User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Job market for physicians, advanced practitioners rebounds after COVID-19 slump: Report
After a year of uncertainty and decline because of the COVID-19 pandemic, according to a recently released report from Merritt Hawkins, the physician search division of AMN Healthcare.
The study is based on an analysis of job search and consulting assignments that the firm conducted on behalf of its health care organization clients from April 1, 2021, to March 31, 2022.
“Search engagements were down a little over 30% in 2020, but by the end of 2021, everything started spiking dramatically to the point of where we were at a 34-year high,” Michael Belkin, divisional vice president with Merritt Hawkins, told this news organization. “The pendulum has gone all the way back. People are more interested in going out and seeing their physicians.”
Demand for physicians was suppressed during the peak of the pandemic, as many hospitals curtailed elective procedures and many patients refrained from entering a medical facility. A large backlog of patients needing care subsequently developed.
This, combined with an aging population and widespread chronic medical conditions, has caused a strong surge in demand for physicians and advanced practitioners, according to the report.
In addition to the volume of searches increasing, physician starting salaries have rebounded from the COVID-19 downturn.
Average starting salaries of 14 physician specialties tracked in 2021/2022 increased, while only 3 decreased. Orthopedic surgeons were offered an average of $565,000 to start, exclusive of signing bonuses and other incentives, up from $546,000 the previous year. Urologists were offered an average of $510,000 to start, up from $497,000; gastroenterologists were offered $474,000, up from $453,000; while radiologists were offered $455,000, up from $401,000.
Similarly, a recent Medscape study based on responses from more than 13,000 U.S. physicians across 29 specialties found that income for all physician specialists increased, with otolaryngologists, gastroenterologists, and dermatologists experiencing the greatest gains.
A new reality
While the job market for physicians and advanced practitioners has seemingly recovered, there are many differences between today’s working environment for clinicians and what existed during the pandemic.
First, specialists are now stepping into the spotlight, a position that primary care clinicians previously held. The majority of Merritt Hawkins’ search engagements (64%) in 2021/2022 were for physician specialists, including cardiologists, gastroenterologists, orthopedic surgeons, neurologists, oncologists, and others. Only 17% of the search engagements were for primary care physicians, down from 18% in 2020/2021 and 20% in 2019/2020.
“We’ve seen specialties bounce back faster. Of course, you’ve got the aging population; you’ve got people that want that specialized care,” Mr. Belkin said.
Advanced practitioners also are playing a more significant role in the postpandemic word. In fact, 19% of Merritt Hawkins’ search engagements were for advanced practitioners, including nurse practitioners (NPs), physician assistants, and certified registered nurse anesthetists, up from 18% the previous year and just 13% the year prior to that, indicating growing demand for nonphysician providers.
NPs, in fact, topped the list of most requested search engagements, underscoring a shift from traditional physician office-based primary care delivery settings toward “convenient care” settings such as urgent care centers and retail clinics that are largely staffed by NPs and other advanced practitioners.
Advanced practitioners are taking on more responsibility for primary care simply because there is a large number of these professionals ready to take on the challenge.
The health care industry was “not able to produce enough primary care physicians over the last decade. So advanced practitioners, I believe, have slowly started to work alongside those primary care physicians. In a lot of areas such as your retail space, your CVS, your Walmart, your Walgreens, your standalone urgent cares, they’ve stepped up,” Mr. Belkin said.
Advanced practitioners also are providing the convenience that consumers are increasingly demanding.
“We are a society that wants things immediately ... but it’s still a challenge to schedule an appointment with a physician. However, it’s less of a challenge to get into a retail clinic or an urgent care center or to schedule something through telehealth,” Mr. Belkin noted.
More than just money
With the job market strong, the challenge for health care organizations is to create competitive recruiting packages. Sure enough, 92% of candidates were offered signing bonuses in 2021/2022 compared with just 61% in 2020/2021.
The financial incentives, however, might not be enough. In this environment, health care organizations need to go beyond simply offering competitive salaries to new recruits. For example, clinicians are seeking flexibility, as many potential hires are seeking remote positions. In fact, 18% of radiology search engagements were for teleradiologists, while 15% of its search engagements for psychiatrists were for telepsychiatrists in 2021/2022.
“Right now, quality of life is a very important factor. It’s work-life balance. It’s sensitivity to the stresses that we just experienced over the last 2.5 years,” Mr. Belkin concluded. “There’s more sensitivity around the culture of the organizations. What’s the leadership like? How did the organization handle the pandemic? How do they respond?”
A version of this article first appeared on Medscape.com.
After a year of uncertainty and decline because of the COVID-19 pandemic, according to a recently released report from Merritt Hawkins, the physician search division of AMN Healthcare.
The study is based on an analysis of job search and consulting assignments that the firm conducted on behalf of its health care organization clients from April 1, 2021, to March 31, 2022.
“Search engagements were down a little over 30% in 2020, but by the end of 2021, everything started spiking dramatically to the point of where we were at a 34-year high,” Michael Belkin, divisional vice president with Merritt Hawkins, told this news organization. “The pendulum has gone all the way back. People are more interested in going out and seeing their physicians.”
Demand for physicians was suppressed during the peak of the pandemic, as many hospitals curtailed elective procedures and many patients refrained from entering a medical facility. A large backlog of patients needing care subsequently developed.
This, combined with an aging population and widespread chronic medical conditions, has caused a strong surge in demand for physicians and advanced practitioners, according to the report.
In addition to the volume of searches increasing, physician starting salaries have rebounded from the COVID-19 downturn.
Average starting salaries of 14 physician specialties tracked in 2021/2022 increased, while only 3 decreased. Orthopedic surgeons were offered an average of $565,000 to start, exclusive of signing bonuses and other incentives, up from $546,000 the previous year. Urologists were offered an average of $510,000 to start, up from $497,000; gastroenterologists were offered $474,000, up from $453,000; while radiologists were offered $455,000, up from $401,000.
Similarly, a recent Medscape study based on responses from more than 13,000 U.S. physicians across 29 specialties found that income for all physician specialists increased, with otolaryngologists, gastroenterologists, and dermatologists experiencing the greatest gains.
A new reality
While the job market for physicians and advanced practitioners has seemingly recovered, there are many differences between today’s working environment for clinicians and what existed during the pandemic.
First, specialists are now stepping into the spotlight, a position that primary care clinicians previously held. The majority of Merritt Hawkins’ search engagements (64%) in 2021/2022 were for physician specialists, including cardiologists, gastroenterologists, orthopedic surgeons, neurologists, oncologists, and others. Only 17% of the search engagements were for primary care physicians, down from 18% in 2020/2021 and 20% in 2019/2020.
“We’ve seen specialties bounce back faster. Of course, you’ve got the aging population; you’ve got people that want that specialized care,” Mr. Belkin said.
Advanced practitioners also are playing a more significant role in the postpandemic word. In fact, 19% of Merritt Hawkins’ search engagements were for advanced practitioners, including nurse practitioners (NPs), physician assistants, and certified registered nurse anesthetists, up from 18% the previous year and just 13% the year prior to that, indicating growing demand for nonphysician providers.
NPs, in fact, topped the list of most requested search engagements, underscoring a shift from traditional physician office-based primary care delivery settings toward “convenient care” settings such as urgent care centers and retail clinics that are largely staffed by NPs and other advanced practitioners.
Advanced practitioners are taking on more responsibility for primary care simply because there is a large number of these professionals ready to take on the challenge.
The health care industry was “not able to produce enough primary care physicians over the last decade. So advanced practitioners, I believe, have slowly started to work alongside those primary care physicians. In a lot of areas such as your retail space, your CVS, your Walmart, your Walgreens, your standalone urgent cares, they’ve stepped up,” Mr. Belkin said.
Advanced practitioners also are providing the convenience that consumers are increasingly demanding.
“We are a society that wants things immediately ... but it’s still a challenge to schedule an appointment with a physician. However, it’s less of a challenge to get into a retail clinic or an urgent care center or to schedule something through telehealth,” Mr. Belkin noted.
More than just money
With the job market strong, the challenge for health care organizations is to create competitive recruiting packages. Sure enough, 92% of candidates were offered signing bonuses in 2021/2022 compared with just 61% in 2020/2021.
The financial incentives, however, might not be enough. In this environment, health care organizations need to go beyond simply offering competitive salaries to new recruits. For example, clinicians are seeking flexibility, as many potential hires are seeking remote positions. In fact, 18% of radiology search engagements were for teleradiologists, while 15% of its search engagements for psychiatrists were for telepsychiatrists in 2021/2022.
“Right now, quality of life is a very important factor. It’s work-life balance. It’s sensitivity to the stresses that we just experienced over the last 2.5 years,” Mr. Belkin concluded. “There’s more sensitivity around the culture of the organizations. What’s the leadership like? How did the organization handle the pandemic? How do they respond?”
A version of this article first appeared on Medscape.com.
After a year of uncertainty and decline because of the COVID-19 pandemic, according to a recently released report from Merritt Hawkins, the physician search division of AMN Healthcare.
The study is based on an analysis of job search and consulting assignments that the firm conducted on behalf of its health care organization clients from April 1, 2021, to March 31, 2022.
“Search engagements were down a little over 30% in 2020, but by the end of 2021, everything started spiking dramatically to the point of where we were at a 34-year high,” Michael Belkin, divisional vice president with Merritt Hawkins, told this news organization. “The pendulum has gone all the way back. People are more interested in going out and seeing their physicians.”
Demand for physicians was suppressed during the peak of the pandemic, as many hospitals curtailed elective procedures and many patients refrained from entering a medical facility. A large backlog of patients needing care subsequently developed.
This, combined with an aging population and widespread chronic medical conditions, has caused a strong surge in demand for physicians and advanced practitioners, according to the report.
In addition to the volume of searches increasing, physician starting salaries have rebounded from the COVID-19 downturn.
Average starting salaries of 14 physician specialties tracked in 2021/2022 increased, while only 3 decreased. Orthopedic surgeons were offered an average of $565,000 to start, exclusive of signing bonuses and other incentives, up from $546,000 the previous year. Urologists were offered an average of $510,000 to start, up from $497,000; gastroenterologists were offered $474,000, up from $453,000; while radiologists were offered $455,000, up from $401,000.
Similarly, a recent Medscape study based on responses from more than 13,000 U.S. physicians across 29 specialties found that income for all physician specialists increased, with otolaryngologists, gastroenterologists, and dermatologists experiencing the greatest gains.
A new reality
While the job market for physicians and advanced practitioners has seemingly recovered, there are many differences between today’s working environment for clinicians and what existed during the pandemic.
First, specialists are now stepping into the spotlight, a position that primary care clinicians previously held. The majority of Merritt Hawkins’ search engagements (64%) in 2021/2022 were for physician specialists, including cardiologists, gastroenterologists, orthopedic surgeons, neurologists, oncologists, and others. Only 17% of the search engagements were for primary care physicians, down from 18% in 2020/2021 and 20% in 2019/2020.
“We’ve seen specialties bounce back faster. Of course, you’ve got the aging population; you’ve got people that want that specialized care,” Mr. Belkin said.
Advanced practitioners also are playing a more significant role in the postpandemic word. In fact, 19% of Merritt Hawkins’ search engagements were for advanced practitioners, including nurse practitioners (NPs), physician assistants, and certified registered nurse anesthetists, up from 18% the previous year and just 13% the year prior to that, indicating growing demand for nonphysician providers.
NPs, in fact, topped the list of most requested search engagements, underscoring a shift from traditional physician office-based primary care delivery settings toward “convenient care” settings such as urgent care centers and retail clinics that are largely staffed by NPs and other advanced practitioners.
Advanced practitioners are taking on more responsibility for primary care simply because there is a large number of these professionals ready to take on the challenge.
The health care industry was “not able to produce enough primary care physicians over the last decade. So advanced practitioners, I believe, have slowly started to work alongside those primary care physicians. In a lot of areas such as your retail space, your CVS, your Walmart, your Walgreens, your standalone urgent cares, they’ve stepped up,” Mr. Belkin said.
Advanced practitioners also are providing the convenience that consumers are increasingly demanding.
“We are a society that wants things immediately ... but it’s still a challenge to schedule an appointment with a physician. However, it’s less of a challenge to get into a retail clinic or an urgent care center or to schedule something through telehealth,” Mr. Belkin noted.
More than just money
With the job market strong, the challenge for health care organizations is to create competitive recruiting packages. Sure enough, 92% of candidates were offered signing bonuses in 2021/2022 compared with just 61% in 2020/2021.
The financial incentives, however, might not be enough. In this environment, health care organizations need to go beyond simply offering competitive salaries to new recruits. For example, clinicians are seeking flexibility, as many potential hires are seeking remote positions. In fact, 18% of radiology search engagements were for teleradiologists, while 15% of its search engagements for psychiatrists were for telepsychiatrists in 2021/2022.
“Right now, quality of life is a very important factor. It’s work-life balance. It’s sensitivity to the stresses that we just experienced over the last 2.5 years,” Mr. Belkin concluded. “There’s more sensitivity around the culture of the organizations. What’s the leadership like? How did the organization handle the pandemic? How do they respond?”
A version of this article first appeared on Medscape.com.
What are your weaknesses?
In a video posted to TikTok by the comedian Will Flanary, MD, better known to his followers as Dr. Glaucomflecken, he imitates a neurosurgical residency interview. With glasses perched on the bridge of his nose, Dr. Glaucomflecken poses as the attending, asking: “What are your weaknesses?”
The residency applicant answers without hesitation: “My physiological need for sleep.” “What are your strengths?” The resident replies with the hard, steely stare of the determined and uninitiated: “My desire to eliminate my physiological need for sleep.”
If you follow Dr. Glaucomflecken on Twitter, you might know the skit I’m referencing. For many physicians and physicians-in-training, what makes the satire successful is its reflection of reality.
Many things have changed in medicine since his time, but the tired trope of the sleepless surgeon hangs on. Undaunted, I spent my second and third year of medical school accumulating accolades, conducting research, and connecting with mentors with the singular goal of joining the surgical ranks.
Midway through my third year, I completed a month-long surgical subinternship designed to give students a taste of what life would look like as an intern. I loved the operating room; it felt like the difference between being on dry land and being underwater. There were fewer distractions – your patient in the spotlight while everything else receded to the shadows.
However, as the month wore on, something stronger took hold. I couldn’t keep my eyes open in the darkened operating rooms and had to decline stools, fearing that I would fall asleep if I sat down.
On early morning prerounds, it’s 4:50 a.m. when I glance at the clock and pull back the curtain, already apologizing. My patient rolls over, flashing a wry smile. “Do you ever go home?” I’ve seen residents respond to this exact question in various ways. I live here. Yes. No. Soon. Not enough. My partner doesn’t think so.
There are days and, yes, years when we are led to believe this is what we live for: to be constantly available to our patients. It feels like a hollow victory when the patient, 2 days out from a total colectomy, begins to worry about your personal life. I ask her how she slept (not enough), any fevers (no), vomiting (no), urinating (I pause – she has a catheter).
My favorite part of these early morning rounds is the pause in my scripted litany of questions to listen to heart and lungs. It never fails to feel sacred: Patients become so quiet and still that I can’t help but think they have faith in me. Without prompting, she slides the back of her hospital gown forward like a curtain, already taking deep breaths so I can hear her lungs.
I look outside. The streetlights are still on, and from the seventh-floor window, I can watch staff making their way through the sliding double-doors, just beyond the yellowed pools of streetlight. I smile. I love medicine. I’m so tired.
For many in medicine, we are treated, and thus behave, as though our ability to manipulate physiology should also apply within the borders of our bodies: commanding less sleep, food, or bathroom breaks.
It places health care workers solidly in the realm of superhuman, living beyond one’s corporeal needs. The pandemic only heightened this misappropriation – adding hero and setting out a pedestal for health care workers to make their ungainly ascent. This kind of unsolicited admiration implicitly implies inhumanness, an otherness.
What would it look like if we started treating ourselves less like physicians and more like patients? I wish I was offering a solution, but really this is just a story.
To students rising through the ranks of medical training, identify what it is you need early and often. I can count on one hand how many physicians I’ve seen take a lunch break – even 10 minutes. Embrace hard work and self-preservation equally. My hope is that if enough of us take this path, it just might become a matter of course.
Dr. Meffert is a resident in the department of emergency medicine, MedStar Georgetown University Hospital, Washington Hospital Center, Washington. Dr. Meffert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a video posted to TikTok by the comedian Will Flanary, MD, better known to his followers as Dr. Glaucomflecken, he imitates a neurosurgical residency interview. With glasses perched on the bridge of his nose, Dr. Glaucomflecken poses as the attending, asking: “What are your weaknesses?”
The residency applicant answers without hesitation: “My physiological need for sleep.” “What are your strengths?” The resident replies with the hard, steely stare of the determined and uninitiated: “My desire to eliminate my physiological need for sleep.”
If you follow Dr. Glaucomflecken on Twitter, you might know the skit I’m referencing. For many physicians and physicians-in-training, what makes the satire successful is its reflection of reality.
Many things have changed in medicine since his time, but the tired trope of the sleepless surgeon hangs on. Undaunted, I spent my second and third year of medical school accumulating accolades, conducting research, and connecting with mentors with the singular goal of joining the surgical ranks.
Midway through my third year, I completed a month-long surgical subinternship designed to give students a taste of what life would look like as an intern. I loved the operating room; it felt like the difference between being on dry land and being underwater. There were fewer distractions – your patient in the spotlight while everything else receded to the shadows.
However, as the month wore on, something stronger took hold. I couldn’t keep my eyes open in the darkened operating rooms and had to decline stools, fearing that I would fall asleep if I sat down.
On early morning prerounds, it’s 4:50 a.m. when I glance at the clock and pull back the curtain, already apologizing. My patient rolls over, flashing a wry smile. “Do you ever go home?” I’ve seen residents respond to this exact question in various ways. I live here. Yes. No. Soon. Not enough. My partner doesn’t think so.
There are days and, yes, years when we are led to believe this is what we live for: to be constantly available to our patients. It feels like a hollow victory when the patient, 2 days out from a total colectomy, begins to worry about your personal life. I ask her how she slept (not enough), any fevers (no), vomiting (no), urinating (I pause – she has a catheter).
My favorite part of these early morning rounds is the pause in my scripted litany of questions to listen to heart and lungs. It never fails to feel sacred: Patients become so quiet and still that I can’t help but think they have faith in me. Without prompting, she slides the back of her hospital gown forward like a curtain, already taking deep breaths so I can hear her lungs.
I look outside. The streetlights are still on, and from the seventh-floor window, I can watch staff making their way through the sliding double-doors, just beyond the yellowed pools of streetlight. I smile. I love medicine. I’m so tired.
For many in medicine, we are treated, and thus behave, as though our ability to manipulate physiology should also apply within the borders of our bodies: commanding less sleep, food, or bathroom breaks.
It places health care workers solidly in the realm of superhuman, living beyond one’s corporeal needs. The pandemic only heightened this misappropriation – adding hero and setting out a pedestal for health care workers to make their ungainly ascent. This kind of unsolicited admiration implicitly implies inhumanness, an otherness.
What would it look like if we started treating ourselves less like physicians and more like patients? I wish I was offering a solution, but really this is just a story.
To students rising through the ranks of medical training, identify what it is you need early and often. I can count on one hand how many physicians I’ve seen take a lunch break – even 10 minutes. Embrace hard work and self-preservation equally. My hope is that if enough of us take this path, it just might become a matter of course.
Dr. Meffert is a resident in the department of emergency medicine, MedStar Georgetown University Hospital, Washington Hospital Center, Washington. Dr. Meffert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a video posted to TikTok by the comedian Will Flanary, MD, better known to his followers as Dr. Glaucomflecken, he imitates a neurosurgical residency interview. With glasses perched on the bridge of his nose, Dr. Glaucomflecken poses as the attending, asking: “What are your weaknesses?”
The residency applicant answers without hesitation: “My physiological need for sleep.” “What are your strengths?” The resident replies with the hard, steely stare of the determined and uninitiated: “My desire to eliminate my physiological need for sleep.”
If you follow Dr. Glaucomflecken on Twitter, you might know the skit I’m referencing. For many physicians and physicians-in-training, what makes the satire successful is its reflection of reality.
Many things have changed in medicine since his time, but the tired trope of the sleepless surgeon hangs on. Undaunted, I spent my second and third year of medical school accumulating accolades, conducting research, and connecting with mentors with the singular goal of joining the surgical ranks.
Midway through my third year, I completed a month-long surgical subinternship designed to give students a taste of what life would look like as an intern. I loved the operating room; it felt like the difference between being on dry land and being underwater. There were fewer distractions – your patient in the spotlight while everything else receded to the shadows.
However, as the month wore on, something stronger took hold. I couldn’t keep my eyes open in the darkened operating rooms and had to decline stools, fearing that I would fall asleep if I sat down.
On early morning prerounds, it’s 4:50 a.m. when I glance at the clock and pull back the curtain, already apologizing. My patient rolls over, flashing a wry smile. “Do you ever go home?” I’ve seen residents respond to this exact question in various ways. I live here. Yes. No. Soon. Not enough. My partner doesn’t think so.
There are days and, yes, years when we are led to believe this is what we live for: to be constantly available to our patients. It feels like a hollow victory when the patient, 2 days out from a total colectomy, begins to worry about your personal life. I ask her how she slept (not enough), any fevers (no), vomiting (no), urinating (I pause – she has a catheter).
My favorite part of these early morning rounds is the pause in my scripted litany of questions to listen to heart and lungs. It never fails to feel sacred: Patients become so quiet and still that I can’t help but think they have faith in me. Without prompting, she slides the back of her hospital gown forward like a curtain, already taking deep breaths so I can hear her lungs.
I look outside. The streetlights are still on, and from the seventh-floor window, I can watch staff making their way through the sliding double-doors, just beyond the yellowed pools of streetlight. I smile. I love medicine. I’m so tired.
For many in medicine, we are treated, and thus behave, as though our ability to manipulate physiology should also apply within the borders of our bodies: commanding less sleep, food, or bathroom breaks.
It places health care workers solidly in the realm of superhuman, living beyond one’s corporeal needs. The pandemic only heightened this misappropriation – adding hero and setting out a pedestal for health care workers to make their ungainly ascent. This kind of unsolicited admiration implicitly implies inhumanness, an otherness.
What would it look like if we started treating ourselves less like physicians and more like patients? I wish I was offering a solution, but really this is just a story.
To students rising through the ranks of medical training, identify what it is you need early and often. I can count on one hand how many physicians I’ve seen take a lunch break – even 10 minutes. Embrace hard work and self-preservation equally. My hope is that if enough of us take this path, it just might become a matter of course.
Dr. Meffert is a resident in the department of emergency medicine, MedStar Georgetown University Hospital, Washington Hospital Center, Washington. Dr. Meffert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Charcoal could be the cure for the common high-fat diet
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
Charcoal won’t let high-fat diet weigh you down
Do you want to be the funniest person alive? Of course you do. It’s really simple too, just one joke can make you the greatest comedian of all time. All you have to do is go camping and cook food over a roaring campfire. When someone drops food into the fire (which they always will), get ready. Once they fish out the offending food, which is almost certainly coated in hot coals, tell them: “Ah, eat it anyway. A little texture never hurt!” Trust us, most hilarious and original gag of all time.
But before your hapless friend brushes off his hot dog and forces a laugh, consider this: Japanese researchers have found that a charcoal supplement can prevent weight gain in mice consuming a high-fat diet. Charcoal is actually quite the helpful substance, and not just for grilling. It’s been used as medicine for hundreds of years and even today is used as a treatment for drug overdose and excess gas and flatulence.
The study involved two groups of mice: One was fed a normal diet, the other a high-fat diet. After 12 weeks, the high-fat diet mice had gained weight. At that point, edible activated charcoal was added to their diet. From that point, weight gain was similar between the two groups, and the amount of bile acid, cholesterol, triglyceride, and fatty acid excreted by the high-fat mice increased by two to four times.
The researchers supported the notion that consuming an activated charcoal supplement before or while eating fatty food could prevent weight gain from said fatty food. Which works out well for the classic American barbecue, which is traditionally both high in fat and charcoal. All you have to do is buy some extra charcoal briquettes to pass around and munch on with your friends. Now that’s a party we can get behind.
There’s awake, and then there’s neurologically awake
Time to toss another urban legend onto the trash heap of history. Say goodbye to the benefits of uninterrupted sleep. It’s a fraud, a fake, a myth, a hit or myth, a swing and a myth, an old wives’ tale. You can stuff it and put it on a shelf next to Bigfoot, the Slender Man, and Twinkies.
We all thought we needed 8 hours of uninterrupted sleep every night, but guess who we forgot to tell? Our brains. They’ve been doing exactly the opposite all along, laughing at us the whole time. Smug SOBs.
To straighten out this mess, let’s bring in a scientist, Celia Kjaerby of the Center for Translational Neuromedicine at the University of Copenhagen: “You may think that sleep is a constant state that you are in, and then you wake up. But there is a lot more to sleep than meets the eye. We have learned that noradrenaline causes you to wake up more than 100 times a night. And that is during perfectly normal sleep.”
Those 100 or so sleep interruptions are so brief that we don’t even notice, but they are very important, according to a study conducted at the university. Those tiny little wake-up calls are “the essence for the part of sleep that makes us wake up rested and which enables us to remember what we learned the day before. ... The very short awakenings are created by waves of norepinephrine [and they] reset the brain so that it is ready to store memory when you dive back into sleep,” lead author Maiken Nedergaard, MD, explained.
The investigators compared the level of noradrenaline in sleeping mice with their electrical activity and found that the hormone constantly increased and decreased in a wavelike pattern. A high level meant that the animal was neurologically awake. Deeper valleys between the high points meant better sleep, and the mice with the “highest number of deep noradrenaline valleys were also the ones with the best memory,” the team said in their written statement.
Not just the best memory, they said, but “super memory.” That, of course, was enough to get the attention of Marvel Comics, so the next Disney superhero blockbuster will feature Nocturna, the queen of the night. Her power? Never forgets. Her archnemesis? The Insomniac. Her catchphrase? “Let me sleep on it.”
Words can hurt, literally
Growing up, we’re sure you heard the “sticks and stones” rhyme. Maybe you’ve even recited it once or twice to defend yourself. Well, forget it, because words can hurt and your brain knows it.
In a new study published in Frontiers in Communication, Marijn Struiksma, PhD, of Utrecht University, and colleagues incorporated the use of electroencephalography (EEG) and skin conductance on 79 women to see how words (specifically insults) actually affect the human body.
Each subject was asked to read three different types of statements: an insult, a compliment, and something factual but neutral. Half of the statements contained the subject’s name and half used somebody else’s. The participants were told that these statements were collected from three men.
Nobody interacted with each other, and the setting was completely clinical, yet the results were unmistakable. The EEG showed an effect in P2 amplitude with repetitive insults, no matter who it was about. Even though the insults weren’t real and the participants were aware of it, the brain still recognized them as hurtful, coming across as “mini slaps in the face,” Dr. Struiksma noted in a written statement.
The researchers noted that more needs to be done to better understand the long-term effects that insults can have and create a deeper understanding between words and emotion, but studying the effects of insults in a real-life setting is ethically tricky. This study is a start.
So, yeah, sticks and stones can break your bones, but words will actually hurt you.
This article was updated 7/21/22.
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.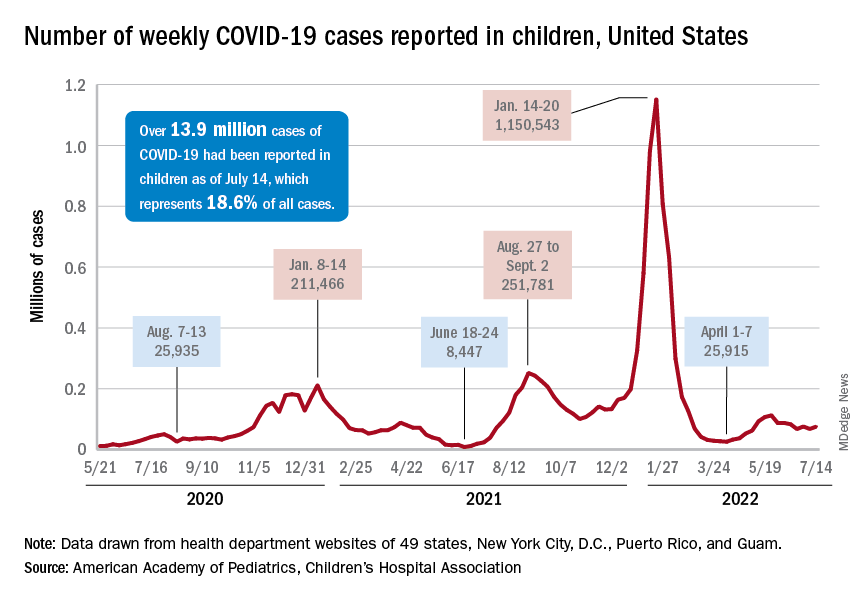
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.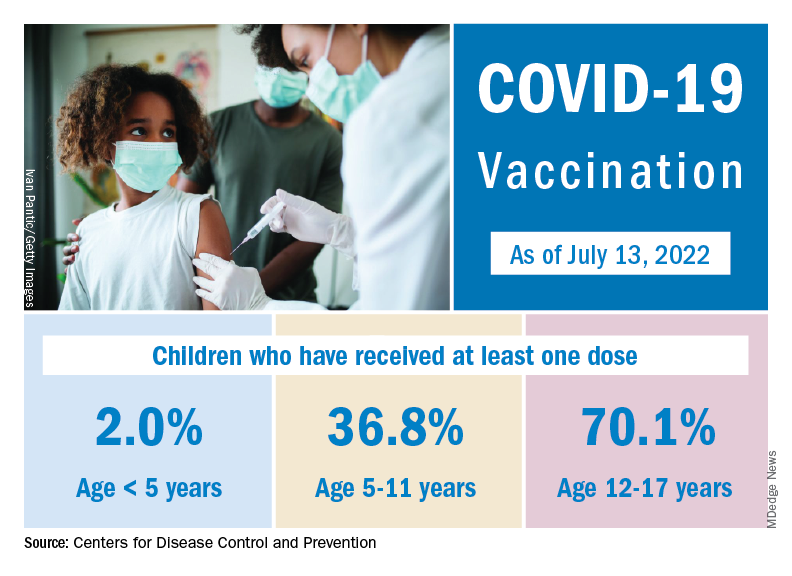
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
RV dysfunction slams survival in acute COVID, flu, pneumonia
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
CDC warns about potentially deadly virus in infants
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Best meds for insomnia identified?
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Many people becoming reinfected as BA.5 dominates new COVID-19 cases
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
Violent patient throws scalding oil on MD; other patient dangers
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.









