User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Cardiogenic shock rate soars in COVID-positive ACS
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
FROM TCT 2020
Brazil confirms death of volunteer in COVID-19 vaccine trial
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
SHM announces 2021 virtual annual conference: SHM Converge
The Society of Hospital Medicine has announced its virtual annual conference for 2021: SHM Converge. Formerly known as Hospital Medicine 2021, SHM Converge will take place virtually from May 3-7, 2021, and will offer a fully digital experience with the same education, professional development, and networking hospitalists have come to expect from SHM’s annual conference.
“This year, COVID-19 has challenged us to embrace change and to innovate to better serve our hospital medicine community,” said Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. “In that spirit, not only are we introducing an exciting new brand for the SHM annual conference, we are unveiling a reimagined experience for attendees, complete with sessions highlighting the latest research, best practices and innovations in the field.”
The SHM Converge schedule features 20 educational tracks, including the addition of four new tracks to support hospital medicine professionals in some of the most relevant topics affecting health care: diagnostic safety; diversity, equity, and inclusion; leadership; and wellness and resilience
Attendees will also have the option to follow many of the most popular tracks from previous SHM annual conferences, including Rapid Fire, Clinical Updates, and High-Value Care, among others. In many sessions, speakers will present the latest data and information available about COVID-19’s impact on the practice of hospital medicine. Precourses will be held on May 3.
SHM Converge will also offer additional professional development opportunities, including the Research, Innovations, and Clinical Vignettes scientific abstract competition and a speed mentoring session. Networking will be an integral component of SHM Converge. Attendees will be able to choose from more than 20 Special Interest forums, live Q&A sessions and networking events through the interactive conference platform.
“While SHM Converge may look a bit different than the SHM annual conference we are accustomed to, I am confident the content will be among the best we have ever offered, spanning a broad range of clinical topics and issues affecting hospitalists and their patients,” said Daniel Steinberg, MD, SFHM, course director for SHM Converge. “This virtual experience will unite hospitalists from around the globe and connect them with renowned faculty members and thought leaders in hospital medicine – as well as with their hospitalist colleagues they look forward to reconnecting with each year.”
Keynote speaker announcements are forthcoming.
Registration for SHM Converge opens in November 2020. Learn more at shmconverge.org.
Members of the media can obtain press passes beginning in November 2020 by contacting [email protected].
The Society of Hospital Medicine has announced its virtual annual conference for 2021: SHM Converge. Formerly known as Hospital Medicine 2021, SHM Converge will take place virtually from May 3-7, 2021, and will offer a fully digital experience with the same education, professional development, and networking hospitalists have come to expect from SHM’s annual conference.
“This year, COVID-19 has challenged us to embrace change and to innovate to better serve our hospital medicine community,” said Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. “In that spirit, not only are we introducing an exciting new brand for the SHM annual conference, we are unveiling a reimagined experience for attendees, complete with sessions highlighting the latest research, best practices and innovations in the field.”
The SHM Converge schedule features 20 educational tracks, including the addition of four new tracks to support hospital medicine professionals in some of the most relevant topics affecting health care: diagnostic safety; diversity, equity, and inclusion; leadership; and wellness and resilience
Attendees will also have the option to follow many of the most popular tracks from previous SHM annual conferences, including Rapid Fire, Clinical Updates, and High-Value Care, among others. In many sessions, speakers will present the latest data and information available about COVID-19’s impact on the practice of hospital medicine. Precourses will be held on May 3.
SHM Converge will also offer additional professional development opportunities, including the Research, Innovations, and Clinical Vignettes scientific abstract competition and a speed mentoring session. Networking will be an integral component of SHM Converge. Attendees will be able to choose from more than 20 Special Interest forums, live Q&A sessions and networking events through the interactive conference platform.
“While SHM Converge may look a bit different than the SHM annual conference we are accustomed to, I am confident the content will be among the best we have ever offered, spanning a broad range of clinical topics and issues affecting hospitalists and their patients,” said Daniel Steinberg, MD, SFHM, course director for SHM Converge. “This virtual experience will unite hospitalists from around the globe and connect them with renowned faculty members and thought leaders in hospital medicine – as well as with their hospitalist colleagues they look forward to reconnecting with each year.”
Keynote speaker announcements are forthcoming.
Registration for SHM Converge opens in November 2020. Learn more at shmconverge.org.
Members of the media can obtain press passes beginning in November 2020 by contacting [email protected].
The Society of Hospital Medicine has announced its virtual annual conference for 2021: SHM Converge. Formerly known as Hospital Medicine 2021, SHM Converge will take place virtually from May 3-7, 2021, and will offer a fully digital experience with the same education, professional development, and networking hospitalists have come to expect from SHM’s annual conference.
“This year, COVID-19 has challenged us to embrace change and to innovate to better serve our hospital medicine community,” said Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. “In that spirit, not only are we introducing an exciting new brand for the SHM annual conference, we are unveiling a reimagined experience for attendees, complete with sessions highlighting the latest research, best practices and innovations in the field.”
The SHM Converge schedule features 20 educational tracks, including the addition of four new tracks to support hospital medicine professionals in some of the most relevant topics affecting health care: diagnostic safety; diversity, equity, and inclusion; leadership; and wellness and resilience
Attendees will also have the option to follow many of the most popular tracks from previous SHM annual conferences, including Rapid Fire, Clinical Updates, and High-Value Care, among others. In many sessions, speakers will present the latest data and information available about COVID-19’s impact on the practice of hospital medicine. Precourses will be held on May 3.
SHM Converge will also offer additional professional development opportunities, including the Research, Innovations, and Clinical Vignettes scientific abstract competition and a speed mentoring session. Networking will be an integral component of SHM Converge. Attendees will be able to choose from more than 20 Special Interest forums, live Q&A sessions and networking events through the interactive conference platform.
“While SHM Converge may look a bit different than the SHM annual conference we are accustomed to, I am confident the content will be among the best we have ever offered, spanning a broad range of clinical topics and issues affecting hospitalists and their patients,” said Daniel Steinberg, MD, SFHM, course director for SHM Converge. “This virtual experience will unite hospitalists from around the globe and connect them with renowned faculty members and thought leaders in hospital medicine – as well as with their hospitalist colleagues they look forward to reconnecting with each year.”
Keynote speaker announcements are forthcoming.
Registration for SHM Converge opens in November 2020. Learn more at shmconverge.org.
Members of the media can obtain press passes beginning in November 2020 by contacting [email protected].
Survey: Acceptance of COVID-19 vaccine dips below 50%
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
When the only clinical choices are ‘lose-lose’
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Latest week brings 44,000 more children with COVID-19
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
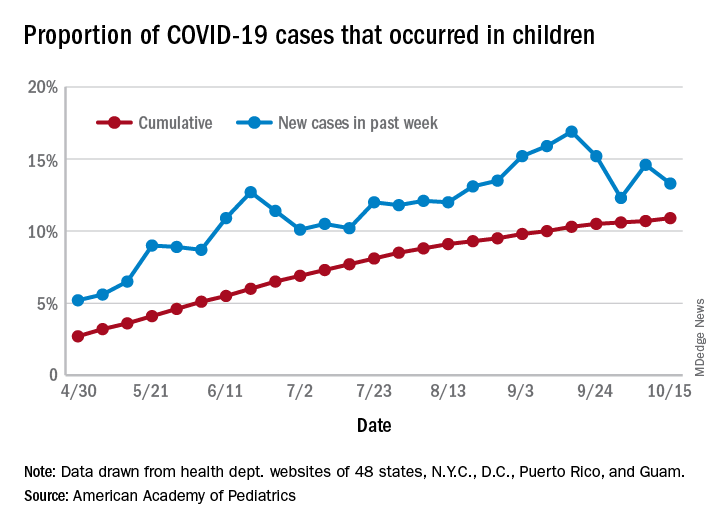
The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
Increasing racial diversity in hospital medicine’s leadership ranks
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
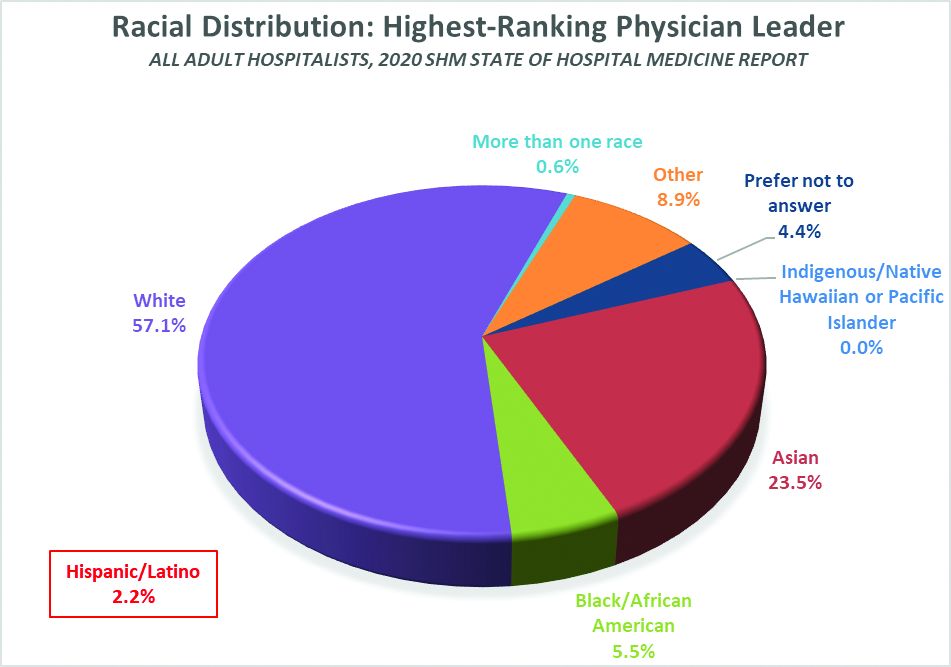
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.
Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.

Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Have you ever done something where you’re not quite sure why you did it at the time, but later on you realize it was part of some larger cosmic purpose, and you go, “Ahhh, now I understand…that’s why!”? Call it a fortuitous coincidence. Or a subconscious act of anticipation. Maybe a little push from God.
Last summer, as SHM’s Practice Analysis Committee was planning the State of Hospital Medicine survey for 2020, we received a request from SHM’s Diversity, Equity & Inclusion (DEI) Special Interest Group (SIG) to include a series of questions related to hospitalist gender, race and ethnic distribution in the new survey. We’ve generally resisted doing things like this because the SoHM is designed to capture data at the group level, not the individual level – and honestly, it’s as much as a lot of groups can do to tell us reliably how many FTEs they have, much less provide details about individual providers. In addition, the survey is already really long, and we are always looking for ways to make it shorter and easier for participants while still collecting the information report users care most about.
But we wanted to take the asks from the DEI SIG seriously, and as we considered their request, we realized that though it wasn’t practical to collect this information for individual hospital medicine group (HMG) members, we could collect it for group leaders. Little did we know last summer that issues of gender and racial diversity and equity would be so front-and-center right now, as we prepare to release the 2020 SoHM Report in early September. Ahhh, now I understand…that’s why – with the prompting of the DEI SIG – we so fortuitously chose to include those questions this year!
Here’s a sneak preview of what we learned. Among SoHM respondents, 57.1% reported that the highest-ranking leader in their HMG is White, and 23.5% of highest-ranking leaders are Asian. Only 5.5% of HMG leaders were Black/African American. Ethnicity was a separate question, and only 2.2% of HMG leaders were reported as Hispanic/Latino.
I have been profoundly moved by the wretched deaths of George Floyd and other people of color at the hands of police in recent months, and by the subsequent protests and our growing national reckoning over issues of racial equity and justice. In my efforts to understand more about race in America, I have been challenged by my friend Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., and others to go beyond just learning about these issues. I want to use my voice to advocate for change, and my actions to participate in effecting change, within the context of my sphere of influence.
So, what does that have to do with the SoHM data on HMG leader demographics? Well, it’s clear that Black and brown people are woefully underrepresented in the ranks of hospital medicine leadership.

Unfortunately, we don’t have good information on racial diversity for hospitalists as a specialty, though I understand that SHM is working on plans to update membership profiles to begin collecting this information. In searching the Internet, I found a 2018 paper from the Journal of Health Care for the Poor and Underserved that studied racial and ethnic distribution of U.S. primary care physicians (doi: 10.1353/hpu.2018.0036). It reported that, in 2012, 7.8% of general internists were Black, along with 5.8% of family medicine/general practice physicians and 6.8% of pediatricians. A separate data set issued by the Association of American Medical Colleges reported that, in 2019, 6.4% of all actively practicing general internal medicine doctors were Black (5.5% of male IM physicians and 7.9% of female IM physicians). While this doesn’t mean hospitalists have the same racial and ethnic distribution, this is probably the best proxy we can come up with.
At first glance, having 5.5% of HMG leaders who are Black doesn’t seem terribly out of line with the reported range of 6.4 to 7.8% in the general population of internal medicine physicians (apologies to the family medicine and pediatric hospitalists reading this, but I’ll confine my discussion to internists for ease and brevity, since they represent the vast majority of the nation’s hospitalists). But do the math. It means Black hospitalists are likely underrepresented in HMG leadership ranks by something like 14% to 29% compared to their likely presence among hospitalists in general.
The real problem, of course, is that according the U.S. Census Bureau, 13.4% of the U.S. population is Black. So even if the racial distribution of HMG leaders catches up to the general hospitalist population, hospital medicine is still woefully underrepresenting the racial and ethnic distribution of our patient population.
The disconnect between the ethnic distribution of HMG leaders vs. hospitalists (based on general internal medicine distribution) is even more pronounced for Latinos. The JHCPU paper reported that, in 2012, 5.6% of general internists were Hispanic. The AAMC data set reported 5.8% of IM doctors were Hispanic/Latino. But only 2.2% of SoHM respondent HMGs reported a Hispanic/Latino leader, which means Latinos are underrepresented by somewhere around 61% or so relative to the likely hospitalist population, and by a whole lot more considering the fact that Latinos make up about 18.5% of the U.S. population.
I’m not saying that a White or Asian doctor can’t provide skilled, compassionate care to a Black or Latino patient, or vice-versa. It happens every day. I guess what I am saying is that we as a country and in the medical profession need to do a better job of creating pathways and promoting careers in medicine for people of color. A JAMA paper from 2019 reported that while the numbers and proportions of minority medical school matriculants has slowly been increasing from 2002 to 2017, the rate of increase was “slower than their age-matched counterparts in the U.S. population, resulting in increased underrepresentation” (doi:10.1001/jamanetworkopen.2019.10490). This means we’re falling behind, not catching up.
We need to make sure that people like Dr. Ryan Brown aren’t discouraged from pursuing medicine by teachers or school counselors because of their skin color or accent, or their gender or sexual orientation. And among those who become doctors, we need to promote hospital medicine as a desirable specialty for people of color and actively invite them in.
In my view, much of this starts with creating more and better paths to leadership within hospital medicine for people of color. Hospital medicine group leaders wield enormous – and increasing – influence, not only within their HMGs and within SHM, but within their institutions and health care systems. We need their voices and their influence to promote diversity within their groups, their institutions, within hospital medicine, and within medicine and the U.S. health care system more broadly.
The Society of Hospital Medicine is already taking steps to promote diversity, equity and inclusion. These include issuing a formal Diversity and Inclusion Statement, creating the DEI SIG, and the recent formation of a Board-designated DEI task force charged with making recommendations to promote DEI within SHM and in hospital medicine more broadly. But I want to challenge SHM to do more, particularly with regard to promoting diversity in leadership. Here are a few ideas to consider:
- Create and sponsor a mentoring program in which hospitalists volunteer to mentor minority junior high and high school students and help them prepare to pursue a career in medicine.
- Develop a formal, structured advocacy or collaboration effort with organizations like AAMC and the Accreditation Council for Graduate Medical Education designed to promote meaningful increases in the proportion of medical school students and residents who are people of color, and in the proportion who choose primary care – and ultimately, hospital medicine.
- Work hard to collect reliable racial, ethnic and gender information about SHM members and consider collaborating with MGMA to incorporate demographic questions into its survey tool for individual hospitalist compensation and productivity data. Challenge us on the Practice Analysis Committee who are responsible for the SoHM survey to continue surveying leadership demographics, and to consider how we can expand our collection of DEI information in 2022.
- Undertake a public relations campaign to highlight to health systems and other employers the under-representation of Black and Latino hospitalists in leadership positions, and to promote conscious efforts to increase those ranks.
- Create scholarships for hospitalists from underrepresented racial and ethnic groups to attend SHM-sponsored leadership development programs such as Leadership Academy, Academic Hospitalist Academy, and Quality and Safety Educators Academy, with the goal of increasing their ranks in positions of influence throughout healthcare. A scholarship program might even include raising funds to help minority hospitalists pursue Master’s-level programs such as an MBA, MHA, or MMM.
- Develop an educational track, mentoring program, or other support initiative for early-career hospitalist leaders and those interested in developing leadership skills, and ensure it gives specific attention to strategies for increasing the proportion of hospitalists of color in leadership positions.
- Review and revise existing SHM documents such as The Key Principles and Characteristics of an Effective Hospital Medicine Group, the Core Competencies in Hospital Medicine, and various white papers and position statements to ensure they address diversity, equity and inclusion – both with regard to the hospital medicine workforce and leadership, and with regard to patient care and eliminating health disparities.
I’m sure there are plenty of other similar actions we can take that I haven’t thought of. But we need to start the conversation about concrete steps our Society, and the medical specialty we represent, can take to foster real change. And then, we need to follow our words up with actions.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Conference Committees and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
COVID-19 antibody response not reduced with diabetes
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE
Link between vitamin D and ICU outcomes unclear
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020