User login
-
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
COVID fatigue is setting in
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
A surge in PTSD may be the ‘new normal’
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
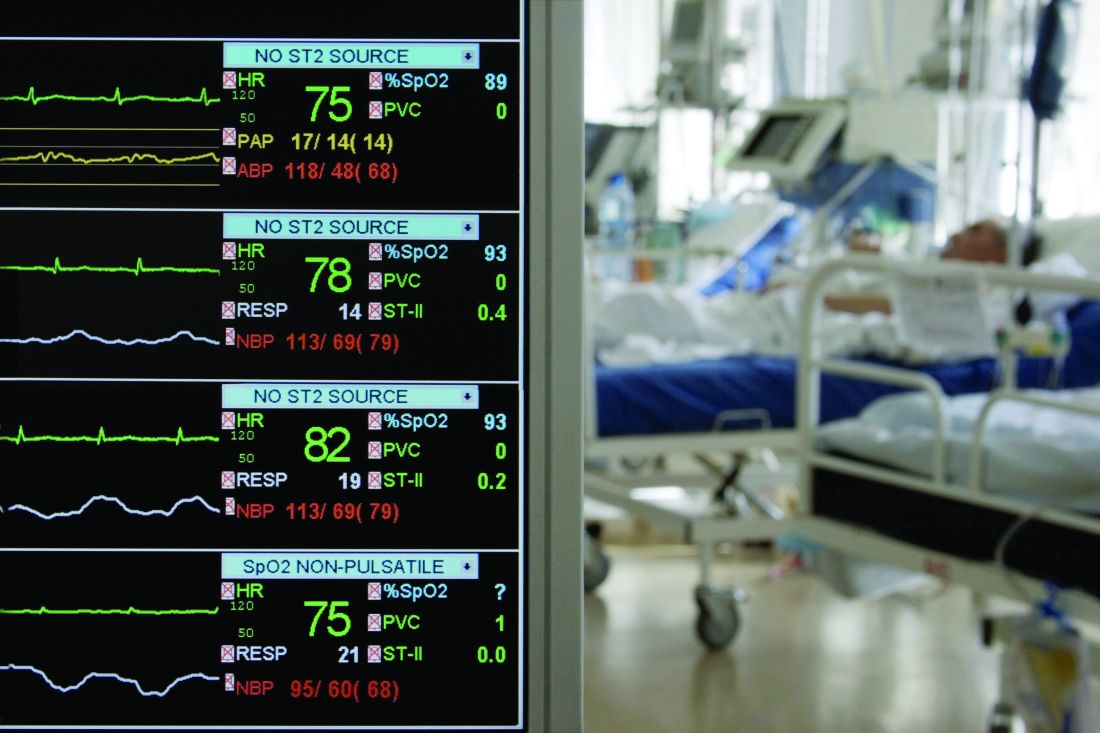
Modify risk factors to manage ICU delirium in patients with COVID-19
, and a bedside risk management strategy based on modifiable risk factors can help prevent lingering effects on cognition, according to an article published in Critical Care.
Several factors can contribute to an increased risk of ICU delirium in COVID-19 patients, wrote Katarzyna Kotfis, MD, of Pomeranian Medical University, Szczecin, Poland, and colleagues.
“In patients with COVID-19, delirium may be a manifestation of direct central nervous system invasion, induction of CNS inflammatory mediators, a secondary effect of other organ system failure, an effect of sedative strategies, prolonged mechanical ventilation time, or environmental factors, including social isolation,” they said.
Delirium in the context of COVID-19 can mean an early sign of infection, so patients should be screened using dedicated psychometric tools, the researchers wrote. Also, COVID-19 has been shown to cause pneumonia in elderly patients, who are at high risk for severe pulmonary disease related to COVID-19 and for ICU delirium generally, they said.
In addition, don’t underestimate the impact of social isolation created by quarantines, the researchers said.
“What is needed now, is not only high-quality ICU care, concentrated on providing adequate respiratory support to critically ill patients, but an identification of the source and degree of mental and spiritual suffering of patients as well as their families to provide the most ethical and person-centered care during this humanitarian crisis,” they emphasized. However, they acknowledged that nonpharmacologic interventions such as mobility outside the ICU room and interactions with family members are limited by the COVID-19 situation.
The researchers noted several mechanisms by which the COVID-19 virus may cause brain damage, including through the dysfunction of the renin-angiotensin system.
“Inflammatory response of the CNS to viral infection seems to be another important reason for poor neurological outcome and occurrence of delirium,” in COVID-19 patients, they said.
As for risk-reduction strategies, the researchers noted that “delirium in mechanically ventilated patients can be reduced dramatically to 50% using a culture of lighter sedation and mobilization via the implementation of the safety bundle called the ABCDEFs promoted by the Society of Critical Care Medicine in their ICU Liberation Collaborative,” although COVID-19 isolation is a barrier, they said.
The ABCDEF bundle consists of Assessment of pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of sedation, Delirium (hyperactive or hypoactive), Early mobility, and Family presence; all of which are challenging in the COVID-19 environment, the researchers said.
They advised implementing easy screening methods for delirium to reduce the burden on medical staff, and emphasized the importance of regular patient orientation, despite social separation from family and caregivers.
“No drugs can be recommended for the prevention or treatment of ICU delirium other than avoidance of overuse of potent psychoactive agents like sedatives and neuromuscular blockers (NMB) unless patients absolutely require such management,” they added.
“Delirium is so common and so hard to manage in the COVID-19 population,” Mangala Narasimhan, DO, of Northwell Health in New Hyde Park, N.Y., said in an interview. Delirium is impacted by many sources including a viral encephalopathy, the amount and duration of sedation medications, and prolonged intubation and hypoxemia, she said. “Managing the delirium allows you to wake the patient up successfully and without a lot of discoordination. This will help with weaning,” she noted. Barriers to delirium management for COVID-19 patients include the length of time on a ventilator, as well as amount of sedatives and paralytics, and the added issues of renal insufficiency, she noted. “How they can be addressed is thoughtful plans on the addition of long-term sedation for withdrawal symptoms, and anxiolytics for the profound anxiety associated with arousal from this type of sedation on ventilators, she said. The take-home message for clinicians is the need to perform weaning trials to manage delirium in the ICU. “We have to combat this delirium in order to be successful in taking these patients off of ventilators,” she said. Dr. Narasimhan added that more research is needed on areas including drug-to-drug interactions, duration of efficacy of various drugs, and how the virus affects the brain.
“Adherence to the ABCDEF bundle can reduce the incidence of delirium, from approximately 75% of mechanically ventilated patients to 50% or less,” David L. Bowton, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., said in an interview.
“Importantly, in most studies, bundle adherence reduces mortality and ICU length of stay and lowers the total cost of care. However, isolation of patients and protection of staff, visitor restrictions, and potentially stressed staffing will likely alter how most institutions approach bundle compliance,” he said. “Gathering input from infection control clinicians and bedside providers from multiple disciplines that consider these factors to critically examine current bundle procedures and workflow will be essential to the creation and/or revision of bundle processes of care that maintain the integrity of the ABCDEF bundle yet preserve staff, patient, and family safety,” he said.
“We did not have strong evidence to suggest an optimal approach to treating delirium before the advent of the COVID-19 pandemic, so I do not believe we know what the best approach is in the current environment,” Dr. Bowton added. “Further, vigilance will be necessary to ensure that altered consciousness or cognition is ICU delirium and not attributable to another cause such as drug withdrawal, drug adverse effect, or primary central nervous system infection or immune response that mandates specific therapy,” he emphasized.
For clinicians, “this study reminds us of the importance of the ABCDEF bundle to improve outcomes of critical illness,” said Dr. Bowton. “It highlights the difficulties of providing frequent reassessment of pain, comfort, reassurance, and reorientation to critically ill patients. To me, it underscores the importance of each institution critically examining staffing needs and staffing roles to mitigate these difficulties and to explore novel methods of maintaining staff-patient and family-patient interactions to enhance compliance with all elements of the ABCDEF bundle while maintaining the safety of staff and families.”
Dr. Bowton added, “When necessary, explicit modifications to existing ABCDEF bundles should be developed and disseminated to provide realistic, readily understood guidance to achieve the best possible compliance with each bundle element. One potentially underrecognized issue will be the large, hopefully temporary, number of people requiring post–critical illness rehabilitation and mental health services,” he said. “In many regions these services are already underfunded and ill-equipped to handle an increased demand for these services,” he noted.
Additional research is needed in many areas, said Dr. Bowton. “While compliance with the ABCDEF bundle decreases the incidence and duration of delirium, decreases ICU length of stay, decreases duration of mechanical ventilation, and improves mortality, many questions remain. Individual elements of the bundle have been inconsistently associated with improved outcomes,” he said. “What is the relative importance of specific elements and what are the mechanisms by which they improve outcomes?” he asked. “We still do not know how to best achieve physical/functional recovery following critical illness, which, in light of these authors’ studies relating persisting physical debility to depression (Lancet Respir Med. 2014; 2[5]:369-79), may be a key component to improving long-term outcomes,” he said.
The study received no specific funding, although several coauthors disclosed grants from agencies including the National Center for Advancing Translational Sciences, National Institute of General Medical Sciences, National Heart, Lung, and Blood Institute, and National Institute on Aging. Dr. Narasimhan and Dr. Bowton had no financial conflicts to disclose.
SOURCE: Kotfis K et al. Critical Care. 2020 Apr 28. doi: 10.1186/s13054-020-02882-x.
, and a bedside risk management strategy based on modifiable risk factors can help prevent lingering effects on cognition, according to an article published in Critical Care.
Several factors can contribute to an increased risk of ICU delirium in COVID-19 patients, wrote Katarzyna Kotfis, MD, of Pomeranian Medical University, Szczecin, Poland, and colleagues.
“In patients with COVID-19, delirium may be a manifestation of direct central nervous system invasion, induction of CNS inflammatory mediators, a secondary effect of other organ system failure, an effect of sedative strategies, prolonged mechanical ventilation time, or environmental factors, including social isolation,” they said.
Delirium in the context of COVID-19 can mean an early sign of infection, so patients should be screened using dedicated psychometric tools, the researchers wrote. Also, COVID-19 has been shown to cause pneumonia in elderly patients, who are at high risk for severe pulmonary disease related to COVID-19 and for ICU delirium generally, they said.
In addition, don’t underestimate the impact of social isolation created by quarantines, the researchers said.
“What is needed now, is not only high-quality ICU care, concentrated on providing adequate respiratory support to critically ill patients, but an identification of the source and degree of mental and spiritual suffering of patients as well as their families to provide the most ethical and person-centered care during this humanitarian crisis,” they emphasized. However, they acknowledged that nonpharmacologic interventions such as mobility outside the ICU room and interactions with family members are limited by the COVID-19 situation.
The researchers noted several mechanisms by which the COVID-19 virus may cause brain damage, including through the dysfunction of the renin-angiotensin system.
“Inflammatory response of the CNS to viral infection seems to be another important reason for poor neurological outcome and occurrence of delirium,” in COVID-19 patients, they said.
As for risk-reduction strategies, the researchers noted that “delirium in mechanically ventilated patients can be reduced dramatically to 50% using a culture of lighter sedation and mobilization via the implementation of the safety bundle called the ABCDEFs promoted by the Society of Critical Care Medicine in their ICU Liberation Collaborative,” although COVID-19 isolation is a barrier, they said.
The ABCDEF bundle consists of Assessment of pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of sedation, Delirium (hyperactive or hypoactive), Early mobility, and Family presence; all of which are challenging in the COVID-19 environment, the researchers said.
They advised implementing easy screening methods for delirium to reduce the burden on medical staff, and emphasized the importance of regular patient orientation, despite social separation from family and caregivers.
“No drugs can be recommended for the prevention or treatment of ICU delirium other than avoidance of overuse of potent psychoactive agents like sedatives and neuromuscular blockers (NMB) unless patients absolutely require such management,” they added.
“Delirium is so common and so hard to manage in the COVID-19 population,” Mangala Narasimhan, DO, of Northwell Health in New Hyde Park, N.Y., said in an interview. Delirium is impacted by many sources including a viral encephalopathy, the amount and duration of sedation medications, and prolonged intubation and hypoxemia, she said. “Managing the delirium allows you to wake the patient up successfully and without a lot of discoordination. This will help with weaning,” she noted. Barriers to delirium management for COVID-19 patients include the length of time on a ventilator, as well as amount of sedatives and paralytics, and the added issues of renal insufficiency, she noted. “How they can be addressed is thoughtful plans on the addition of long-term sedation for withdrawal symptoms, and anxiolytics for the profound anxiety associated with arousal from this type of sedation on ventilators, she said. The take-home message for clinicians is the need to perform weaning trials to manage delirium in the ICU. “We have to combat this delirium in order to be successful in taking these patients off of ventilators,” she said. Dr. Narasimhan added that more research is needed on areas including drug-to-drug interactions, duration of efficacy of various drugs, and how the virus affects the brain.
“Adherence to the ABCDEF bundle can reduce the incidence of delirium, from approximately 75% of mechanically ventilated patients to 50% or less,” David L. Bowton, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., said in an interview.
“Importantly, in most studies, bundle adherence reduces mortality and ICU length of stay and lowers the total cost of care. However, isolation of patients and protection of staff, visitor restrictions, and potentially stressed staffing will likely alter how most institutions approach bundle compliance,” he said. “Gathering input from infection control clinicians and bedside providers from multiple disciplines that consider these factors to critically examine current bundle procedures and workflow will be essential to the creation and/or revision of bundle processes of care that maintain the integrity of the ABCDEF bundle yet preserve staff, patient, and family safety,” he said.
“We did not have strong evidence to suggest an optimal approach to treating delirium before the advent of the COVID-19 pandemic, so I do not believe we know what the best approach is in the current environment,” Dr. Bowton added. “Further, vigilance will be necessary to ensure that altered consciousness or cognition is ICU delirium and not attributable to another cause such as drug withdrawal, drug adverse effect, or primary central nervous system infection or immune response that mandates specific therapy,” he emphasized.
For clinicians, “this study reminds us of the importance of the ABCDEF bundle to improve outcomes of critical illness,” said Dr. Bowton. “It highlights the difficulties of providing frequent reassessment of pain, comfort, reassurance, and reorientation to critically ill patients. To me, it underscores the importance of each institution critically examining staffing needs and staffing roles to mitigate these difficulties and to explore novel methods of maintaining staff-patient and family-patient interactions to enhance compliance with all elements of the ABCDEF bundle while maintaining the safety of staff and families.”
Dr. Bowton added, “When necessary, explicit modifications to existing ABCDEF bundles should be developed and disseminated to provide realistic, readily understood guidance to achieve the best possible compliance with each bundle element. One potentially underrecognized issue will be the large, hopefully temporary, number of people requiring post–critical illness rehabilitation and mental health services,” he said. “In many regions these services are already underfunded and ill-equipped to handle an increased demand for these services,” he noted.
Additional research is needed in many areas, said Dr. Bowton. “While compliance with the ABCDEF bundle decreases the incidence and duration of delirium, decreases ICU length of stay, decreases duration of mechanical ventilation, and improves mortality, many questions remain. Individual elements of the bundle have been inconsistently associated with improved outcomes,” he said. “What is the relative importance of specific elements and what are the mechanisms by which they improve outcomes?” he asked. “We still do not know how to best achieve physical/functional recovery following critical illness, which, in light of these authors’ studies relating persisting physical debility to depression (Lancet Respir Med. 2014; 2[5]:369-79), may be a key component to improving long-term outcomes,” he said.
The study received no specific funding, although several coauthors disclosed grants from agencies including the National Center for Advancing Translational Sciences, National Institute of General Medical Sciences, National Heart, Lung, and Blood Institute, and National Institute on Aging. Dr. Narasimhan and Dr. Bowton had no financial conflicts to disclose.
SOURCE: Kotfis K et al. Critical Care. 2020 Apr 28. doi: 10.1186/s13054-020-02882-x.
, and a bedside risk management strategy based on modifiable risk factors can help prevent lingering effects on cognition, according to an article published in Critical Care.
Several factors can contribute to an increased risk of ICU delirium in COVID-19 patients, wrote Katarzyna Kotfis, MD, of Pomeranian Medical University, Szczecin, Poland, and colleagues.
“In patients with COVID-19, delirium may be a manifestation of direct central nervous system invasion, induction of CNS inflammatory mediators, a secondary effect of other organ system failure, an effect of sedative strategies, prolonged mechanical ventilation time, or environmental factors, including social isolation,” they said.
Delirium in the context of COVID-19 can mean an early sign of infection, so patients should be screened using dedicated psychometric tools, the researchers wrote. Also, COVID-19 has been shown to cause pneumonia in elderly patients, who are at high risk for severe pulmonary disease related to COVID-19 and for ICU delirium generally, they said.
In addition, don’t underestimate the impact of social isolation created by quarantines, the researchers said.
“What is needed now, is not only high-quality ICU care, concentrated on providing adequate respiratory support to critically ill patients, but an identification of the source and degree of mental and spiritual suffering of patients as well as their families to provide the most ethical and person-centered care during this humanitarian crisis,” they emphasized. However, they acknowledged that nonpharmacologic interventions such as mobility outside the ICU room and interactions with family members are limited by the COVID-19 situation.
The researchers noted several mechanisms by which the COVID-19 virus may cause brain damage, including through the dysfunction of the renin-angiotensin system.
“Inflammatory response of the CNS to viral infection seems to be another important reason for poor neurological outcome and occurrence of delirium,” in COVID-19 patients, they said.
As for risk-reduction strategies, the researchers noted that “delirium in mechanically ventilated patients can be reduced dramatically to 50% using a culture of lighter sedation and mobilization via the implementation of the safety bundle called the ABCDEFs promoted by the Society of Critical Care Medicine in their ICU Liberation Collaborative,” although COVID-19 isolation is a barrier, they said.
The ABCDEF bundle consists of Assessment of pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of sedation, Delirium (hyperactive or hypoactive), Early mobility, and Family presence; all of which are challenging in the COVID-19 environment, the researchers said.
They advised implementing easy screening methods for delirium to reduce the burden on medical staff, and emphasized the importance of regular patient orientation, despite social separation from family and caregivers.
“No drugs can be recommended for the prevention or treatment of ICU delirium other than avoidance of overuse of potent psychoactive agents like sedatives and neuromuscular blockers (NMB) unless patients absolutely require such management,” they added.
“Delirium is so common and so hard to manage in the COVID-19 population,” Mangala Narasimhan, DO, of Northwell Health in New Hyde Park, N.Y., said in an interview. Delirium is impacted by many sources including a viral encephalopathy, the amount and duration of sedation medications, and prolonged intubation and hypoxemia, she said. “Managing the delirium allows you to wake the patient up successfully and without a lot of discoordination. This will help with weaning,” she noted. Barriers to delirium management for COVID-19 patients include the length of time on a ventilator, as well as amount of sedatives and paralytics, and the added issues of renal insufficiency, she noted. “How they can be addressed is thoughtful plans on the addition of long-term sedation for withdrawal symptoms, and anxiolytics for the profound anxiety associated with arousal from this type of sedation on ventilators, she said. The take-home message for clinicians is the need to perform weaning trials to manage delirium in the ICU. “We have to combat this delirium in order to be successful in taking these patients off of ventilators,” she said. Dr. Narasimhan added that more research is needed on areas including drug-to-drug interactions, duration of efficacy of various drugs, and how the virus affects the brain.
“Adherence to the ABCDEF bundle can reduce the incidence of delirium, from approximately 75% of mechanically ventilated patients to 50% or less,” David L. Bowton, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., said in an interview.
“Importantly, in most studies, bundle adherence reduces mortality and ICU length of stay and lowers the total cost of care. However, isolation of patients and protection of staff, visitor restrictions, and potentially stressed staffing will likely alter how most institutions approach bundle compliance,” he said. “Gathering input from infection control clinicians and bedside providers from multiple disciplines that consider these factors to critically examine current bundle procedures and workflow will be essential to the creation and/or revision of bundle processes of care that maintain the integrity of the ABCDEF bundle yet preserve staff, patient, and family safety,” he said.
“We did not have strong evidence to suggest an optimal approach to treating delirium before the advent of the COVID-19 pandemic, so I do not believe we know what the best approach is in the current environment,” Dr. Bowton added. “Further, vigilance will be necessary to ensure that altered consciousness or cognition is ICU delirium and not attributable to another cause such as drug withdrawal, drug adverse effect, or primary central nervous system infection or immune response that mandates specific therapy,” he emphasized.
For clinicians, “this study reminds us of the importance of the ABCDEF bundle to improve outcomes of critical illness,” said Dr. Bowton. “It highlights the difficulties of providing frequent reassessment of pain, comfort, reassurance, and reorientation to critically ill patients. To me, it underscores the importance of each institution critically examining staffing needs and staffing roles to mitigate these difficulties and to explore novel methods of maintaining staff-patient and family-patient interactions to enhance compliance with all elements of the ABCDEF bundle while maintaining the safety of staff and families.”
Dr. Bowton added, “When necessary, explicit modifications to existing ABCDEF bundles should be developed and disseminated to provide realistic, readily understood guidance to achieve the best possible compliance with each bundle element. One potentially underrecognized issue will be the large, hopefully temporary, number of people requiring post–critical illness rehabilitation and mental health services,” he said. “In many regions these services are already underfunded and ill-equipped to handle an increased demand for these services,” he noted.
Additional research is needed in many areas, said Dr. Bowton. “While compliance with the ABCDEF bundle decreases the incidence and duration of delirium, decreases ICU length of stay, decreases duration of mechanical ventilation, and improves mortality, many questions remain. Individual elements of the bundle have been inconsistently associated with improved outcomes,” he said. “What is the relative importance of specific elements and what are the mechanisms by which they improve outcomes?” he asked. “We still do not know how to best achieve physical/functional recovery following critical illness, which, in light of these authors’ studies relating persisting physical debility to depression (Lancet Respir Med. 2014; 2[5]:369-79), may be a key component to improving long-term outcomes,” he said.
The study received no specific funding, although several coauthors disclosed grants from agencies including the National Center for Advancing Translational Sciences, National Institute of General Medical Sciences, National Heart, Lung, and Blood Institute, and National Institute on Aging. Dr. Narasimhan and Dr. Bowton had no financial conflicts to disclose.
SOURCE: Kotfis K et al. Critical Care. 2020 Apr 28. doi: 10.1186/s13054-020-02882-x.
FROM CRITICAL CARE
U.S. is poised to produce a COVID-19 vaccine, but don’t expect it soon
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
Manufacturers will begin producing COVID-19 vaccine doses in anticipation of approval so that if a product gets the okay for usage, distribution can begin quickly, according to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
“We will be producing vaccine at risk, which means we’ll be [investing] considerable resources in developing doses even before we know any given candidate or candidates work,” he testified during a May 12, 2020, hearing of the Senate Health, Education, Labor, and Pensions Committee.
During the hearing, Dr. Fauci did not elaborate on how the production at risk would be undertaken, what criteria would be in place for selecting which candidates would be in the pipeline, or how much would be spent on the advanced production of these vaccines.
And while Dr. Fauci, a member of the White House coronavirus task force, remained optimistic that one or more vaccine candidates would ultimately be viable, he cautioned that there remain many unknowns that could slow the development of a vaccine for COVID-19.
“I must warn that there’s also the possibility of negative consequences that certain vaccines can actually enhance the negative effect of the infection,” he said. “The big unknown is efficacy. Will it be present or absent and how durable will it be?”
It’s unlikely that either a vaccine or an effective treatment will be available in the next 3 months, Dr. Fauci told the committee.
Sen. Lamar Alexander (R-Tenn.), the committee chairman, asked Dr. Fauci what he would say to college, primary, and secondary school administrators about how the availability of treatments and vaccines could influence the ability to reopen campuses to students. Dr. Fauci replied that the idea of having treatments or a vaccine available to facilitate the reentry of students in the fall term would be “a bit of a bridge too far.”
The emphasis in the coming months should be on testing, contact tracing, and isolation of those infected with the virus, Dr. Fauci said.
States vary in vulnerability to COVID-19 impact
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
West Virginia’s large elderly population and high rates of chronic kidney disease, cardiovascular disease, diabetes, and COPD make it the most vulnerable state to the coronavirus, according to a new analysis.

Vulnerability to the virus “isn’t just health related, though, as many people are harmed by the economic effects of the pandemic,” personal finance website WalletHub said May 12.
“It’s important for the U.S. to dedicate a large portion of its resources to providing medical support during the coronavirus pandemic, but we should also support people who don’t have adequate housing or enough money to survive the pandemic,” said WalletHub analyst Jill Gonzalez.
WalletHub graded each state on 28 measures – including share of obese adults, share of homes lacking access to basic hygienic facilities, and biggest increases in unemployment because of COVID-19 – grouped into three dimensions of vulnerability: medical (60% of the total score), housing (15%), and financial (25%).
Using those measures, Louisiana is the most vulnerable state after West Virginia, followed by Mississippi, Arkansas, and Alabama. All 5 states finished in the top 6 for medical vulnerability, and 4 were in the top 10 for financial vulnerability, but only 1 (Arkansas) was in the top 10 for housing vulnerability, WalletHub said.
Among the three vulnerability dimensions, West Virginia was first in medical, Hawaii (33rd overall) was first in housing, and Louisiana was first in financial. Utah is the least vulnerable state, overall, and the least vulnerable states in each dimension are, respectively, Colorado (50th overall), the District of Columbia (29th overall), and Iowa (45th overall), the report showed.
A look at the individual metrics WalletHub used shows some serious disparities:
- New Jersey’s unemployment recipiency rate of 57.2%, the highest in the country, is 6.1 times higher than North Carolina’s 9.3%.
- The highest uninsured rate, 17.4% in Texas, is 6.2 times higher than in Massachusetts, which is the lowest at 2.8%.
- In California, the share of the homeless population that is unsheltered (71.7%) is more than 33 times higher than in North Dakota (2.2%).
“The financial damage caused by COVID-19 is leaving many Americans without the means to pay their bills and purchase necessities. … The U.S. must continue to support its financially vulnerable populations even after the virus has subsided,” Ms. Gonzalez said.
Bronchoscopy guideline for COVID-19 pandemic: Use sparingly
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
With little evidence available on the role of bronchoscopy during the COVID-19 pandemic, an expert panel has published a guideline recommending its spare use in COVID-19 patients and those with suspected COVID-19 infection.
The panel stated that in the context of the COVID-19 crisis, bronchoscopy and other aerosol-generating procedures put health care workers (HCWs) at particularly high risk of exposure and infection. They recommended deferring bronchoscopy in nonurgent cases, and advised practitioners to wear personal protective equipment when performing bronchoscopy, even on asymptomatic patients.
The guideline and expert panel report have been published online in the journal Chest. CHEST and the American Association for Bronchology and Interventional Pulmonology participated in selecting the 14 panelists. “The recommendation and suggestions outlined in this document were specifically created to address what were felt to be clinically common and urgent questions that frontline clinicians are likely to face,” wrote lead author and panel cochair Momen M. Wahidi, MD, MBA, of Duke University, Durham, N.C., and colleagues.
Only one of the six recommendations is based on graded evidence; the remainder are ungraded consensus-based statements. The guideline consists of the following recommendations for performing or using bronchoscopy:
- HCWs in the procedure or recovery rooms should wear either an N-95 respirator or powered air-purifying respirator (PAPR) when performing bronchoscopy on patients suspected or confirmed to have COVID-19. They should wear personal protective equipment (PPE) that includes a face shield, gown, and gloves, and they should discard N-95 respirators after performing bronchoscopy.
- A nasopharyngeal specimen in COVID-19 suspects should be obtained before performing bronchoscopy. If the patient has severe or progressive disease that requires intubation but an additional specimen is needed to confirm COVID-19 or another diagnosis that could change the treatment course, an option would be lower-respiratory specimen from the endotracheal aspirate or bronchoscopy with bronchoalveolar lavage.
- HCWs should wear an N-95 or PAPR when doing bronchoscopy on asymptomatic patients in an area with community spread of COVID-19 – again, with the PPE designated in the first recommendation.
- Test for COVID-19 before doing bronchoscopy on asymptomatic patients. Defer nonurgent bronchoscopy if the test is positive. If it’s negative, follow the recommendations regarding respirators and PPE when doing bronchoscopy.
- Perform timely bronchoscopy when indicated even in an area with known community spread of COVID-19. This is the only graded recommendation among the six (Grade 2C) and may be the most nuanced. Local teams should develop strategies for using bronchoscopy in their setting, taking into account local resources and availability of PPE, and they should send noninfected cancer patients from resource-depleted hospitals to other centers.
- Base the timing of bronchoscopy in patients recovering after COVID-19 on the indication for the procedure, disease severity, and time duration since symptoms resolved. The recommendation noted that the exact timing is still unknown, but that a wait of at least 30 days after symptoms recede is “reasonable.”
The expert panel added a noteworthy caveat to the recommendations. “We would like to stress that these protective strategies can be rendered completely ineffective if proper training on donning and doffing is not provided to HCW,” Dr. Wahidi and colleagues wrote. “Proper personnel instruction and practice for wearing PPE should receive as much attention by health facilities as the chosen strategy for protection.”
Dr. Wahidi and colleagues have no financial relationships to disclose.
SOURCE: Wahidi MM et al. CHEST. 2020 Apr 30. doi: 10.1016/j.chest.2020.04.036.
FROM CHEST
Many hydroxychloroquine COVID-19 prophylaxis trials lack ECG screening
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
REPORTING FROM JACC
COVID-19: Telehealth at the forefront of the pandemic
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
Protective levels of vitamin D achievable in SCD with oral supplementation
Sickle cell disease is associated with worse long-term bone health than that of the general population, and SCD patients are more likely to experience vitamin D [25(OH)D] deficiency. Oral vitamin D3 supplementation can achieve protective levels in children with sickle cell disease, and a daily dose was able to achieved optimal blood levels, according to a report published online in Bone.
The researchers performed a prospective, longitudinal, single-center study of 80 children with SCD. They collected demographic, clinical, and management data, as well as 25(OH)D levels. Bone densitometries (DXA) were also collected.
Among the 80 patients were included in the analysis, there were significant differences between the means of 25(OH)D levels based on whether the patient started prophylactic treatment as an infant or not (35.7 vs. 27.9 ng/mL, respectively [P = .014]), according to the researchers.
They also found that, in multivariate analysis, an oral 800 IU daily dose of vitamin D3 was shown to be a protective factor (P = .044) in reaching optimal 25(OH)D blood levels (≥ 30 ng/mL).
Kaplan-Meier analysis showed that those patients younger than 10 years of age reached optimal levels significantly earlier than older patients when on supplementation (P = .002), as did those patients who were not being treated with hydroxyurea (P = .039), the researchers wrote.
Significant differences were seen between the mean bone mineral density in both DXAs performed when comparing suboptimal vs. optimal blood levels of 25(OH)D (0.54 g/cm2 vs. 0.64 g/cm2, respectively, P = .001), for the initial DXA, and for the most recent DXA (0.59 g/cm2 vs. 0.77 g/cm2, respectively, P = .044). “VitD3 prophylaxis is a safe practice in SCD. It is important to start this prophylactic treatment when the child is an infant. The daily regimen with 800 IU could be more effective for reaching levels ≥ 30 ng/mL, and, especially in preadolescent and adolescent patients, we should raise awareness about the importance of good bone health,” the authors concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Garrido C et al. Bone. 2020;133: doi.org/10.1016/j.bone.2020.115228.
Sickle cell disease is associated with worse long-term bone health than that of the general population, and SCD patients are more likely to experience vitamin D [25(OH)D] deficiency. Oral vitamin D3 supplementation can achieve protective levels in children with sickle cell disease, and a daily dose was able to achieved optimal blood levels, according to a report published online in Bone.
The researchers performed a prospective, longitudinal, single-center study of 80 children with SCD. They collected demographic, clinical, and management data, as well as 25(OH)D levels. Bone densitometries (DXA) were also collected.
Among the 80 patients were included in the analysis, there were significant differences between the means of 25(OH)D levels based on whether the patient started prophylactic treatment as an infant or not (35.7 vs. 27.9 ng/mL, respectively [P = .014]), according to the researchers.
They also found that, in multivariate analysis, an oral 800 IU daily dose of vitamin D3 was shown to be a protective factor (P = .044) in reaching optimal 25(OH)D blood levels (≥ 30 ng/mL).
Kaplan-Meier analysis showed that those patients younger than 10 years of age reached optimal levels significantly earlier than older patients when on supplementation (P = .002), as did those patients who were not being treated with hydroxyurea (P = .039), the researchers wrote.
Significant differences were seen between the mean bone mineral density in both DXAs performed when comparing suboptimal vs. optimal blood levels of 25(OH)D (0.54 g/cm2 vs. 0.64 g/cm2, respectively, P = .001), for the initial DXA, and for the most recent DXA (0.59 g/cm2 vs. 0.77 g/cm2, respectively, P = .044). “VitD3 prophylaxis is a safe practice in SCD. It is important to start this prophylactic treatment when the child is an infant. The daily regimen with 800 IU could be more effective for reaching levels ≥ 30 ng/mL, and, especially in preadolescent and adolescent patients, we should raise awareness about the importance of good bone health,” the authors concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Garrido C et al. Bone. 2020;133: doi.org/10.1016/j.bone.2020.115228.
Sickle cell disease is associated with worse long-term bone health than that of the general population, and SCD patients are more likely to experience vitamin D [25(OH)D] deficiency. Oral vitamin D3 supplementation can achieve protective levels in children with sickle cell disease, and a daily dose was able to achieved optimal blood levels, according to a report published online in Bone.
The researchers performed a prospective, longitudinal, single-center study of 80 children with SCD. They collected demographic, clinical, and management data, as well as 25(OH)D levels. Bone densitometries (DXA) were also collected.
Among the 80 patients were included in the analysis, there were significant differences between the means of 25(OH)D levels based on whether the patient started prophylactic treatment as an infant or not (35.7 vs. 27.9 ng/mL, respectively [P = .014]), according to the researchers.
They also found that, in multivariate analysis, an oral 800 IU daily dose of vitamin D3 was shown to be a protective factor (P = .044) in reaching optimal 25(OH)D blood levels (≥ 30 ng/mL).
Kaplan-Meier analysis showed that those patients younger than 10 years of age reached optimal levels significantly earlier than older patients when on supplementation (P = .002), as did those patients who were not being treated with hydroxyurea (P = .039), the researchers wrote.
Significant differences were seen between the mean bone mineral density in both DXAs performed when comparing suboptimal vs. optimal blood levels of 25(OH)D (0.54 g/cm2 vs. 0.64 g/cm2, respectively, P = .001), for the initial DXA, and for the most recent DXA (0.59 g/cm2 vs. 0.77 g/cm2, respectively, P = .044). “VitD3 prophylaxis is a safe practice in SCD. It is important to start this prophylactic treatment when the child is an infant. The daily regimen with 800 IU could be more effective for reaching levels ≥ 30 ng/mL, and, especially in preadolescent and adolescent patients, we should raise awareness about the importance of good bone health,” the authors concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Garrido C et al. Bone. 2020;133: doi.org/10.1016/j.bone.2020.115228.
FROM BONE