User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.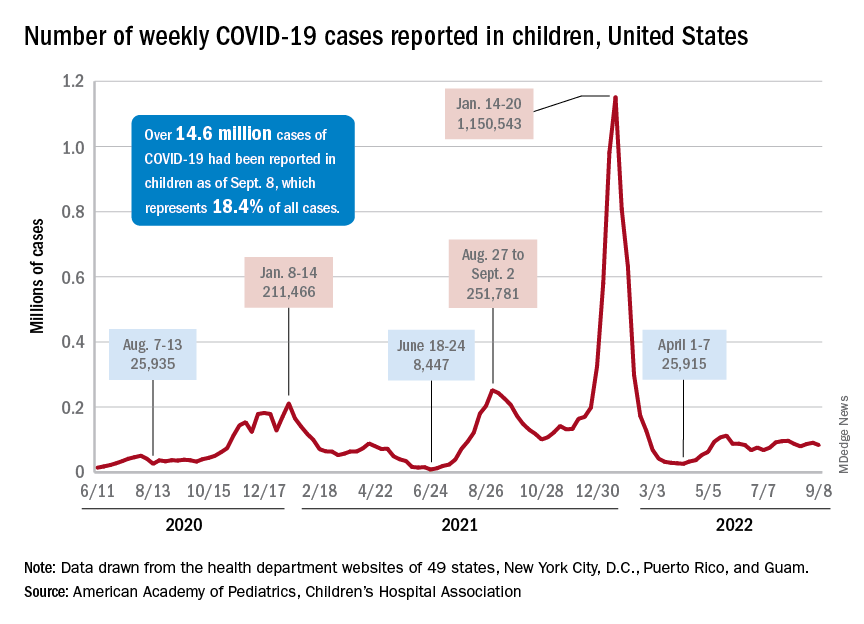
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
Integrase inhibitors and gestational weight gain: Should women worry?
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
CDC warns of enterovirus strain linked to polio-like condition
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Post-COVID fatigue, exercise intolerance signal ME/CFS
A new study provides yet more evidence that a significant subset of people who experience persistent fatigue and exercise intolerance following COVID-19 will meet diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Data from the prospective observational study of 42 patients with “post-COVID-19 syndrome (PCS),” including persistent fatigue and exercise intolerance, suggest that a large proportion will meet strict diagnostic criteria for ME/CFS, including the hallmark postexertional malaise (PEM). Still others may experience similar disability but lack duration and/or severity requirements for the diagnosis.
Moreover, disease severity and symptom burden were found similar in those with ME/CFS following COVID-19 and in a group of 19 age- and sex-matched individuals with ME/CFS that wasn’t associated with COVID-19.
“The major finding is that ME/CFS is indeed part of the spectrum of the post-COVID syndrome and very similar to the ME/CFS we know after other infectious triggers,” senior author Carmen Scheibenbogen, MD, acting director of the Institute for Medical Immunology at the Charité University Medicine Campus Virchow-Klinikum, Berlin, told this news organization.
Importantly, from a clinical standpoint, both diminished hand-grip strength (HGS) and orthostatic intolerance were common across all patient groups, as were several laboratory values, Claudia Kedor, MD, and colleagues at Charité report in the paper, published online in Nature Communications.
Of the 42 with PCS, including persistent fatigue and exercise intolerance lasting at least 6 months, 19 met the rigorous Canadian Consensus Criteria (CCC) for ME/CFS, established in 2003, which require PEM, along with sleep dysfunction, significant persistent fatigue, pain, and several other symptoms from neurological/cognitive, autonomic, neuroendocrine, and immune categories that persist for at least 6 months.
Of the 23 who did not meet the CCC criteria, 18 still experienced PEM but for less than the required 14 hours set by the authors based on recent data. The original CCC had suggested 24 hours as the PEM duration. Eight subjects met all the Canadian criteria except for the neurological/cognitive symptoms. None of the 42 had evidence of severe depression.
The previously widely used 1994 “Fukuda” criteria for ME/CFS are no longer recommended because they don’t require PEM, which is now considered a key symptom. The more recent 2015 Institute (now Academy) of Medicine criteria don’t define the length of PEM, the authors note in the paper.
Dr. Scheibenbogen said, “Post-COVID has a spectrum of syndromes and conditions. We see that a subset of patients have similar symptoms of ME/CFS but don’t fulfill the CCC, although they may meet less stringent criteria. We think this is of relevance for both diagnostic markers and development of therapy, because there may be different pathomechanisms between the subsets of post-COVID patients.”
She pointed to other studies from her group suggesting that inflammation is present early in post-COVID (not yet published), while in the subset that goes on to ME/CFS, autoantibodies or endothelial dysfunction play a more important role. «At the moment, it’s quite complex, and I don’t think in the end we will have just one pathomechanism. So I think we’ll need to develop various treatment strategies.”
Asked to comment on the new data, Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School, senior physician at Brigham and Women’s Hospital, both in Boston, and editor in chief of the Harvard Health Letter, told this news organization, “This paper adds to the evidence that an illness with symptoms that meet criteria for ME/CFS can follow COVID-19 in nearly half of those patients who have lingering symptoms. This can occur even in people who initially have only mild symptoms from COVID-19, although it is more likely to happen in the people who are sickest when they first get COVID-19. And those who meet criteria for ME/CFS were seriously impaired in their ability to function, [both] at work and at home.”
But, Dr. Komaroff also cautioned, “the study does not help in determining what fraction of all people who are infected with SARS-CoV-2 go on to develop a condition like ME/CFS, nor how long that condition will last. It is crucial that we get answers to these questions, as the impact on the economy, the health care system, and the disability system could be substantial.”
He pointed to a recent report from the Brookings Institution (2022 Aug 24. “New data shows long Covid is keeping as many as 4 million people out of work” Katie Bach) “finding that “long COVID may be a major contributor to the shortage of job applicants plaguing many businesses.”
Biomarkers include hand-grip strength, orthostatic intolerance, lab measures
Hand-grip strength, as assessed by 10 repeat grips at maximum force and repeated after 60 minutes, were lower for all those meeting ME/CFS criteria, compared with the healthy controls. Hand-grip strength parameters were also positively correlated with laboratory hemoglobin measures in both PCS groups who did and didn’t meet the Canadian ME/CFS criteria.
A total of three patients with PCS who didn’t meet ME/CFS criteria and seven with PCS who met ME/CFS criteria had sitting blood pressures of greater than 140 mm Hg systolic and/or greater than 90 mm Hg diastolic. Five patients with PCS – four who met ME/CFS criteria and one who didn’t – fulfilled criteria for postural orthostatic tachycardia syndrome. Orthostatic hypotension was diagnosed in a total of seven with PCS, including one who did not meet ME/CFS criteria and the rest who did.
Among significant laboratory findings, mannose-binding lectin deficiency, which is associated with increased infection susceptibility and found in only about 6% of historical controls, was found more frequently in both of the PCS cohorts (17% of those with ME/CFS and 23% of those without) than it has been in the past among those with ME/CFS, compared with historical controls (15%).
There was only slight elevation in C-reactive protein, the most commonly measured marker of inflammation. However, another marker indicating inflammation within the last 3-4 months, interleukin 8 assessed in erythrocytes, was above normal in 37% with PCS and ME/CFS and in 48% with PCS who did not meet the ME/CFS criteria.
Elevated antinuclear antibodies, anti–thyroid peroxidase antibodies, vitamin D deficiencies, and folic acid deficiencies were all seen in small numbers of the PCS patients. Angiotensin-converting enzyme 1 levels were below the normal range in 31% of all patients.
“We must anticipate that this pandemic has the potential to dramatically increase the number of ME/CFS patients,” Dr. Kedor and colleagues write. “At the same time, it offers the unique chance to identify ME/CFS patients in a very early stage of disease and apply interventions such as pacing and coping early with a better therapeutic prognosis. Further, it is an unprecedented opportunity to understand the underlying pathomechanism and characterize targets for specific treatment approaches.”
Dr. Scheibenbogen and Dr. Komaroff reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study provides yet more evidence that a significant subset of people who experience persistent fatigue and exercise intolerance following COVID-19 will meet diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Data from the prospective observational study of 42 patients with “post-COVID-19 syndrome (PCS),” including persistent fatigue and exercise intolerance, suggest that a large proportion will meet strict diagnostic criteria for ME/CFS, including the hallmark postexertional malaise (PEM). Still others may experience similar disability but lack duration and/or severity requirements for the diagnosis.
Moreover, disease severity and symptom burden were found similar in those with ME/CFS following COVID-19 and in a group of 19 age- and sex-matched individuals with ME/CFS that wasn’t associated with COVID-19.
“The major finding is that ME/CFS is indeed part of the spectrum of the post-COVID syndrome and very similar to the ME/CFS we know after other infectious triggers,” senior author Carmen Scheibenbogen, MD, acting director of the Institute for Medical Immunology at the Charité University Medicine Campus Virchow-Klinikum, Berlin, told this news organization.
Importantly, from a clinical standpoint, both diminished hand-grip strength (HGS) and orthostatic intolerance were common across all patient groups, as were several laboratory values, Claudia Kedor, MD, and colleagues at Charité report in the paper, published online in Nature Communications.
Of the 42 with PCS, including persistent fatigue and exercise intolerance lasting at least 6 months, 19 met the rigorous Canadian Consensus Criteria (CCC) for ME/CFS, established in 2003, which require PEM, along with sleep dysfunction, significant persistent fatigue, pain, and several other symptoms from neurological/cognitive, autonomic, neuroendocrine, and immune categories that persist for at least 6 months.
Of the 23 who did not meet the CCC criteria, 18 still experienced PEM but for less than the required 14 hours set by the authors based on recent data. The original CCC had suggested 24 hours as the PEM duration. Eight subjects met all the Canadian criteria except for the neurological/cognitive symptoms. None of the 42 had evidence of severe depression.
The previously widely used 1994 “Fukuda” criteria for ME/CFS are no longer recommended because they don’t require PEM, which is now considered a key symptom. The more recent 2015 Institute (now Academy) of Medicine criteria don’t define the length of PEM, the authors note in the paper.
Dr. Scheibenbogen said, “Post-COVID has a spectrum of syndromes and conditions. We see that a subset of patients have similar symptoms of ME/CFS but don’t fulfill the CCC, although they may meet less stringent criteria. We think this is of relevance for both diagnostic markers and development of therapy, because there may be different pathomechanisms between the subsets of post-COVID patients.”
She pointed to other studies from her group suggesting that inflammation is present early in post-COVID (not yet published), while in the subset that goes on to ME/CFS, autoantibodies or endothelial dysfunction play a more important role. «At the moment, it’s quite complex, and I don’t think in the end we will have just one pathomechanism. So I think we’ll need to develop various treatment strategies.”
Asked to comment on the new data, Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School, senior physician at Brigham and Women’s Hospital, both in Boston, and editor in chief of the Harvard Health Letter, told this news organization, “This paper adds to the evidence that an illness with symptoms that meet criteria for ME/CFS can follow COVID-19 in nearly half of those patients who have lingering symptoms. This can occur even in people who initially have only mild symptoms from COVID-19, although it is more likely to happen in the people who are sickest when they first get COVID-19. And those who meet criteria for ME/CFS were seriously impaired in their ability to function, [both] at work and at home.”
But, Dr. Komaroff also cautioned, “the study does not help in determining what fraction of all people who are infected with SARS-CoV-2 go on to develop a condition like ME/CFS, nor how long that condition will last. It is crucial that we get answers to these questions, as the impact on the economy, the health care system, and the disability system could be substantial.”
He pointed to a recent report from the Brookings Institution (2022 Aug 24. “New data shows long Covid is keeping as many as 4 million people out of work” Katie Bach) “finding that “long COVID may be a major contributor to the shortage of job applicants plaguing many businesses.”
Biomarkers include hand-grip strength, orthostatic intolerance, lab measures
Hand-grip strength, as assessed by 10 repeat grips at maximum force and repeated after 60 minutes, were lower for all those meeting ME/CFS criteria, compared with the healthy controls. Hand-grip strength parameters were also positively correlated with laboratory hemoglobin measures in both PCS groups who did and didn’t meet the Canadian ME/CFS criteria.
A total of three patients with PCS who didn’t meet ME/CFS criteria and seven with PCS who met ME/CFS criteria had sitting blood pressures of greater than 140 mm Hg systolic and/or greater than 90 mm Hg diastolic. Five patients with PCS – four who met ME/CFS criteria and one who didn’t – fulfilled criteria for postural orthostatic tachycardia syndrome. Orthostatic hypotension was diagnosed in a total of seven with PCS, including one who did not meet ME/CFS criteria and the rest who did.
Among significant laboratory findings, mannose-binding lectin deficiency, which is associated with increased infection susceptibility and found in only about 6% of historical controls, was found more frequently in both of the PCS cohorts (17% of those with ME/CFS and 23% of those without) than it has been in the past among those with ME/CFS, compared with historical controls (15%).
There was only slight elevation in C-reactive protein, the most commonly measured marker of inflammation. However, another marker indicating inflammation within the last 3-4 months, interleukin 8 assessed in erythrocytes, was above normal in 37% with PCS and ME/CFS and in 48% with PCS who did not meet the ME/CFS criteria.
Elevated antinuclear antibodies, anti–thyroid peroxidase antibodies, vitamin D deficiencies, and folic acid deficiencies were all seen in small numbers of the PCS patients. Angiotensin-converting enzyme 1 levels were below the normal range in 31% of all patients.
“We must anticipate that this pandemic has the potential to dramatically increase the number of ME/CFS patients,” Dr. Kedor and colleagues write. “At the same time, it offers the unique chance to identify ME/CFS patients in a very early stage of disease and apply interventions such as pacing and coping early with a better therapeutic prognosis. Further, it is an unprecedented opportunity to understand the underlying pathomechanism and characterize targets for specific treatment approaches.”
Dr. Scheibenbogen and Dr. Komaroff reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study provides yet more evidence that a significant subset of people who experience persistent fatigue and exercise intolerance following COVID-19 will meet diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Data from the prospective observational study of 42 patients with “post-COVID-19 syndrome (PCS),” including persistent fatigue and exercise intolerance, suggest that a large proportion will meet strict diagnostic criteria for ME/CFS, including the hallmark postexertional malaise (PEM). Still others may experience similar disability but lack duration and/or severity requirements for the diagnosis.
Moreover, disease severity and symptom burden were found similar in those with ME/CFS following COVID-19 and in a group of 19 age- and sex-matched individuals with ME/CFS that wasn’t associated with COVID-19.
“The major finding is that ME/CFS is indeed part of the spectrum of the post-COVID syndrome and very similar to the ME/CFS we know after other infectious triggers,” senior author Carmen Scheibenbogen, MD, acting director of the Institute for Medical Immunology at the Charité University Medicine Campus Virchow-Klinikum, Berlin, told this news organization.
Importantly, from a clinical standpoint, both diminished hand-grip strength (HGS) and orthostatic intolerance were common across all patient groups, as were several laboratory values, Claudia Kedor, MD, and colleagues at Charité report in the paper, published online in Nature Communications.
Of the 42 with PCS, including persistent fatigue and exercise intolerance lasting at least 6 months, 19 met the rigorous Canadian Consensus Criteria (CCC) for ME/CFS, established in 2003, which require PEM, along with sleep dysfunction, significant persistent fatigue, pain, and several other symptoms from neurological/cognitive, autonomic, neuroendocrine, and immune categories that persist for at least 6 months.
Of the 23 who did not meet the CCC criteria, 18 still experienced PEM but for less than the required 14 hours set by the authors based on recent data. The original CCC had suggested 24 hours as the PEM duration. Eight subjects met all the Canadian criteria except for the neurological/cognitive symptoms. None of the 42 had evidence of severe depression.
The previously widely used 1994 “Fukuda” criteria for ME/CFS are no longer recommended because they don’t require PEM, which is now considered a key symptom. The more recent 2015 Institute (now Academy) of Medicine criteria don’t define the length of PEM, the authors note in the paper.
Dr. Scheibenbogen said, “Post-COVID has a spectrum of syndromes and conditions. We see that a subset of patients have similar symptoms of ME/CFS but don’t fulfill the CCC, although they may meet less stringent criteria. We think this is of relevance for both diagnostic markers and development of therapy, because there may be different pathomechanisms between the subsets of post-COVID patients.”
She pointed to other studies from her group suggesting that inflammation is present early in post-COVID (not yet published), while in the subset that goes on to ME/CFS, autoantibodies or endothelial dysfunction play a more important role. «At the moment, it’s quite complex, and I don’t think in the end we will have just one pathomechanism. So I think we’ll need to develop various treatment strategies.”
Asked to comment on the new data, Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School, senior physician at Brigham and Women’s Hospital, both in Boston, and editor in chief of the Harvard Health Letter, told this news organization, “This paper adds to the evidence that an illness with symptoms that meet criteria for ME/CFS can follow COVID-19 in nearly half of those patients who have lingering symptoms. This can occur even in people who initially have only mild symptoms from COVID-19, although it is more likely to happen in the people who are sickest when they first get COVID-19. And those who meet criteria for ME/CFS were seriously impaired in their ability to function, [both] at work and at home.”
But, Dr. Komaroff also cautioned, “the study does not help in determining what fraction of all people who are infected with SARS-CoV-2 go on to develop a condition like ME/CFS, nor how long that condition will last. It is crucial that we get answers to these questions, as the impact on the economy, the health care system, and the disability system could be substantial.”
He pointed to a recent report from the Brookings Institution (2022 Aug 24. “New data shows long Covid is keeping as many as 4 million people out of work” Katie Bach) “finding that “long COVID may be a major contributor to the shortage of job applicants plaguing many businesses.”
Biomarkers include hand-grip strength, orthostatic intolerance, lab measures
Hand-grip strength, as assessed by 10 repeat grips at maximum force and repeated after 60 minutes, were lower for all those meeting ME/CFS criteria, compared with the healthy controls. Hand-grip strength parameters were also positively correlated with laboratory hemoglobin measures in both PCS groups who did and didn’t meet the Canadian ME/CFS criteria.
A total of three patients with PCS who didn’t meet ME/CFS criteria and seven with PCS who met ME/CFS criteria had sitting blood pressures of greater than 140 mm Hg systolic and/or greater than 90 mm Hg diastolic. Five patients with PCS – four who met ME/CFS criteria and one who didn’t – fulfilled criteria for postural orthostatic tachycardia syndrome. Orthostatic hypotension was diagnosed in a total of seven with PCS, including one who did not meet ME/CFS criteria and the rest who did.
Among significant laboratory findings, mannose-binding lectin deficiency, which is associated with increased infection susceptibility and found in only about 6% of historical controls, was found more frequently in both of the PCS cohorts (17% of those with ME/CFS and 23% of those without) than it has been in the past among those with ME/CFS, compared with historical controls (15%).
There was only slight elevation in C-reactive protein, the most commonly measured marker of inflammation. However, another marker indicating inflammation within the last 3-4 months, interleukin 8 assessed in erythrocytes, was above normal in 37% with PCS and ME/CFS and in 48% with PCS who did not meet the ME/CFS criteria.
Elevated antinuclear antibodies, anti–thyroid peroxidase antibodies, vitamin D deficiencies, and folic acid deficiencies were all seen in small numbers of the PCS patients. Angiotensin-converting enzyme 1 levels were below the normal range in 31% of all patients.
“We must anticipate that this pandemic has the potential to dramatically increase the number of ME/CFS patients,” Dr. Kedor and colleagues write. “At the same time, it offers the unique chance to identify ME/CFS patients in a very early stage of disease and apply interventions such as pacing and coping early with a better therapeutic prognosis. Further, it is an unprecedented opportunity to understand the underlying pathomechanism and characterize targets for specific treatment approaches.”
Dr. Scheibenbogen and Dr. Komaroff reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
N.Y. governor declares state disaster emergency to boost polio vaccination
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
Risk factors linked to post–COVID vaccination death identified
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
FROM JAMA NETWORK OPEN
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
The potential problem(s) with a once-a-year COVID vaccine
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comments from the White House this week suggesting a once-a-year COVID-19 shot for most Americans, “just like your annual flu shot,” were met with backlash from many who say COVID and influenza come from different viruses and need different schedules.
Remarks, from “capitulation” to too few data, hit the airwaves and social media.
Some, however, agree with the White House vision and say that asking people to get one shot in the fall instead of periodic pushes for boosters will raise public confidence and buy-in and reduce consumer confusion.
Health leaders, including Bob Wachter, MD, chair of the department of medicine at the University of California, San Francisco, say they like the framing of the concept – that people who are not high-risk should plan each year for a COVID shot and a flu shot.
& we need strategy to bump uptake,” Dr. Wachter tweeted this week.
But the numbers of Americans seeking boosters remain low. Only one-third of all eligible people 50 years and older have gotten a second COVID booster, according to the Centers for Disease Control and Prevention. About half of those who got the original two shots got a first booster.
Meanwhile, the United States is still averaging about 70,000 new COVID cases and more than 300 deaths every day.
The suggested change in approach comes as Pfizer/BioNTech and Moderna roll out their new boosters that target Omicron subvariants BA.4 and BA.5 after the CDC recommended their use and the U.S. Food and Drug Administration approved emergency use authorization.
“As the virus continues to change, we will now be able to update our vaccines annually to target the dominant variant,” President Joe Biden said in a statement promoting the yearly approach.
Some say annual shot premature
Other experts say it’s too soon to tell whether an annual approach will work.
“We have no data to support that current vaccines, including the new BA.5 booster, will provide durable protection beyond 4-6 months. It would be good to aspire to this objective, and much longer duration or protection, but that will likely require next generation and nasal vaccines,” said Eric Topol, MD, Medscape’s editor-in-chief and founder and director of the Scripps Research Translational Institute.
A report in Nature Reviews Immunology states, “Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection” and potentially “can prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms.”
Dr. Topol tweeted after the White House statements, “[An annual vaccine] has the ring of Covid capitulation.”
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., told this news organization that he cautions against interpreting the White House comments as official policy.
“This is the difficulty of having public health announcements come out of Washington,” he said. “They ought to come out of the CDC.”
He says there is a reasonable analogy between COVID and influenza, but warns, “don’t push the analogy.”
They are both serious respiratory viruses that can cause much illness and death in essentially the same populations, he notes. These are the older, frail people, people who have underlying illnesses or are immunocompromised.
Both viruses also mutate. But there the paths diverge.
“We’ve gotten into a pattern of annually updating the influenza vaccine because it is such a singularly seasonal virus,” Dr. Schaffner said. “Basically it disappears during the summer. We’ve had plenty of COVID during the summers.”
For COVID, he said, “We will need a periodic booster. Could this be annually? That would certainly make it easier.” But it’s too soon to tell, he said.
Dr. Schaffner noted that several manufacturers are working on a combined flu/COVID vaccine.
Just a ‘first step’ toward annual shot
The currently updated COVID vaccine may be the first step toward an annual vaccine, but it’s only the first step, Dr. Schaffner said. “We haven’t committed to further steps yet because we’re watching this virus.”
Syra Madad, DHSc, MSc, an infectious disease epidemiologist at Harvard University’s Belfer Center for Science and International Affairs, Cambridge, Mass., and the New York City hospital system, told this news organization that arguments on both sides make sense.
Having a single message once a year can help eliminate the considerable confusion involving people on individual timelines with different levels of immunity and separate campaigns for COVID and flu shots coming at different times of the year.
“Communication around vaccines is very muddled and that shows in our overall vaccination rates, particularly booster rates,” she says. “The overall strategy is hopeful and makes sense if we’re going to progress that way based on data.”
However, she said that the data are just not there yet to show it’s time for an annual vaccine. First, scientists will need to see how long protection lasts with the Omicron-specific vaccine and how well and how long it protects against severe disease and death as well as infection.
COVID is less predictable than influenza and the influenza vaccine has been around for decades, Dr. Madad noted. With influenza, the patterns are more easily anticipated with their “ladder-like pattern,” she said. “COVID-19 is not like that.”
What is hopeful, she said, “is that we’ve been in the Omicron dynasty since November of 2021. I’m hopeful that we’ll stick with that particular variant.”
Dr. Topol, Dr. Schaffner, and Dr. Madad declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Flashy, blingy doc sabotages his own malpractice trial in rural farm town
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.




