User login
-
What to do if an employee tests positive for COVID-19
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
One-third of health care workers leery of getting COVID-19 vaccine, survey shows
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
PPE protected critical care staff from COVID-19 transmission
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
FROM CCC50
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.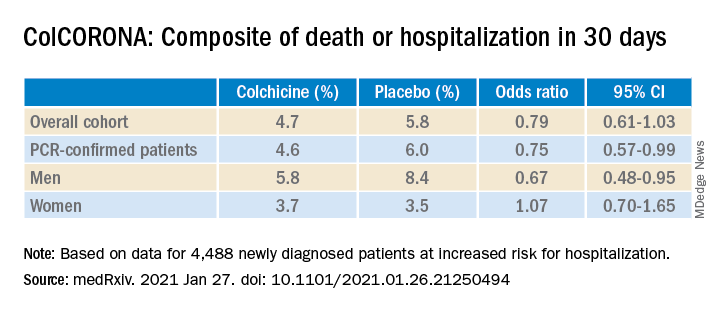
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Study: COVID cases have been ‘severely undercounted’
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
COVID-19: Peginterferon lambda may prevent clinical deterioration, shorten viral shedding
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
FROM THE LANCET RESPIRATORY MEDICINE
Some COVID-19 vaccine reactions could be pseudoallergic, experts say
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
‘We can do better’: COVID-19 vaccination in patients with cancer
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
U.K. COVID-19 variant doubling every 10 days in the U.S.: Study
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.