User login
In Case You Missed It: COVID
No increase seen in children’s cumulative COVID-19 burden
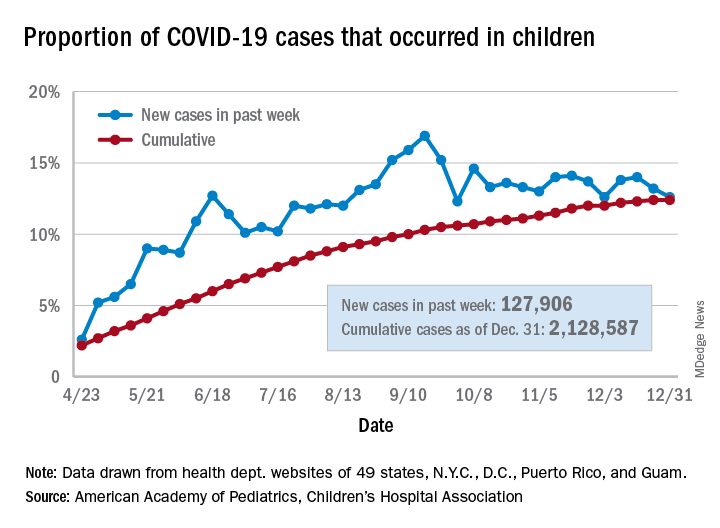
Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.
COVID-19 vaccines: The rollout, the risks, and the reason to still wear a mask
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
Is it safe to be pregnant during the COVID-19 pandemic?
Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
FDA warns about risk for false negatives from Curative COVID test
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
Social isolation at the time of social distancing
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Pandemic packed a year of distress into 1 month
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.
“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.

“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
The first month of the coronavirus pandemic created almost as much psychological distress among American adults as they had experienced in the year before February 2019, according to the results of two representative surveys.

“The 30-day prevalence of SD [serious distress] in May 2020 did not differ from the past-year prevalence of SD assessed with the same instrument [the Kessler-6 distress scale] in February 2019. In other words, equal numbers of people experienced SD in 30-days during the pandemic as experienced SD over an entire year prior to the pandemic,” Joshua Breslau, PhD, and associates at the Rand Corporation wrote in Preventive Medicine.
In May of 2020, the prevalence of SD was 10.1% in the previous month among 1,870 adults aged 20 years and older who had participated in the two Rand American Life Panel surveys, the first occurring in February 2019. In that earlier poll, 10.9% of the 2,555 respondents said that they experienced SD in the worst month of the previous year, the investigators said.
The prevalence of overall psychological distress increased by 12.8% from February 2019 to May 2020, with increases higher among women (17.7%) than men (10.6%); adults under age 60 years, compared with those over 60 (see graph); and Hispanics, compared with other races/ethnicities. Disparities also were seen among income groups: Distress rose 10.2% for those earning over $100,000, compared with 15.4% for those making less than $35,000 and 18.2% for Americans earning between $35,000 and $60,000, the researchers reported.
A high level of stress in the prepandemic survey strongly predicted serious distress during the pandemic. “Risk for SD during the pandemic among those with SD during a year before the pandemic was almost 3 times higher than among those reporting mild/moderate distress and 15 times higher than among those reporting no/low distress during the prepandemic year,” they noted.
Distress levels often return to normal after a disaster, Dr. Breslau and associates pointed out, but “the pandemic’s influence on economic stressors, disruption of usual activities and subsequent effects on population health may continue for an extended period and affect different regions of the country at different points in time.”
SOURCE: Breslau J et al. Prev Med. 2020 Dec 31. doi: 10.1016/j.ypmed.2020.106362.
FROM PREVENTIVE MEDICINE
Microvascular injury of brain, olfactory bulb seen in COVID-19
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
Experts debate wisdom of delaying second COVID-19 vaccine dose
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New evidence shows that COVID-19 invades the brain
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
U.S. hits 20 million cases as COVID variant spreads
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.