User login
-
Hematologic manifestations of COVID-19
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
FROM NATURE MEDICINE
MIS-C is a serious immune-mediated response to COVID-19 infection
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
FROM PHM20 VIRTUAL
Physician recruitment drops by 30% because of pandemic
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
NFL’s only physician player opts out of 2020 season over COVID
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.
E.U. gives thumbs up for belantamab in R/R multiple myeloma
The first-in-class drug belantamab mafodotin (Blenrep, GlaxoSmithKline) has been recommended for conditional marketing approval in the European Union (EU) for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
The product was accepted into the European Medicines Agency (EMA) PRIME program for medicines that have potential to address unmet medical needs, the agency noted.
Belantamab mafodotin was also recently recommended for U.S. approval when a Food and Drug Administration advisory committee voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
Specifically, these patients with refractory or relapsed multiple myeloma should have already tried treatment with one of the three major classes of drugs, namely an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA said. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
Belantamab mafodotin has a novel mechanism of action: It targets B-cell maturation antigen (BCMA), a protein present on the surface of virtually all multiple myeloma cells, but is absent from normal B-cells, thus “making it an ideal drug target,” the agency remarked.
The product is an antibody–drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethylauristatin F (mcMMAF). It homes in on BCMA on myeloma cell surfaces, and once inside the myeloma cell, the cytotoxic agent is released leading to apoptosis, the “programmed” death of the cancerous plasma cells, the agency explained.
Results from open-label study
The recommendation for conditional marketing authorization comes from the EMA Committee for Medicinal Products for Human Use (CHMP) and was based on a phase 2, open-label, randomized, two-arm study, DREAMM-2.
The study investigated the efficacy and safety of two doses of belantamab mafodotin in patients with multiple myeloma who still had active disease after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results were published in December in The Lancet Oncology. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The EMA has requested further clinical data, including final results from the phase 2 study, as well as results from a confirmatory phase 3 trial comparing belantamab mafodotin with pomalidomide plus low-dose dexamethasone (a standard treatment option for relapsed and refractory multiple myeloma).
Ocular toxicity
One of the most common side effects of the new drug experienced by participants in clinical trials was keratopathy, which affects the cornea. This ocular toxicity was seen at both drug doses.
The EMA noted that patients taking the drug would need to undergo specific ophthalmic examinations so that any findings can be promptly and adequately managed. As for all medicines, a risk management plan (RMP) will ensure rigorous safety monitoring of the medicine once authorized across the European Union, it added.
At the FDA advisory committee meeting, it was noted that 44% of patients in the group that received the 2.5-mg/kg dose experienced at least one episode of severe keratopathy. In some patients, the ocular side effects caused severe vision loss that interfered with patients’ activities of daily living, such as driving and reading, FDA staff said.
For the United States, the manufacturer proposed a risk evaluation and mitigation strategy (REMS) for the detection and treatment of potential complications of belantamab. This includes recommendations for ophthalmic examinations, including assessment of best corrected visual acuity prior to each treatment cycle and promptly for patients with worsening symptoms.
One of the FDA advisory committee panelists, Gita Thanarajasingam, MD, assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated with the exception of ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some than current treatments, she said.
“It’s reasonable to leave open the option for decision-making. Patients can express their values and preferences,” Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk–benefit discussion in a REMS program.”
Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep, GlaxoSmithKline) has been recommended for conditional marketing approval in the European Union (EU) for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
The product was accepted into the European Medicines Agency (EMA) PRIME program for medicines that have potential to address unmet medical needs, the agency noted.
Belantamab mafodotin was also recently recommended for U.S. approval when a Food and Drug Administration advisory committee voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
Specifically, these patients with refractory or relapsed multiple myeloma should have already tried treatment with one of the three major classes of drugs, namely an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA said. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
Belantamab mafodotin has a novel mechanism of action: It targets B-cell maturation antigen (BCMA), a protein present on the surface of virtually all multiple myeloma cells, but is absent from normal B-cells, thus “making it an ideal drug target,” the agency remarked.
The product is an antibody–drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethylauristatin F (mcMMAF). It homes in on BCMA on myeloma cell surfaces, and once inside the myeloma cell, the cytotoxic agent is released leading to apoptosis, the “programmed” death of the cancerous plasma cells, the agency explained.
Results from open-label study
The recommendation for conditional marketing authorization comes from the EMA Committee for Medicinal Products for Human Use (CHMP) and was based on a phase 2, open-label, randomized, two-arm study, DREAMM-2.
The study investigated the efficacy and safety of two doses of belantamab mafodotin in patients with multiple myeloma who still had active disease after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results were published in December in The Lancet Oncology. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The EMA has requested further clinical data, including final results from the phase 2 study, as well as results from a confirmatory phase 3 trial comparing belantamab mafodotin with pomalidomide plus low-dose dexamethasone (a standard treatment option for relapsed and refractory multiple myeloma).
Ocular toxicity
One of the most common side effects of the new drug experienced by participants in clinical trials was keratopathy, which affects the cornea. This ocular toxicity was seen at both drug doses.
The EMA noted that patients taking the drug would need to undergo specific ophthalmic examinations so that any findings can be promptly and adequately managed. As for all medicines, a risk management plan (RMP) will ensure rigorous safety monitoring of the medicine once authorized across the European Union, it added.
At the FDA advisory committee meeting, it was noted that 44% of patients in the group that received the 2.5-mg/kg dose experienced at least one episode of severe keratopathy. In some patients, the ocular side effects caused severe vision loss that interfered with patients’ activities of daily living, such as driving and reading, FDA staff said.
For the United States, the manufacturer proposed a risk evaluation and mitigation strategy (REMS) for the detection and treatment of potential complications of belantamab. This includes recommendations for ophthalmic examinations, including assessment of best corrected visual acuity prior to each treatment cycle and promptly for patients with worsening symptoms.
One of the FDA advisory committee panelists, Gita Thanarajasingam, MD, assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated with the exception of ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some than current treatments, she said.
“It’s reasonable to leave open the option for decision-making. Patients can express their values and preferences,” Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk–benefit discussion in a REMS program.”
Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep, GlaxoSmithKline) has been recommended for conditional marketing approval in the European Union (EU) for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
The product was accepted into the European Medicines Agency (EMA) PRIME program for medicines that have potential to address unmet medical needs, the agency noted.
Belantamab mafodotin was also recently recommended for U.S. approval when a Food and Drug Administration advisory committee voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
Specifically, these patients with refractory or relapsed multiple myeloma should have already tried treatment with one of the three major classes of drugs, namely an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA said. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
Belantamab mafodotin has a novel mechanism of action: It targets B-cell maturation antigen (BCMA), a protein present on the surface of virtually all multiple myeloma cells, but is absent from normal B-cells, thus “making it an ideal drug target,” the agency remarked.
The product is an antibody–drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethylauristatin F (mcMMAF). It homes in on BCMA on myeloma cell surfaces, and once inside the myeloma cell, the cytotoxic agent is released leading to apoptosis, the “programmed” death of the cancerous plasma cells, the agency explained.
Results from open-label study
The recommendation for conditional marketing authorization comes from the EMA Committee for Medicinal Products for Human Use (CHMP) and was based on a phase 2, open-label, randomized, two-arm study, DREAMM-2.
The study investigated the efficacy and safety of two doses of belantamab mafodotin in patients with multiple myeloma who still had active disease after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results were published in December in The Lancet Oncology. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The EMA has requested further clinical data, including final results from the phase 2 study, as well as results from a confirmatory phase 3 trial comparing belantamab mafodotin with pomalidomide plus low-dose dexamethasone (a standard treatment option for relapsed and refractory multiple myeloma).
Ocular toxicity
One of the most common side effects of the new drug experienced by participants in clinical trials was keratopathy, which affects the cornea. This ocular toxicity was seen at both drug doses.
The EMA noted that patients taking the drug would need to undergo specific ophthalmic examinations so that any findings can be promptly and adequately managed. As for all medicines, a risk management plan (RMP) will ensure rigorous safety monitoring of the medicine once authorized across the European Union, it added.
At the FDA advisory committee meeting, it was noted that 44% of patients in the group that received the 2.5-mg/kg dose experienced at least one episode of severe keratopathy. In some patients, the ocular side effects caused severe vision loss that interfered with patients’ activities of daily living, such as driving and reading, FDA staff said.
For the United States, the manufacturer proposed a risk evaluation and mitigation strategy (REMS) for the detection and treatment of potential complications of belantamab. This includes recommendations for ophthalmic examinations, including assessment of best corrected visual acuity prior to each treatment cycle and promptly for patients with worsening symptoms.
One of the FDA advisory committee panelists, Gita Thanarajasingam, MD, assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated with the exception of ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some than current treatments, she said.
“It’s reasonable to leave open the option for decision-making. Patients can express their values and preferences,” Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk–benefit discussion in a REMS program.”
Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it” with belantamab.
This article first appeared on Medscape.com.
EMA gives green light to avapritinib for GIST, acalabrutinib for CLL
The CHMP recommended granting conditional marketing authorization for avapritinib (Ayvakit, Blueprint Medicines) for use in adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations. About 6%-10% of GIST tumors harbor this mutation, and avapritinib is a selective and potent inhibitor of KIT and PDGFRA mutant kinases.
The CHMP also adopted a positive opinion for acalabrutinib (Calquence, AstraZeneca) for the treatment of chronic lymphocytic leukemia (CLL) as monotherapy in patients who are treatment-naive or have received at least one prior therapy.
The CHMP opinion on both drugs will be reviewed by the European Commission, which has the authority to grant marketing authorization for medicinal products in the EU.
Detailed recommendations for the use of both drugs will be provided in the summary of product characteristics, which will be published in the European public assessment report and made available in all official EU languages after the products receive marketing authorization by the European Commission.
First targeted therapy for mutation
If approved by the European Commission, avapritinib would be the first treatment in the EU indicated for patients with PDGFRA D842V-mutant GIST.
Avapritinib was approved by the US Food and Drug Administration (FDA) earlier this year for the aforementioned indication. The FDA approval was based on findings from the phase 1 NAVIGATOR trial, which included 43 patients with GIST harboring a PDGFRA exon 18 mutation, including 38 patients with the most common mutation, PDGFRA D842V.
For patients harboring a PDGFRA exon 18 mutation, the overall response rate (ORR) was 84%, with 7% having a complete response and 77% having a partial response. Patients with the PDGFRA D842V mutation achieved an ORR of 89%, with 8% having a complete response and 82% having a partial response.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies ... Today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, in a statement at the time of approval.
The most common side effects (≥ 20% of patients) observed in patients taking avapritinib include nausea, fatigue, anemia, periorbital edema, face edema, hyperbilirubinemia, diarrhea, vomiting, peripheral edema, increased lacrimation, decreased appetite, and memory impairment. There may also be a risk of intracranial hemorrhage, in which case the dose should be reduced or the drug should be discontinued.
In the EU, conditional marketing authorization is granted to a medicinal product that fulfills an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required, the CHMP notes on its website.
Avapritinib had received an orphan medicine designation during development, which the EMA will review to determine if the designation can be maintained.
New treatment for CLL
Acalabrutinib is already approved in the United States, Canada, and Australia for the treatment of CLL and small lymphocytic lymphoma. The product was approved at the same time by all three regulatory authorities last year. In the United States, acalabrutinib had previously been approved for use in mantle cell lymphoma.
The CHMP’s positive opinion of acalabrutinib is based on results from two phase 3 trials, ELEVATE TN and ASCEND.
In the ASCEND trial, acalabrutinib was compared with investigator’s choice of idelalisib or bendamustine with rituximab. The trial, which involved 310 patients with relapsed/refractory CLL, showed that acalabrutinib improved progression-free survival (PFS).
At a median follow-up of 16.1 months, the median PFS was not reached with acalabrutinib and was 16.5 months with investigator’s choice of therapy (P < .0001).
The most commonly reported adverse events seen with acalabrutinib were respiratory tract infections, headache, bruising, contusion, diarrhea, nausea, rash, musculoskeletal pain, fatigue, decreased hemoglobin, and decreased platelets.
In the ELEVATE TN trial, acalabrutinib was given alone or combined with obinutuzumab and compared to chlorambucil plus obinutuzumab in patients with previously untreated CLL. There were 535 patients randomized to receive acalabrutinib alone (n = 179), acalabrutinib plus obinutuzumab (n = 179), and chlorambucil plus obinutuzumab (n = 177).
At a median follow-up of 28 months, the median PFS was not reached with acalabrutinib alone or with acalabrutinib plus obinutuzumab, but the median PFS was 22.6 months in the chlorambucil-obinutuzumab arm (P < .0001 for both comparisons).
The most common adverse events in the acalabrutinib arms were headache, diarrhea, neutropenia, and nausea.
A version of this article first appeared on Medscape.com.
The CHMP recommended granting conditional marketing authorization for avapritinib (Ayvakit, Blueprint Medicines) for use in adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations. About 6%-10% of GIST tumors harbor this mutation, and avapritinib is a selective and potent inhibitor of KIT and PDGFRA mutant kinases.
The CHMP also adopted a positive opinion for acalabrutinib (Calquence, AstraZeneca) for the treatment of chronic lymphocytic leukemia (CLL) as monotherapy in patients who are treatment-naive or have received at least one prior therapy.
The CHMP opinion on both drugs will be reviewed by the European Commission, which has the authority to grant marketing authorization for medicinal products in the EU.
Detailed recommendations for the use of both drugs will be provided in the summary of product characteristics, which will be published in the European public assessment report and made available in all official EU languages after the products receive marketing authorization by the European Commission.
First targeted therapy for mutation
If approved by the European Commission, avapritinib would be the first treatment in the EU indicated for patients with PDGFRA D842V-mutant GIST.
Avapritinib was approved by the US Food and Drug Administration (FDA) earlier this year for the aforementioned indication. The FDA approval was based on findings from the phase 1 NAVIGATOR trial, which included 43 patients with GIST harboring a PDGFRA exon 18 mutation, including 38 patients with the most common mutation, PDGFRA D842V.
For patients harboring a PDGFRA exon 18 mutation, the overall response rate (ORR) was 84%, with 7% having a complete response and 77% having a partial response. Patients with the PDGFRA D842V mutation achieved an ORR of 89%, with 8% having a complete response and 82% having a partial response.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies ... Today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, in a statement at the time of approval.
The most common side effects (≥ 20% of patients) observed in patients taking avapritinib include nausea, fatigue, anemia, periorbital edema, face edema, hyperbilirubinemia, diarrhea, vomiting, peripheral edema, increased lacrimation, decreased appetite, and memory impairment. There may also be a risk of intracranial hemorrhage, in which case the dose should be reduced or the drug should be discontinued.
In the EU, conditional marketing authorization is granted to a medicinal product that fulfills an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required, the CHMP notes on its website.
Avapritinib had received an orphan medicine designation during development, which the EMA will review to determine if the designation can be maintained.
New treatment for CLL
Acalabrutinib is already approved in the United States, Canada, and Australia for the treatment of CLL and small lymphocytic lymphoma. The product was approved at the same time by all three regulatory authorities last year. In the United States, acalabrutinib had previously been approved for use in mantle cell lymphoma.
The CHMP’s positive opinion of acalabrutinib is based on results from two phase 3 trials, ELEVATE TN and ASCEND.
In the ASCEND trial, acalabrutinib was compared with investigator’s choice of idelalisib or bendamustine with rituximab. The trial, which involved 310 patients with relapsed/refractory CLL, showed that acalabrutinib improved progression-free survival (PFS).
At a median follow-up of 16.1 months, the median PFS was not reached with acalabrutinib and was 16.5 months with investigator’s choice of therapy (P < .0001).
The most commonly reported adverse events seen with acalabrutinib were respiratory tract infections, headache, bruising, contusion, diarrhea, nausea, rash, musculoskeletal pain, fatigue, decreased hemoglobin, and decreased platelets.
In the ELEVATE TN trial, acalabrutinib was given alone or combined with obinutuzumab and compared to chlorambucil plus obinutuzumab in patients with previously untreated CLL. There were 535 patients randomized to receive acalabrutinib alone (n = 179), acalabrutinib plus obinutuzumab (n = 179), and chlorambucil plus obinutuzumab (n = 177).
At a median follow-up of 28 months, the median PFS was not reached with acalabrutinib alone or with acalabrutinib plus obinutuzumab, but the median PFS was 22.6 months in the chlorambucil-obinutuzumab arm (P < .0001 for both comparisons).
The most common adverse events in the acalabrutinib arms were headache, diarrhea, neutropenia, and nausea.
A version of this article first appeared on Medscape.com.
The CHMP recommended granting conditional marketing authorization for avapritinib (Ayvakit, Blueprint Medicines) for use in adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations. About 6%-10% of GIST tumors harbor this mutation, and avapritinib is a selective and potent inhibitor of KIT and PDGFRA mutant kinases.
The CHMP also adopted a positive opinion for acalabrutinib (Calquence, AstraZeneca) for the treatment of chronic lymphocytic leukemia (CLL) as monotherapy in patients who are treatment-naive or have received at least one prior therapy.
The CHMP opinion on both drugs will be reviewed by the European Commission, which has the authority to grant marketing authorization for medicinal products in the EU.
Detailed recommendations for the use of both drugs will be provided in the summary of product characteristics, which will be published in the European public assessment report and made available in all official EU languages after the products receive marketing authorization by the European Commission.
First targeted therapy for mutation
If approved by the European Commission, avapritinib would be the first treatment in the EU indicated for patients with PDGFRA D842V-mutant GIST.
Avapritinib was approved by the US Food and Drug Administration (FDA) earlier this year for the aforementioned indication. The FDA approval was based on findings from the phase 1 NAVIGATOR trial, which included 43 patients with GIST harboring a PDGFRA exon 18 mutation, including 38 patients with the most common mutation, PDGFRA D842V.
For patients harboring a PDGFRA exon 18 mutation, the overall response rate (ORR) was 84%, with 7% having a complete response and 77% having a partial response. Patients with the PDGFRA D842V mutation achieved an ORR of 89%, with 8% having a complete response and 82% having a partial response.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies ... Today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, in a statement at the time of approval.
The most common side effects (≥ 20% of patients) observed in patients taking avapritinib include nausea, fatigue, anemia, periorbital edema, face edema, hyperbilirubinemia, diarrhea, vomiting, peripheral edema, increased lacrimation, decreased appetite, and memory impairment. There may also be a risk of intracranial hemorrhage, in which case the dose should be reduced or the drug should be discontinued.
In the EU, conditional marketing authorization is granted to a medicinal product that fulfills an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required, the CHMP notes on its website.
Avapritinib had received an orphan medicine designation during development, which the EMA will review to determine if the designation can be maintained.
New treatment for CLL
Acalabrutinib is already approved in the United States, Canada, and Australia for the treatment of CLL and small lymphocytic lymphoma. The product was approved at the same time by all three regulatory authorities last year. In the United States, acalabrutinib had previously been approved for use in mantle cell lymphoma.
The CHMP’s positive opinion of acalabrutinib is based on results from two phase 3 trials, ELEVATE TN and ASCEND.
In the ASCEND trial, acalabrutinib was compared with investigator’s choice of idelalisib or bendamustine with rituximab. The trial, which involved 310 patients with relapsed/refractory CLL, showed that acalabrutinib improved progression-free survival (PFS).
At a median follow-up of 16.1 months, the median PFS was not reached with acalabrutinib and was 16.5 months with investigator’s choice of therapy (P < .0001).
The most commonly reported adverse events seen with acalabrutinib were respiratory tract infections, headache, bruising, contusion, diarrhea, nausea, rash, musculoskeletal pain, fatigue, decreased hemoglobin, and decreased platelets.
In the ELEVATE TN trial, acalabrutinib was given alone or combined with obinutuzumab and compared to chlorambucil plus obinutuzumab in patients with previously untreated CLL. There were 535 patients randomized to receive acalabrutinib alone (n = 179), acalabrutinib plus obinutuzumab (n = 179), and chlorambucil plus obinutuzumab (n = 177).
At a median follow-up of 28 months, the median PFS was not reached with acalabrutinib alone or with acalabrutinib plus obinutuzumab, but the median PFS was 22.6 months in the chlorambucil-obinutuzumab arm (P < .0001 for both comparisons).
The most common adverse events in the acalabrutinib arms were headache, diarrhea, neutropenia, and nausea.
A version of this article first appeared on Medscape.com.
FDA okays new CAR T therapy, first for mantle cell lymphoma
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
US News releases latest top hospitals list, adds COVID heroes
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
COVID-19 fears would keep most Hispanics with stroke, MI symptoms home
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.
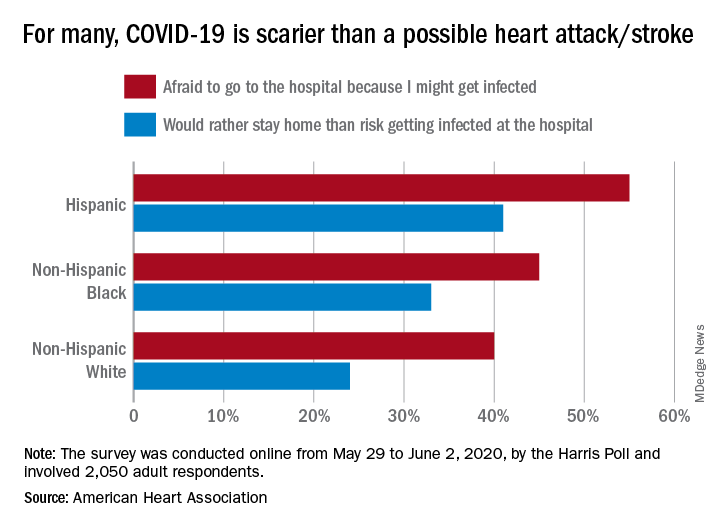
Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.

Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.

Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
Cleaner data confirm severe COVID-19 link to diabetes, hypertension
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
FROM JOURNAL OF THE ENDOCRINE SOCIETY