User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Patients fend for themselves to access highly touted COVID antibody treatments
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Think twice before intensifying BP regimen in older hospitalized patients
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
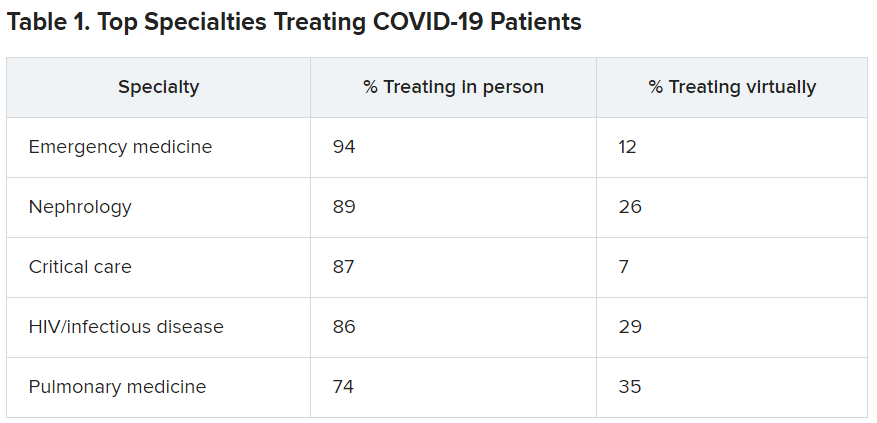
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
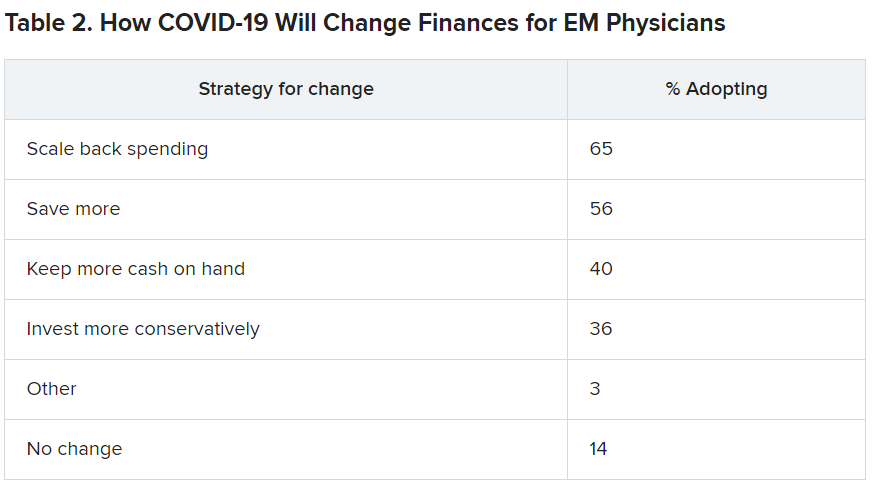
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
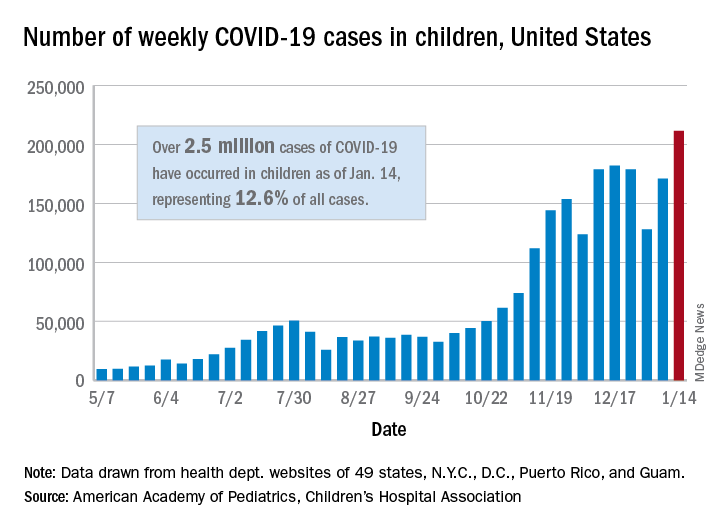
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Biomarker HF risk score envisioned as SGLT2 inhibitor lodestar in diabetes
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
HHS will drop buprenorphine waiver rule for most physicians
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Covert stroke after noncardiac surgery linked with cognitive decline
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Finding meaning in ‘Lean’?
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5
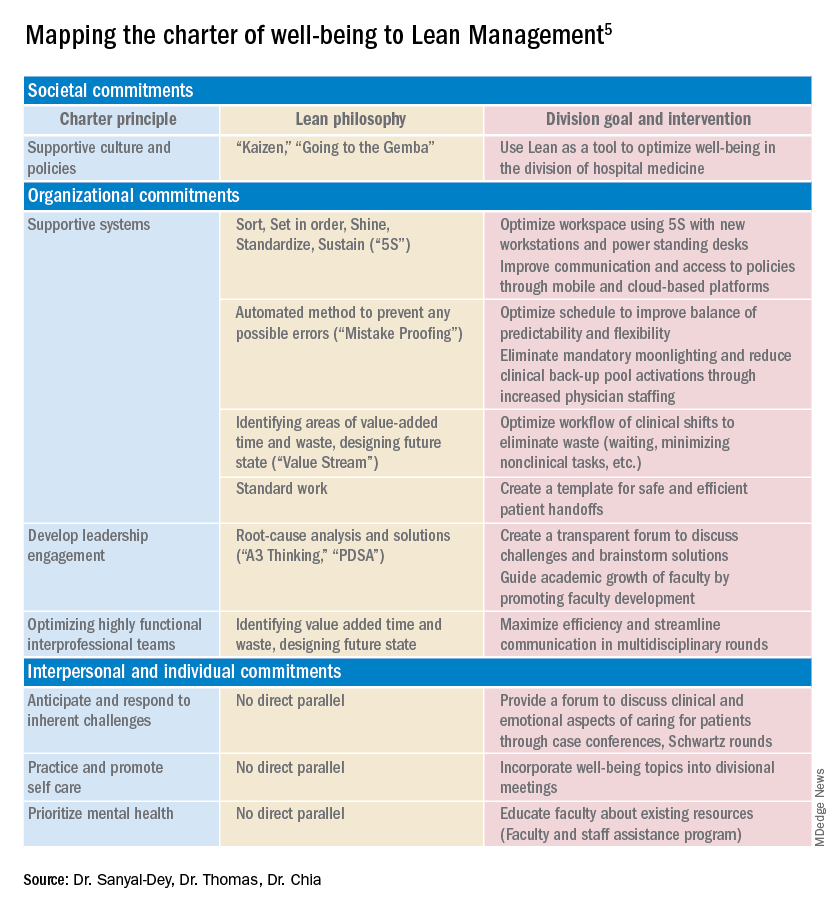
As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
Using systems improvement strategies to support the Quadruple Aim
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.








