User login
-
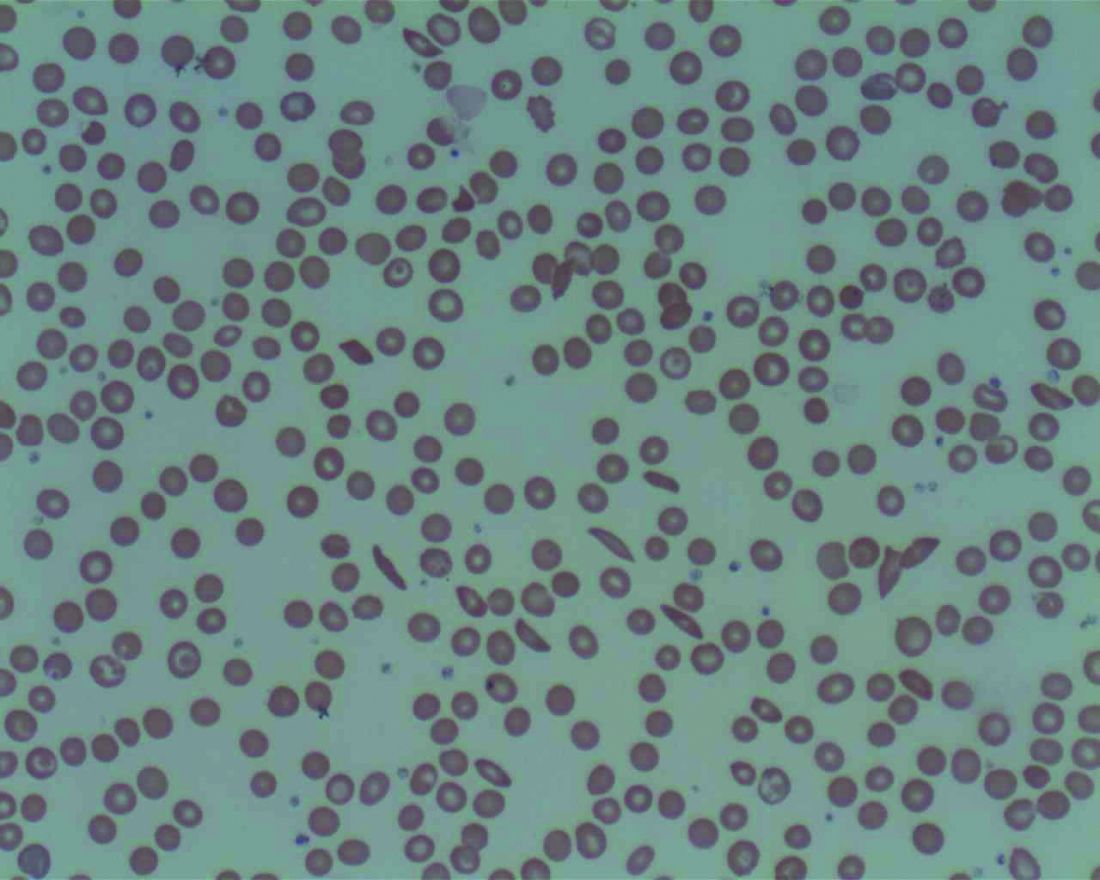
Orthopedic problems in children can be the first indication of acute lymphoblastic leukemia
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
FROM THE JOURNAL OF ORTHOPAEDICS
Children’s share of new COVID-19 cases is on the rise
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
FROM PEDIATRICS
Pandemic poses new challenges for rural doctors
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
Landmark sickle cell report targets massive failures, calls for action
The National Academies of Science, Engineering, and Medicine have just released a 522-page report, but it’s not the usual compilation of guidelines for treatment of a disease. Instead, the authors of “Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action” argue in stark terms that the American society has colossally failed individuals living with sickle cell disease (SCD), who are mostly Black or Brown. A dramatic overhaul of the country’s medical and societal priorities is needed to turn things around to improve health and longevity among this rare disease population.
The findings from the NASEM report are explicit: “There has been substantial success in increasing the survival of children with SCD, but this success had not been translated to similar success as they become adults.” One factor posited to contribute to the slow progress in the improvement of quality and quantity of life for adults living with this disease is the fact that “SCD is largely a disease of African Americans, and as such exists in a context of racial discrimination, health and other societal disparities, mistrust of the health care system, and the effects of poverty.” The report also cites the substantial evidence that those with SCD may receive poorer quality of care.
The report’s 14 authors were made up of an ad hoc committee formed at the request of the Department of Health & Human Services’ Office of Minority Health. The office asked NASEM to convene the committee to develop a strategic plan and blueprint for the United States and others regarding SCD.
The NASEM SCD committee members “realized that we can’t address the medical components of SCD if we don’t explore societal issues and why it’s been so hard to get good care for people with sickle cell disease,” hematologist and report coauthor Ifeyinwa (Ify) Osunkwo, MD, professor of medicine and pediatrics at Atrium Health and director of the Sickle Cell Disease Enterprise, Levine Cancer Institute, Charlotte, N.C., said in an interview. Dr. Osunkwo is also the medical editor of Hematology News.
“After almost a year of meetings and digging into the background and history of SCD care, we came out with very comprehensive summary of where we were and where we want to be,” she said. “The report provides short-, intermediate- and long-term recommendations and identifies which entity and organization should be responsible for implementing them.”
The report authors, led by pediatrician and committee chair Marie Clare McCormick, MD, of the Harvard School of Public Health, Boston, stated that about 100,000 people in the United States and millions worldwide live with SCD. The disease kills more than 700 people per year in the United States, and treatment costs an estimated $2 billion a year.
When judged by disability-adjusted life-years lost – a measurement of expected healthy years of life without an illness – the impact of SCD on individuals is estimated to be greater than a long list of other diseases such as Alzheimer’s disease, breast cancer, type 1 diabetes, and AIDS/HIV, the report noted.
“The health care needs of individuals living with SCD have been neglected by the U.S. and global health care systems, causing them and their families to suffer,” the report said. “Many of the complications that afflict individuals with SCD, particularly pain, are invisible. Pain is only diagnosed by self-reports, and in SCD there are few to no external indicators of the pain experience. Nevertheless, the pain can be excruciatingly severe and requires treatment with strong analgesics.”
There’s even more misery to the story of SCD, the report said, and Dr. Osunkwo agreed. “It’s not just about pain. These individuals suffer from multiple organ-system complications that are physical but also psychological and societal. They experience a lot of disparities in every aspect of their lives. You’re sick, so then you can’t get a job or health insurance, you can’t get Social Security benefits. You can’t get the type of health care you need nor can you access the other forms of support you need and often you are judged as a drug seeker for complaining of pain or repeatedly seeking acute care for unresolved pain.”
Multiple factors exacerbate the experience of people living with SCD in America, the report said. “Because of systemic racism, unconscious bias, and the stigma associated with the diagnosis, the disease brings with it a much broader burden.”
Dr. Osunkwo put it this way: “SCD is a disease that mostly affects Brown and Black people, and that gets layered into the whole discrimination issues that Black and Brown people face compounding the health burden from their disease.”
The report added that “the SCD community has developed a significant lack of trust in the health care system due to the nearly universal stigma and lack of belief in their reports of pain, a lack of trust that has been further reinforced by historical events, such as the Tuskegee experiment.”
The report highlighted research that finds that Blacks “are more likely to receive a lower quality of pain management than white patients and may be perceived as having drug-seeking behavior.”
The report also identified gaps in treatment, noting that “many SCD complications are not restricted to any one organ system, and the impact of the disease on [quality of life] can be profound but hard to define and compartmentalize.”
Dr. Osunkwo said medical professionals often fail to understand the full breadth of the disease. “There’s no particular look to SCD. When you have cancer, you come in, and you look like you’re sick because you’re bald. Everyone clues into that cancer look and knows it’s lethal, that you’re may likely die early. We don’t have that “look that generates empathy” for SCD, and people don’t understand the burden on those affected. They don’t understand or appreciate that SCD shortens your lifespan as well ... that people living with SCD die 3 decades earlier than their ethnically matched peers. Also, SCD is associated with a lot of pain, and pain and the treatment of pain with opioids makes people [health care providers] uncomfortable unless it’s cancer pain.”
She added: “People also assume that, if it’s not pain, it’s not SCD even though SCD can cause leg ulcers and blood clots and even affect the tonsils, or lead to a stroke. When a disease complexity is too difficult for providers to understand, they either avoid it or don’t do anything for the patient.”
Screening and surveillance for SCD and sickle cell trait is insufficient, the report said, and the potential cost of missed childhood cases is large. Detecting the condition at birth allows the implementation of appropriate comprehensive care and treatment to prevent early death from infections and strokes. As the authors noted, “tremendous strides have been made in the past few decades in the care of children with SCD, which have led to almost all children in high-income settings surviving to adulthood.” However, there remains gaps in care coordination and follow-up of babies screened at birth and even bigger gaps in translating these life span gains to adults particularly around the period of transition from pediatrics to adult care when there appears to be a spike in morbidity and mortality.
The report summarized current treatments for SCD and noted “an influx of pipeline products” after years of little progress and identifies “a need for targeted SCD therapies that address the underlying cause of the disease.”
While treatment recommendations exist, Dr. Osunkwo said, “the evidence for them is very poor and many SCD complications have no evidence-based guidelines for providers to follow. We need more research to provide high quality evidence to make guidelines for SCD treatment stronger and more robust.”
In its final section, the report offers a “strategic plan and blueprint for sickle cell disease action.” It offers several strategies to achieve the vision of “long healthy productive lives for those living with sickle cell disease and sickle cell trait”:
- Establish and fund a research agenda to inform effective programs and policies across the life span.
- Implement efforts to advance understanding of the full impact of sickle cell trait on individuals and society.
- Address barriers to accessing current and pipeline therapies for SCD.
- Improve SCD awareness and strengthen advocacy efforts.
- Increase the number of qualified health professionals providing SCD care.
- Strengthen the evidence base for interventions and disease management and implement widespread efforts to monitor the quality of SCD care.
- Establish organized systems of care assuring both clinical and nonclinical supportive services to all persons living with SCD.
- Establish a national system to collect and link data to characterize the burden of disease, outcomes, and the needs of those with SCD across the life span.
“Right now, the average lifespan for SCD is in the mid-40s to mid-50s,” Dr. Osunkwo said. “That’s a horrible statistic. Even if we just take up half of these recommendations, people will live longer with SCD, and they’ll be more productive and contribute more to society. If we value a cancer life the same as a sickle cell life, we’ll be halfway across the finish line. But the stigma of SCD being a Black and Brown problem is going to be the hardest to confront as it requires a systemic change in our culture as a country and a health care system.”
Still, she said, the commissioning of the report “shows that there is a desire to understand the issue in better detail and try to mitigate it.”
Dr. Osunkwo and Dr. McCormick had no relevant disclosures.
SOURCE: National Academies of Sciences, Engineering, and Medicine. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. Washington, D.C.: National Academies Press, 2020.
The National Academies of Science, Engineering, and Medicine have just released a 522-page report, but it’s not the usual compilation of guidelines for treatment of a disease. Instead, the authors of “Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action” argue in stark terms that the American society has colossally failed individuals living with sickle cell disease (SCD), who are mostly Black or Brown. A dramatic overhaul of the country’s medical and societal priorities is needed to turn things around to improve health and longevity among this rare disease population.
The findings from the NASEM report are explicit: “There has been substantial success in increasing the survival of children with SCD, but this success had not been translated to similar success as they become adults.” One factor posited to contribute to the slow progress in the improvement of quality and quantity of life for adults living with this disease is the fact that “SCD is largely a disease of African Americans, and as such exists in a context of racial discrimination, health and other societal disparities, mistrust of the health care system, and the effects of poverty.” The report also cites the substantial evidence that those with SCD may receive poorer quality of care.
The report’s 14 authors were made up of an ad hoc committee formed at the request of the Department of Health & Human Services’ Office of Minority Health. The office asked NASEM to convene the committee to develop a strategic plan and blueprint for the United States and others regarding SCD.
The NASEM SCD committee members “realized that we can’t address the medical components of SCD if we don’t explore societal issues and why it’s been so hard to get good care for people with sickle cell disease,” hematologist and report coauthor Ifeyinwa (Ify) Osunkwo, MD, professor of medicine and pediatrics at Atrium Health and director of the Sickle Cell Disease Enterprise, Levine Cancer Institute, Charlotte, N.C., said in an interview. Dr. Osunkwo is also the medical editor of Hematology News.
“After almost a year of meetings and digging into the background and history of SCD care, we came out with very comprehensive summary of where we were and where we want to be,” she said. “The report provides short-, intermediate- and long-term recommendations and identifies which entity and organization should be responsible for implementing them.”
The report authors, led by pediatrician and committee chair Marie Clare McCormick, MD, of the Harvard School of Public Health, Boston, stated that about 100,000 people in the United States and millions worldwide live with SCD. The disease kills more than 700 people per year in the United States, and treatment costs an estimated $2 billion a year.
When judged by disability-adjusted life-years lost – a measurement of expected healthy years of life without an illness – the impact of SCD on individuals is estimated to be greater than a long list of other diseases such as Alzheimer’s disease, breast cancer, type 1 diabetes, and AIDS/HIV, the report noted.
“The health care needs of individuals living with SCD have been neglected by the U.S. and global health care systems, causing them and their families to suffer,” the report said. “Many of the complications that afflict individuals with SCD, particularly pain, are invisible. Pain is only diagnosed by self-reports, and in SCD there are few to no external indicators of the pain experience. Nevertheless, the pain can be excruciatingly severe and requires treatment with strong analgesics.”
There’s even more misery to the story of SCD, the report said, and Dr. Osunkwo agreed. “It’s not just about pain. These individuals suffer from multiple organ-system complications that are physical but also psychological and societal. They experience a lot of disparities in every aspect of their lives. You’re sick, so then you can’t get a job or health insurance, you can’t get Social Security benefits. You can’t get the type of health care you need nor can you access the other forms of support you need and often you are judged as a drug seeker for complaining of pain or repeatedly seeking acute care for unresolved pain.”
Multiple factors exacerbate the experience of people living with SCD in America, the report said. “Because of systemic racism, unconscious bias, and the stigma associated with the diagnosis, the disease brings with it a much broader burden.”
Dr. Osunkwo put it this way: “SCD is a disease that mostly affects Brown and Black people, and that gets layered into the whole discrimination issues that Black and Brown people face compounding the health burden from their disease.”
The report added that “the SCD community has developed a significant lack of trust in the health care system due to the nearly universal stigma and lack of belief in their reports of pain, a lack of trust that has been further reinforced by historical events, such as the Tuskegee experiment.”
The report highlighted research that finds that Blacks “are more likely to receive a lower quality of pain management than white patients and may be perceived as having drug-seeking behavior.”
The report also identified gaps in treatment, noting that “many SCD complications are not restricted to any one organ system, and the impact of the disease on [quality of life] can be profound but hard to define and compartmentalize.”
Dr. Osunkwo said medical professionals often fail to understand the full breadth of the disease. “There’s no particular look to SCD. When you have cancer, you come in, and you look like you’re sick because you’re bald. Everyone clues into that cancer look and knows it’s lethal, that you’re may likely die early. We don’t have that “look that generates empathy” for SCD, and people don’t understand the burden on those affected. They don’t understand or appreciate that SCD shortens your lifespan as well ... that people living with SCD die 3 decades earlier than their ethnically matched peers. Also, SCD is associated with a lot of pain, and pain and the treatment of pain with opioids makes people [health care providers] uncomfortable unless it’s cancer pain.”
She added: “People also assume that, if it’s not pain, it’s not SCD even though SCD can cause leg ulcers and blood clots and even affect the tonsils, or lead to a stroke. When a disease complexity is too difficult for providers to understand, they either avoid it or don’t do anything for the patient.”
Screening and surveillance for SCD and sickle cell trait is insufficient, the report said, and the potential cost of missed childhood cases is large. Detecting the condition at birth allows the implementation of appropriate comprehensive care and treatment to prevent early death from infections and strokes. As the authors noted, “tremendous strides have been made in the past few decades in the care of children with SCD, which have led to almost all children in high-income settings surviving to adulthood.” However, there remains gaps in care coordination and follow-up of babies screened at birth and even bigger gaps in translating these life span gains to adults particularly around the period of transition from pediatrics to adult care when there appears to be a spike in morbidity and mortality.
The report summarized current treatments for SCD and noted “an influx of pipeline products” after years of little progress and identifies “a need for targeted SCD therapies that address the underlying cause of the disease.”
While treatment recommendations exist, Dr. Osunkwo said, “the evidence for them is very poor and many SCD complications have no evidence-based guidelines for providers to follow. We need more research to provide high quality evidence to make guidelines for SCD treatment stronger and more robust.”
In its final section, the report offers a “strategic plan and blueprint for sickle cell disease action.” It offers several strategies to achieve the vision of “long healthy productive lives for those living with sickle cell disease and sickle cell trait”:
- Establish and fund a research agenda to inform effective programs and policies across the life span.
- Implement efforts to advance understanding of the full impact of sickle cell trait on individuals and society.
- Address barriers to accessing current and pipeline therapies for SCD.
- Improve SCD awareness and strengthen advocacy efforts.
- Increase the number of qualified health professionals providing SCD care.
- Strengthen the evidence base for interventions and disease management and implement widespread efforts to monitor the quality of SCD care.
- Establish organized systems of care assuring both clinical and nonclinical supportive services to all persons living with SCD.
- Establish a national system to collect and link data to characterize the burden of disease, outcomes, and the needs of those with SCD across the life span.
“Right now, the average lifespan for SCD is in the mid-40s to mid-50s,” Dr. Osunkwo said. “That’s a horrible statistic. Even if we just take up half of these recommendations, people will live longer with SCD, and they’ll be more productive and contribute more to society. If we value a cancer life the same as a sickle cell life, we’ll be halfway across the finish line. But the stigma of SCD being a Black and Brown problem is going to be the hardest to confront as it requires a systemic change in our culture as a country and a health care system.”
Still, she said, the commissioning of the report “shows that there is a desire to understand the issue in better detail and try to mitigate it.”
Dr. Osunkwo and Dr. McCormick had no relevant disclosures.
SOURCE: National Academies of Sciences, Engineering, and Medicine. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. Washington, D.C.: National Academies Press, 2020.
The National Academies of Science, Engineering, and Medicine have just released a 522-page report, but it’s not the usual compilation of guidelines for treatment of a disease. Instead, the authors of “Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action” argue in stark terms that the American society has colossally failed individuals living with sickle cell disease (SCD), who are mostly Black or Brown. A dramatic overhaul of the country’s medical and societal priorities is needed to turn things around to improve health and longevity among this rare disease population.
The findings from the NASEM report are explicit: “There has been substantial success in increasing the survival of children with SCD, but this success had not been translated to similar success as they become adults.” One factor posited to contribute to the slow progress in the improvement of quality and quantity of life for adults living with this disease is the fact that “SCD is largely a disease of African Americans, and as such exists in a context of racial discrimination, health and other societal disparities, mistrust of the health care system, and the effects of poverty.” The report also cites the substantial evidence that those with SCD may receive poorer quality of care.
The report’s 14 authors were made up of an ad hoc committee formed at the request of the Department of Health & Human Services’ Office of Minority Health. The office asked NASEM to convene the committee to develop a strategic plan and blueprint for the United States and others regarding SCD.
The NASEM SCD committee members “realized that we can’t address the medical components of SCD if we don’t explore societal issues and why it’s been so hard to get good care for people with sickle cell disease,” hematologist and report coauthor Ifeyinwa (Ify) Osunkwo, MD, professor of medicine and pediatrics at Atrium Health and director of the Sickle Cell Disease Enterprise, Levine Cancer Institute, Charlotte, N.C., said in an interview. Dr. Osunkwo is also the medical editor of Hematology News.
“After almost a year of meetings and digging into the background and history of SCD care, we came out with very comprehensive summary of where we were and where we want to be,” she said. “The report provides short-, intermediate- and long-term recommendations and identifies which entity and organization should be responsible for implementing them.”
The report authors, led by pediatrician and committee chair Marie Clare McCormick, MD, of the Harvard School of Public Health, Boston, stated that about 100,000 people in the United States and millions worldwide live with SCD. The disease kills more than 700 people per year in the United States, and treatment costs an estimated $2 billion a year.
When judged by disability-adjusted life-years lost – a measurement of expected healthy years of life without an illness – the impact of SCD on individuals is estimated to be greater than a long list of other diseases such as Alzheimer’s disease, breast cancer, type 1 diabetes, and AIDS/HIV, the report noted.
“The health care needs of individuals living with SCD have been neglected by the U.S. and global health care systems, causing them and their families to suffer,” the report said. “Many of the complications that afflict individuals with SCD, particularly pain, are invisible. Pain is only diagnosed by self-reports, and in SCD there are few to no external indicators of the pain experience. Nevertheless, the pain can be excruciatingly severe and requires treatment with strong analgesics.”
There’s even more misery to the story of SCD, the report said, and Dr. Osunkwo agreed. “It’s not just about pain. These individuals suffer from multiple organ-system complications that are physical but also psychological and societal. They experience a lot of disparities in every aspect of their lives. You’re sick, so then you can’t get a job or health insurance, you can’t get Social Security benefits. You can’t get the type of health care you need nor can you access the other forms of support you need and often you are judged as a drug seeker for complaining of pain or repeatedly seeking acute care for unresolved pain.”
Multiple factors exacerbate the experience of people living with SCD in America, the report said. “Because of systemic racism, unconscious bias, and the stigma associated with the diagnosis, the disease brings with it a much broader burden.”
Dr. Osunkwo put it this way: “SCD is a disease that mostly affects Brown and Black people, and that gets layered into the whole discrimination issues that Black and Brown people face compounding the health burden from their disease.”
The report added that “the SCD community has developed a significant lack of trust in the health care system due to the nearly universal stigma and lack of belief in their reports of pain, a lack of trust that has been further reinforced by historical events, such as the Tuskegee experiment.”
The report highlighted research that finds that Blacks “are more likely to receive a lower quality of pain management than white patients and may be perceived as having drug-seeking behavior.”
The report also identified gaps in treatment, noting that “many SCD complications are not restricted to any one organ system, and the impact of the disease on [quality of life] can be profound but hard to define and compartmentalize.”
Dr. Osunkwo said medical professionals often fail to understand the full breadth of the disease. “There’s no particular look to SCD. When you have cancer, you come in, and you look like you’re sick because you’re bald. Everyone clues into that cancer look and knows it’s lethal, that you’re may likely die early. We don’t have that “look that generates empathy” for SCD, and people don’t understand the burden on those affected. They don’t understand or appreciate that SCD shortens your lifespan as well ... that people living with SCD die 3 decades earlier than their ethnically matched peers. Also, SCD is associated with a lot of pain, and pain and the treatment of pain with opioids makes people [health care providers] uncomfortable unless it’s cancer pain.”
She added: “People also assume that, if it’s not pain, it’s not SCD even though SCD can cause leg ulcers and blood clots and even affect the tonsils, or lead to a stroke. When a disease complexity is too difficult for providers to understand, they either avoid it or don’t do anything for the patient.”
Screening and surveillance for SCD and sickle cell trait is insufficient, the report said, and the potential cost of missed childhood cases is large. Detecting the condition at birth allows the implementation of appropriate comprehensive care and treatment to prevent early death from infections and strokes. As the authors noted, “tremendous strides have been made in the past few decades in the care of children with SCD, which have led to almost all children in high-income settings surviving to adulthood.” However, there remains gaps in care coordination and follow-up of babies screened at birth and even bigger gaps in translating these life span gains to adults particularly around the period of transition from pediatrics to adult care when there appears to be a spike in morbidity and mortality.
The report summarized current treatments for SCD and noted “an influx of pipeline products” after years of little progress and identifies “a need for targeted SCD therapies that address the underlying cause of the disease.”
While treatment recommendations exist, Dr. Osunkwo said, “the evidence for them is very poor and many SCD complications have no evidence-based guidelines for providers to follow. We need more research to provide high quality evidence to make guidelines for SCD treatment stronger and more robust.”
In its final section, the report offers a “strategic plan and blueprint for sickle cell disease action.” It offers several strategies to achieve the vision of “long healthy productive lives for those living with sickle cell disease and sickle cell trait”:
- Establish and fund a research agenda to inform effective programs and policies across the life span.
- Implement efforts to advance understanding of the full impact of sickle cell trait on individuals and society.
- Address barriers to accessing current and pipeline therapies for SCD.
- Improve SCD awareness and strengthen advocacy efforts.
- Increase the number of qualified health professionals providing SCD care.
- Strengthen the evidence base for interventions and disease management and implement widespread efforts to monitor the quality of SCD care.
- Establish organized systems of care assuring both clinical and nonclinical supportive services to all persons living with SCD.
- Establish a national system to collect and link data to characterize the burden of disease, outcomes, and the needs of those with SCD across the life span.
“Right now, the average lifespan for SCD is in the mid-40s to mid-50s,” Dr. Osunkwo said. “That’s a horrible statistic. Even if we just take up half of these recommendations, people will live longer with SCD, and they’ll be more productive and contribute more to society. If we value a cancer life the same as a sickle cell life, we’ll be halfway across the finish line. But the stigma of SCD being a Black and Brown problem is going to be the hardest to confront as it requires a systemic change in our culture as a country and a health care system.”
Still, she said, the commissioning of the report “shows that there is a desire to understand the issue in better detail and try to mitigate it.”
Dr. Osunkwo and Dr. McCormick had no relevant disclosures.
SOURCE: National Academies of Sciences, Engineering, and Medicine. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. Washington, D.C.: National Academies Press, 2020.
J&J’s one-shot COVID-19 vaccine advances to phase 3 testing
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
Initial response to novel agents preps MM patients for favorable transplant outcomes
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
For patients with multiple myeloma, triplet induction with novel agents beat doublet with regards to early results, according to Lalit Kumar, MD, and colleagues at the All India Institute of Medical sciences Myeloma Group, New Delhi.
The study analyzed 326 multiple myeloma patients who received high-dose, novel agent–based induction therapy prior to autologous stem cell transplant (ASCT) at a single institution, according to a report published in Clinical Lymphoma, Myeloma and Leukemia.
Between January 2005 and December 2018, 326 consecutive patients underwent high-dose chemotherapy and autologous stem cell transplant. The median age of the patients was 52 years; 66% were men, nearly 33% had Revised ISS III disease; almost 16% had high-risk cytogenetics and 23% underwent transplant in second remission after salvage therapy for relapse. A total of 194 patients (59.5%) received induction with two novel agents (thalidomide/dexamethasone, n = 95; lenalidomide/dexamethasone, n = 63; bortezomib/dexamethasone, n = 36) and 132 (40.5%) received three drugs (bortezomib/lenalidomide/dexamethasone, n = 53; bortezomib/liposomal doxorubicin/dexamethasone, n = 42; bortezomib/thalidomide/dexamethasone, n = 31; other n = 3).
Outcomes favorable
After transplant 227 (69.8%) patients achieved a complete response; 48 (14.7%) had a very good partial response, 32 (9.8%) had a partial response, and 9 (2.8%) patients had stable disease. Ten (3.1%) patients died of transplant-related complications (before day 100). Triplet induction beat doublet with regards to early response (95.4% vs. 84.02% [doublets], P < .003), stem cell mobilization (88.6% vs. 76.8%, P < .005) and lower day-100 transplant-related mortality (P < .001), However, at a median follow-up of 62.5 months, the median overall response rate (97.5 months triplet vs. 100.0 months doublet) and the median progression free survival (54.5 months vs. 57 months) were not statistically different between the two induction-treatment groups.
Patients who had undergone transplant in a recent period (2016-18) had a better outcome, compared with initial years, which possibly reflects a combined effect of learning curve, use of triplets, and gradual reduction in day-100 mortality, the authors stated.
“Whether newer regimens incorporating monoclonal antibodies (associated with higher [complete response] rate and [minimal residual disease] negativity) would result in further improvement in survival, needs to be determined in future studies,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Kumar L et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 18. doi: 10.1016/j.clml.2020.08.021.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
Three major COVID vaccine developers release detailed trial protocols
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Smart health devices – promises and pitfalls
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
What needs to be done before the data deluge hits the office
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Two new protein biomarkers may serve as prognostic indicators for outcomes in CLL
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
FROM EXPERIMENTAL HEMATOLOGY