Focus in Murrysville, Pa. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the Grand Canyon, norovirus gives new meaning to ‘leave no trace’
Ain’t gastroenteritis grand?
The Grand Canyon is perhaps America’s greatest natural wonder. The mile-deep gorge of epic proportions, carved over eons by the Colorado River, elicits superlatives of the highest order from those seeing it for the first time. In the past few months, though, visitors to the Grand Canyon have been experiencing a rather more unpleasant sort of reaction: Involuntary bowel evacuation.
Since April, more than 150 river rafters and backcountry campers have fallen ill with bouts of acute gastroenteritis, likely caused by norovirus. Hey, a viral outbreak and our old friend SARS-CoV-2 isn’t involved! Hopefully it won’t get jealous. Whatever the culprit is, however, it got everywhere, as clusters of illness have popped up in unconnected parts of the park and some hikers have been restricted to a smaller portion of the park to avoid further disease spread. The majority of cases occurred in May, so it’s hoped that the outbreak is dying down, but the park remains on alert.
Now, acute gastroenteritis is certainly an unpleasant disease, but it isn’t typically a life-threatening one. There are, however, a couple of unique factors complicating this outbreak. For one, the Grand Canyon is in Arizona (duh), which can get rather hot in the summer months. Expelling waste from both ends becomes rather more dangerous when the thermometer reads over a hundred degrees, and there have been reports of multiple helicopter rescues.
That’s pretty bad, but in a way, they’re the lucky ones. How can we explain this … see, when you visit the Grand Canyon, you’re expected to follow the general rules of Leave No Trace. That means several things, but essentially, if you bring it in, you have to bring it out. Yes, that includes the various consequences of an acute gastroenteritis attack.
Forget spooky campfire stories and hungry wildlife lurking in the night, because true horror is scraping your friend’s diarrhea off the walls of the Grand Canyon into a plastic bag and stuffing it into your backpack. Probably not the sublime one-on-one Grand Canyon experience that people are expecting.
Give us a pee! ... for stem cell retrieval
Getting cells for regenerative stem cell treatment has traditionally been painful and difficult – usually they are retrieved by surgical means from bone marrow or fat tissue – but there may be an easier way.
Just pee in a cup.
Apparently, human urine contains stem cells with the potential to be used for regenerative effects. The magic ingredient? The enzyme telomerase, which “is essential for the self-renewal and potential of different types of stem cells” and is related to longevity, according to researchers at Wake Forest Institute for Regenerative Medicine.
They looked into how regenerative telomerase activity is for various capabilities beyond chromosomal stability, and whether these stem cells can become other kinds of cells for optimal tissue repair. Turns out they could, acting as a “distinct subpopulation” that has the ability not only to grow cells but also to morph into other cells, they said in a written statement.
Safety is also an issue. “Being able to use a patient’s own stem cells for therapy is considered advantageous because they do not induce immune responses or rejection,” said Anthony Atala, MD, a coauthor of the study published in Frontiers in Cell and Developmental Biology.
So less risk, easier retrieval, and great regenerative results. If this takes off, the other methods of retrieval could get flushed down the toilet.
Politicians playing the long game, literally
Before we get started with actual information, here’s a joke about politicians:
What do you call a lawyer with an IQ of 100? Your Honor.
What do you call a lawyer with an IQ of 50? Senator.
Politics is a dirty business, no doubt, so why do people do it? Is it for the prestige? Seems like everyone hates politicians, so it’s probably not that. Is it their selfless concern for the well-being of others? Probably not that either. Is it for the money? Most members of Congress have more corporate sponsors than a NASCAR driver, but we’re going to pass on that one as well.
Once again, science gives us the real answer: Longevity. Politicians live longer than the rest of us, and that longevity gap is getting wider.
Investigators looked at data from 11 industrialized countries, some of it going back to 1817, and found that politicians in the United States can expect to live about 7 years longer than the national average. The difference is around 3 years in Switzerland, 4.5 years in Germany, and 6 years in France.
“For almost all countries, politicians had similar rates of mortality to the general population in the late 19th and early 20th centuries. Throughout the 20th century, differences in mortality rates widened significantly across all countries, so that politicians had an increasing survival advantage over the general population,” they said in a written statement.
Income inequality could be a factor, but the longevity gains made by politicians, which started before the 1940s, predate the rise of their earnings relative to the rest of the population, which didn’t really get going until the 1980s, the investigators noted.
Whatever the reason, we have this closing thought regarding our long-lived lawmakers: What’s the difference between a politician and a snail? One is a slimy pest that leaves a trail everywhere. The other is a snail.
Land of the free, home of obesity
In the United States, it seems, people are becoming more comfortable with obesity. TikTok and Instagram trends often try to show the world that all sizes are beautiful. There’s also the growing popularity of the dad bod.
America, it has been said, is the land of the free. We love our freedom, and we value our individualism. If an obese man orders three meals from McDonald’s just for himself, no one is going to stop him. Many Americans also have more access to the food they want at any given time, even while they are moving around a lot less because of their sedentary lifestyles.
According to a recent study cited by the New York Post, however, America is not the only country battling obesity. Egypt and Mexico, for example, also have men with higher BMIs who cherish their individualism and the right to eat what they want, Plamen Akaliyski, PhD, of University Carlos III of Madrid, and associates, said in Social Science & Medicine.
Women are not as likely to think the same way. “Men in particular think, ‘I’m an individual, don’t tell me what to do. I’m going to eat what I want,’ ” bariatric surgeon George A. Fielding, MD, said in the Post article. Dr. Fielding also noted that women are three times more likely than men to seek bariatric surgery.
Dr. Akaliyski and associates found that Asian countries such as Japan, Singapore, and South Korea – countries that value thrift, discipline, self control, and delaying gratification – have lower rates of obesity.
So yes, we can go to the drive through of a fast food restaurant whenever we want and order whatever we want, but can doesn’t always mean should.
Ain’t gastroenteritis grand?
The Grand Canyon is perhaps America’s greatest natural wonder. The mile-deep gorge of epic proportions, carved over eons by the Colorado River, elicits superlatives of the highest order from those seeing it for the first time. In the past few months, though, visitors to the Grand Canyon have been experiencing a rather more unpleasant sort of reaction: Involuntary bowel evacuation.
Since April, more than 150 river rafters and backcountry campers have fallen ill with bouts of acute gastroenteritis, likely caused by norovirus. Hey, a viral outbreak and our old friend SARS-CoV-2 isn’t involved! Hopefully it won’t get jealous. Whatever the culprit is, however, it got everywhere, as clusters of illness have popped up in unconnected parts of the park and some hikers have been restricted to a smaller portion of the park to avoid further disease spread. The majority of cases occurred in May, so it’s hoped that the outbreak is dying down, but the park remains on alert.
Now, acute gastroenteritis is certainly an unpleasant disease, but it isn’t typically a life-threatening one. There are, however, a couple of unique factors complicating this outbreak. For one, the Grand Canyon is in Arizona (duh), which can get rather hot in the summer months. Expelling waste from both ends becomes rather more dangerous when the thermometer reads over a hundred degrees, and there have been reports of multiple helicopter rescues.
That’s pretty bad, but in a way, they’re the lucky ones. How can we explain this … see, when you visit the Grand Canyon, you’re expected to follow the general rules of Leave No Trace. That means several things, but essentially, if you bring it in, you have to bring it out. Yes, that includes the various consequences of an acute gastroenteritis attack.
Forget spooky campfire stories and hungry wildlife lurking in the night, because true horror is scraping your friend’s diarrhea off the walls of the Grand Canyon into a plastic bag and stuffing it into your backpack. Probably not the sublime one-on-one Grand Canyon experience that people are expecting.
Give us a pee! ... for stem cell retrieval
Getting cells for regenerative stem cell treatment has traditionally been painful and difficult – usually they are retrieved by surgical means from bone marrow or fat tissue – but there may be an easier way.
Just pee in a cup.
Apparently, human urine contains stem cells with the potential to be used for regenerative effects. The magic ingredient? The enzyme telomerase, which “is essential for the self-renewal and potential of different types of stem cells” and is related to longevity, according to researchers at Wake Forest Institute for Regenerative Medicine.
They looked into how regenerative telomerase activity is for various capabilities beyond chromosomal stability, and whether these stem cells can become other kinds of cells for optimal tissue repair. Turns out they could, acting as a “distinct subpopulation” that has the ability not only to grow cells but also to morph into other cells, they said in a written statement.
Safety is also an issue. “Being able to use a patient’s own stem cells for therapy is considered advantageous because they do not induce immune responses or rejection,” said Anthony Atala, MD, a coauthor of the study published in Frontiers in Cell and Developmental Biology.
So less risk, easier retrieval, and great regenerative results. If this takes off, the other methods of retrieval could get flushed down the toilet.
Politicians playing the long game, literally
Before we get started with actual information, here’s a joke about politicians:
What do you call a lawyer with an IQ of 100? Your Honor.
What do you call a lawyer with an IQ of 50? Senator.
Politics is a dirty business, no doubt, so why do people do it? Is it for the prestige? Seems like everyone hates politicians, so it’s probably not that. Is it their selfless concern for the well-being of others? Probably not that either. Is it for the money? Most members of Congress have more corporate sponsors than a NASCAR driver, but we’re going to pass on that one as well.
Once again, science gives us the real answer: Longevity. Politicians live longer than the rest of us, and that longevity gap is getting wider.
Investigators looked at data from 11 industrialized countries, some of it going back to 1817, and found that politicians in the United States can expect to live about 7 years longer than the national average. The difference is around 3 years in Switzerland, 4.5 years in Germany, and 6 years in France.
“For almost all countries, politicians had similar rates of mortality to the general population in the late 19th and early 20th centuries. Throughout the 20th century, differences in mortality rates widened significantly across all countries, so that politicians had an increasing survival advantage over the general population,” they said in a written statement.
Income inequality could be a factor, but the longevity gains made by politicians, which started before the 1940s, predate the rise of their earnings relative to the rest of the population, which didn’t really get going until the 1980s, the investigators noted.
Whatever the reason, we have this closing thought regarding our long-lived lawmakers: What’s the difference between a politician and a snail? One is a slimy pest that leaves a trail everywhere. The other is a snail.
Land of the free, home of obesity
In the United States, it seems, people are becoming more comfortable with obesity. TikTok and Instagram trends often try to show the world that all sizes are beautiful. There’s also the growing popularity of the dad bod.
America, it has been said, is the land of the free. We love our freedom, and we value our individualism. If an obese man orders three meals from McDonald’s just for himself, no one is going to stop him. Many Americans also have more access to the food they want at any given time, even while they are moving around a lot less because of their sedentary lifestyles.
According to a recent study cited by the New York Post, however, America is not the only country battling obesity. Egypt and Mexico, for example, also have men with higher BMIs who cherish their individualism and the right to eat what they want, Plamen Akaliyski, PhD, of University Carlos III of Madrid, and associates, said in Social Science & Medicine.
Women are not as likely to think the same way. “Men in particular think, ‘I’m an individual, don’t tell me what to do. I’m going to eat what I want,’ ” bariatric surgeon George A. Fielding, MD, said in the Post article. Dr. Fielding also noted that women are three times more likely than men to seek bariatric surgery.
Dr. Akaliyski and associates found that Asian countries such as Japan, Singapore, and South Korea – countries that value thrift, discipline, self control, and delaying gratification – have lower rates of obesity.
So yes, we can go to the drive through of a fast food restaurant whenever we want and order whatever we want, but can doesn’t always mean should.
Ain’t gastroenteritis grand?
The Grand Canyon is perhaps America’s greatest natural wonder. The mile-deep gorge of epic proportions, carved over eons by the Colorado River, elicits superlatives of the highest order from those seeing it for the first time. In the past few months, though, visitors to the Grand Canyon have been experiencing a rather more unpleasant sort of reaction: Involuntary bowel evacuation.
Since April, more than 150 river rafters and backcountry campers have fallen ill with bouts of acute gastroenteritis, likely caused by norovirus. Hey, a viral outbreak and our old friend SARS-CoV-2 isn’t involved! Hopefully it won’t get jealous. Whatever the culprit is, however, it got everywhere, as clusters of illness have popped up in unconnected parts of the park and some hikers have been restricted to a smaller portion of the park to avoid further disease spread. The majority of cases occurred in May, so it’s hoped that the outbreak is dying down, but the park remains on alert.
Now, acute gastroenteritis is certainly an unpleasant disease, but it isn’t typically a life-threatening one. There are, however, a couple of unique factors complicating this outbreak. For one, the Grand Canyon is in Arizona (duh), which can get rather hot in the summer months. Expelling waste from both ends becomes rather more dangerous when the thermometer reads over a hundred degrees, and there have been reports of multiple helicopter rescues.
That’s pretty bad, but in a way, they’re the lucky ones. How can we explain this … see, when you visit the Grand Canyon, you’re expected to follow the general rules of Leave No Trace. That means several things, but essentially, if you bring it in, you have to bring it out. Yes, that includes the various consequences of an acute gastroenteritis attack.
Forget spooky campfire stories and hungry wildlife lurking in the night, because true horror is scraping your friend’s diarrhea off the walls of the Grand Canyon into a plastic bag and stuffing it into your backpack. Probably not the sublime one-on-one Grand Canyon experience that people are expecting.
Give us a pee! ... for stem cell retrieval
Getting cells for regenerative stem cell treatment has traditionally been painful and difficult – usually they are retrieved by surgical means from bone marrow or fat tissue – but there may be an easier way.
Just pee in a cup.
Apparently, human urine contains stem cells with the potential to be used for regenerative effects. The magic ingredient? The enzyme telomerase, which “is essential for the self-renewal and potential of different types of stem cells” and is related to longevity, according to researchers at Wake Forest Institute for Regenerative Medicine.
They looked into how regenerative telomerase activity is for various capabilities beyond chromosomal stability, and whether these stem cells can become other kinds of cells for optimal tissue repair. Turns out they could, acting as a “distinct subpopulation” that has the ability not only to grow cells but also to morph into other cells, they said in a written statement.
Safety is also an issue. “Being able to use a patient’s own stem cells for therapy is considered advantageous because they do not induce immune responses or rejection,” said Anthony Atala, MD, a coauthor of the study published in Frontiers in Cell and Developmental Biology.
So less risk, easier retrieval, and great regenerative results. If this takes off, the other methods of retrieval could get flushed down the toilet.
Politicians playing the long game, literally
Before we get started with actual information, here’s a joke about politicians:
What do you call a lawyer with an IQ of 100? Your Honor.
What do you call a lawyer with an IQ of 50? Senator.
Politics is a dirty business, no doubt, so why do people do it? Is it for the prestige? Seems like everyone hates politicians, so it’s probably not that. Is it their selfless concern for the well-being of others? Probably not that either. Is it for the money? Most members of Congress have more corporate sponsors than a NASCAR driver, but we’re going to pass on that one as well.
Once again, science gives us the real answer: Longevity. Politicians live longer than the rest of us, and that longevity gap is getting wider.
Investigators looked at data from 11 industrialized countries, some of it going back to 1817, and found that politicians in the United States can expect to live about 7 years longer than the national average. The difference is around 3 years in Switzerland, 4.5 years in Germany, and 6 years in France.
“For almost all countries, politicians had similar rates of mortality to the general population in the late 19th and early 20th centuries. Throughout the 20th century, differences in mortality rates widened significantly across all countries, so that politicians had an increasing survival advantage over the general population,” they said in a written statement.
Income inequality could be a factor, but the longevity gains made by politicians, which started before the 1940s, predate the rise of their earnings relative to the rest of the population, which didn’t really get going until the 1980s, the investigators noted.
Whatever the reason, we have this closing thought regarding our long-lived lawmakers: What’s the difference between a politician and a snail? One is a slimy pest that leaves a trail everywhere. The other is a snail.
Land of the free, home of obesity
In the United States, it seems, people are becoming more comfortable with obesity. TikTok and Instagram trends often try to show the world that all sizes are beautiful. There’s also the growing popularity of the dad bod.
America, it has been said, is the land of the free. We love our freedom, and we value our individualism. If an obese man orders three meals from McDonald’s just for himself, no one is going to stop him. Many Americans also have more access to the food they want at any given time, even while they are moving around a lot less because of their sedentary lifestyles.
According to a recent study cited by the New York Post, however, America is not the only country battling obesity. Egypt and Mexico, for example, also have men with higher BMIs who cherish their individualism and the right to eat what they want, Plamen Akaliyski, PhD, of University Carlos III of Madrid, and associates, said in Social Science & Medicine.
Women are not as likely to think the same way. “Men in particular think, ‘I’m an individual, don’t tell me what to do. I’m going to eat what I want,’ ” bariatric surgeon George A. Fielding, MD, said in the Post article. Dr. Fielding also noted that women are three times more likely than men to seek bariatric surgery.
Dr. Akaliyski and associates found that Asian countries such as Japan, Singapore, and South Korea – countries that value thrift, discipline, self control, and delaying gratification – have lower rates of obesity.
So yes, we can go to the drive through of a fast food restaurant whenever we want and order whatever we want, but can doesn’t always mean should.
More evidence the flu vaccine may guard against Alzheimer’s
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
COVID-19 tied to increased risk for Alzheimer’s disease and Parkinson’s disease
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NEUROLOGY
White House expands access to monkeypox vaccines
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
FDA panel backs adding Omicron component to COVID boosters
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
COVID subvariants could cause ‘substantial’ summer cases
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
Children and COVID: Vaccination off to slow start for the newly eligible
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.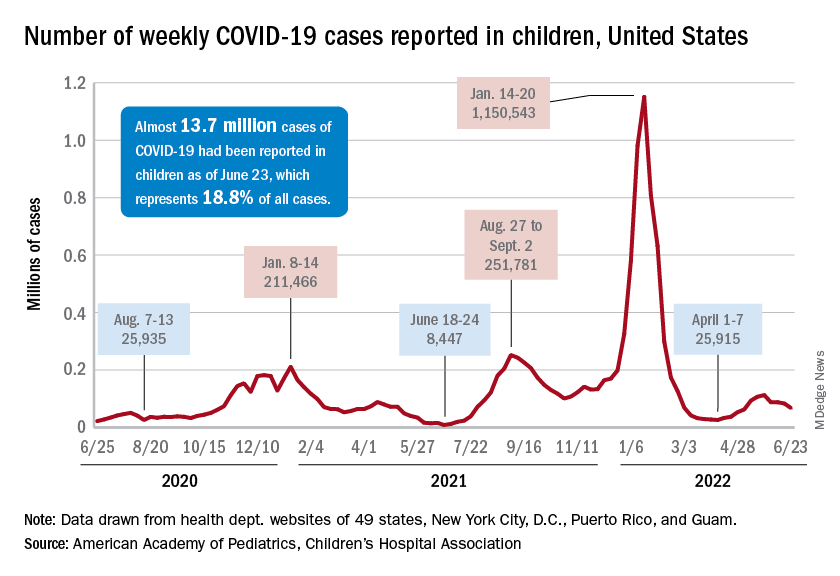
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.




