User login
-
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.
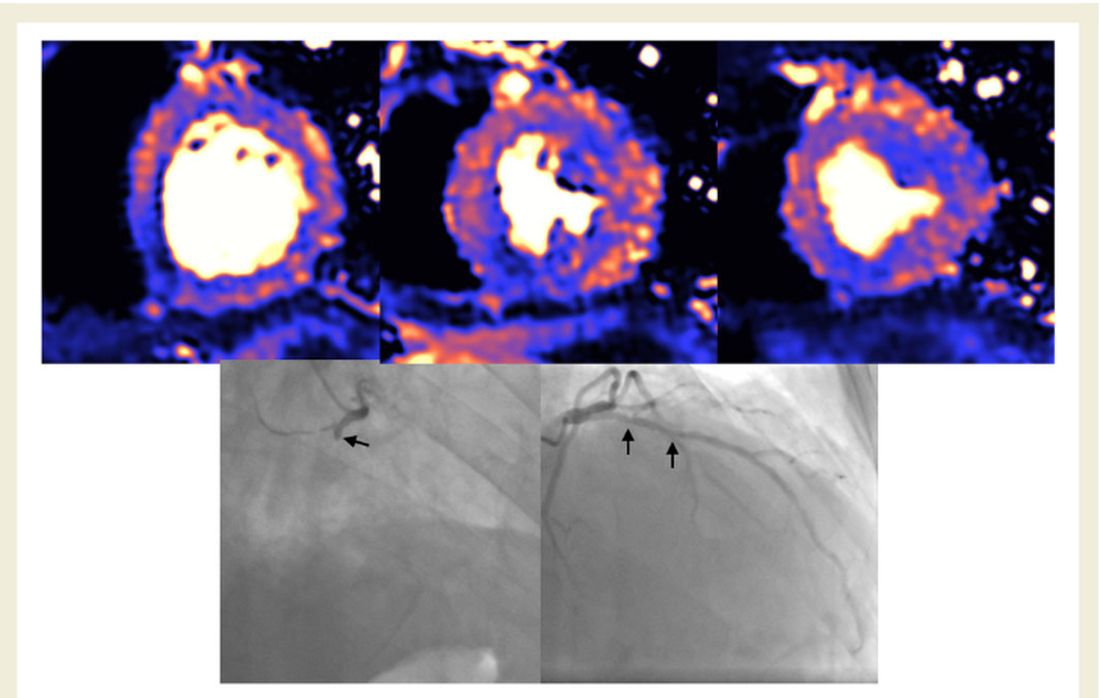
No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
Janssen/J&J COVID-19 vaccine cuts transmission, new data show
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Safety profiles of hemophilia agents vary widely
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
Despite their similar functions, each current and emerging therapy for treating hemophilia has a unique safety profile, and each needs to be weighed apart from agents both within and outside its pharmacologic class, a hemophilia specialist said.
“My view is that each new molecule coming to the hemophilia space, including variant factor molecules, needs to be scrutinized separately, without class assumptions or extrapolations, and it’s clear that thrombosis risk has become a priority safety consideration,” said Dan Hart, MBChB, MRCP, FRCPath, PhD, from Barts and the London School of Medicine and Dentistry.
He reviewed the comparative safety of standard and novel therapies for hemophilia at the annual congress of the European Association for Haemophilia and Allied Disorders.
Factor inhibitors
Inhibitors occur in both hemophilia A and hemophilia B, and are primarily seen in patients with childhood exposure to factor concentrates. Inhibitors, which include anti–factor VIII and factor IX alloantibodies, are more common among patients with severe hemophilia and those with more disruptive factor VIII and factor IX mutations.
“There can be transient vs. persistent inhibitors, and arguably the more you look, the more you find, but clinically we never miss high-titer inhibitors that have a big impact on individuals and the subsequent decisions about management,” he said.
Hamster vs. human
It’s currently unclear whether there is an immunologic advantage for previously untreated patients to be started on factor VIII concentrates derived from recombinant human cells lines, or from products derived from Chinese hamster ovary (CHO) or baby hamster kidney (BHK) cell lines, Dr. Hart said.
“We need to ensure that we’re not selective about comparator choice for new products in the absence of head-to-head studies,” he said.
Route of administration matters
Inhibitors appear to be a more common occurrence among patients who received factor concentrates subcutaneously, compared with intravenously, Dr. Hart noted, pointing to a 2011 study indicating a background annual risk of 5 cases of inhibitor development per 1,000 treatment years in previously treated patients who received intravenous therapy (Blood. 2011 Jun 9;117[23]:6367-70).
In contrast, in a phase 1 trial of subcutaneous turoctocog alfa pegol, 5 out of 26 patients had detectable N8-GP–binding antibodies after 42-91 exposure days. Of these patients, one developed an inhibitor to factor VIII, and anti–N8-GP antibody appearance was associated with a decline in factor VIII plasma activity in four of the five patients. In addition, five patients reported a total of nine bleeding episodes requiring treatment during prophylaxis. As a result of this trial, further clinical development of the subcutaneous version was suspended. (J Thromb Haemost. 2020 Feb;18[2]:341-51).
Other subcutaneously administered factors are currently in development, Dr. Hart noted.
Nonfactor inhibitors?
“The nonfactor agents do have the risk of generating antibodies: Monoclonal antibodies outside the hemophilia setting provoke antidrug antibodies,” he said.
Although there is no consensus regarding which assay can best monitor antidrug antibodies (ADA), enzyme-linked immunosorbent assay (ELISA) can detect neutralizing antibodies and other antibodies.
In the hemophilia setting, surrogate markers for loss of drug efficacy include longer activated partial thromboplastin time (ATTP) or a drop in serum drug levels. Worsening bleeding phenotype can also be a marker for loss of efficacy, albeit an imperfect one.
Emicizumab (Hemlibara), the first nonfactor monoclonal agent to make it to market, has the largest dataset available, and evidence suggests a rate of neutralizing antibodies with this agent of less than 1% in the HAVEN clinical trial series, but 5.2% in the single-arm STASEY trial.
“We shouldn’t assume that other biophenotypics will have a similar ADA rate, and this needs to be evaluated for each molecule, as it will need to be for other monoclonals” such as anti–tissue factor pathway (TFPI) antibodies, Dr. Hart emphasized.
Pegylation
Pegylated compounds include polyethylene glycol, an inert polymer, covalently bound to the therapeutic protein to extend its half-life, and theoretically, reduce immunogenicity.
Many patients may already have exposure to pegylated products in the form of peginterferon to treat hepatitis C, consumer products such as toothpaste, cough medicine, and cosmetics, and, more recently, in vaccines against COVID-19.
Safety considerations with pegylated agents in hemophilia include concerns about accumulation of polyethylene glycol (PEG), although “some of the preclinical models looking at excretion of PEG are difficult to interpret in my view, and people debate about whether studies are long enough, but it’s undoubtedly the case that toxicology dosing is order of magnitude higher than the routine dosing in hemophilia,” he said.
After more than 5 years of experience with pegylated products there is no clinical evidence of concern, although “it’s not clear, actually, what we’re looking for, whether it’s a clinical parameter, or imaging or histological parameter.”
Patients may also not have lifelong exposure to pegylated products, as it is unlikely that they will stay on the same product for decades, Dr. Hart said.
Thrombosis
As of June 30, 2020, more than 7,200 persons with hemophilia have received emicizumab, and there have been 23 reported thrombotic events, 19 of which occurred in the postmarketing period. Of the reported cases, six patients had a myocardial infarction, and all of these patients had at least one cardiovascular risk factor.
The antithrombin agent fitusiran was associated with one fatal thrombotic event in a phase 2, open-label extension trial, leading to a pause and resumption with mitigation protocols, but that trial has since been paused again because of additional, nonfatal thrombotic events.
Nonfatal thrombotic events have also occurred in clinical trials for the investigational anti-TFPI monoclonal antibodies BAY 1093884 and concizumab, but none have thus far been reported in phase 3 trial of marstacimab.
“We need renewed efforts for prospective reporting and independent review of all adverse events of all agents, old and new: This will need some guidance nationally and internationally, and I think the relevant trial [serious adverse events] need to be reported in peer review literature, and clinicaltrials.gov updated in a timely manner, regardless of whether that strategy was successful or unsuccessful,” Dr. Hart said.
Risk with longer-acting agents?
In the question and answer following his presentation, Christoph Königs, MD, PhD, from University Hospital Frankfurt, asked whether there was potential for increased thrombosis risk with second-generation extended half-life (EHL) molecules in clinical trials.
“As we edge towards normalization of hemostasis, clearly the other non–hemophilia dependent issues of thrombosis risk come into play,” Dr. Hart acknowledged. “I think it will be an inevitability that there will be events, and we need to understand what the denominators are – hence my pitch for there being a renewed effort to try and collate sufficient data that we can really define events happening with people treated with standard half-life [products] through into the novel agents,” he said.
Dr. Hart disclosed grant/research support and speaker bureau activities for Bayer, Octapharma, Takeda, and others. Dr. Königs has reported no relevant disclosures.
FROM EAHAD 2021
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
Using engineered T cells reduced acute, chronic GVHD
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
FROM TCT 2021
Transplant-related mortality higher with CD34 selection
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
FROM TCT 2021
Large study finds trans men on testosterone at risk for blood clots
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel ddPCR assay precisely measures CAR T-cells after infusion
A novel quantitative assay used with flow cytometry helps to precisely measure chimeric antigen receptor (CAR) T-cell engraftment and in vivo expansion to predict patient outcomes after CAR T-cell infusion, according to researchers at the Fondazione IRCCS Istituto Nazionale Tumorion in Milan.
Higher frequencies of CAR-positive T cells at day 9 after infusion, as measured using the polymerase chain reaction (PCR)-based assay, accurately distinguished responders from nonresponders, Paolo Corradini, MD, said at the 3rd European CAR T-cell Meeting.
The findings, first presented in December at the American Society of Hematology annual conference, suggest the assay could improve treatment decision-making, Dr. Corradini of the University of Milan said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association
He and his colleagues prospectively collected samples from 16 patients with diffuse large B-cell lymphoma, 5 with transformed follicular lymphoma, and 7 with primary mediastinal B-cell lymphoma who were treated with either axicabtagene ciloleucel (axi-cel; Yescarta) or tisagenlecleucel (tisa-cal; Kymriah) between November 2019 and July 2020. CAR T cells were monitored using flow cytometry.
Pivotal trial data and subsequent findings with respect to tisa-cel and axi-cel have demonstrated that CAR T-cell engraftment and in vivo expansion have a crucial impact on disease response and toxicity: a cut-off value of CAR+ cells at day 9 greater than 24.5/microliters distinguished responders from nonresponders with a sensitivity of 87.5% and specificity of 81%, Dr. Corradini noted.
“But we have also devised a methodology by digital droplet PCR (ddPCR) recently that correlates perfectly with the flow cytometry data,” he said, adding that the assay is “easy and allowed precise enumeration of the CAR T cells in the blood of the patient.”
The R square (coefficient of determination) for ddPCR and flow cytometry was 0.9995 and 0.9997 for tisa-cel and axi-cel, respectively (P < .0001 for each). This is particularly useful for assessing whether low CAR T-cell levels on flow cytometry are background signals resulting from nonspecific binding of the antibodies or true low levels, and the findings therefore have implications for improving clinical decision-making and outcomes in CAR T-cell therapy recipients, he said.
A novel quantitative assay used with flow cytometry helps to precisely measure chimeric antigen receptor (CAR) T-cell engraftment and in vivo expansion to predict patient outcomes after CAR T-cell infusion, according to researchers at the Fondazione IRCCS Istituto Nazionale Tumorion in Milan.
Higher frequencies of CAR-positive T cells at day 9 after infusion, as measured using the polymerase chain reaction (PCR)-based assay, accurately distinguished responders from nonresponders, Paolo Corradini, MD, said at the 3rd European CAR T-cell Meeting.
The findings, first presented in December at the American Society of Hematology annual conference, suggest the assay could improve treatment decision-making, Dr. Corradini of the University of Milan said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association
He and his colleagues prospectively collected samples from 16 patients with diffuse large B-cell lymphoma, 5 with transformed follicular lymphoma, and 7 with primary mediastinal B-cell lymphoma who were treated with either axicabtagene ciloleucel (axi-cel; Yescarta) or tisagenlecleucel (tisa-cal; Kymriah) between November 2019 and July 2020. CAR T cells were monitored using flow cytometry.
Pivotal trial data and subsequent findings with respect to tisa-cel and axi-cel have demonstrated that CAR T-cell engraftment and in vivo expansion have a crucial impact on disease response and toxicity: a cut-off value of CAR+ cells at day 9 greater than 24.5/microliters distinguished responders from nonresponders with a sensitivity of 87.5% and specificity of 81%, Dr. Corradini noted.
“But we have also devised a methodology by digital droplet PCR (ddPCR) recently that correlates perfectly with the flow cytometry data,” he said, adding that the assay is “easy and allowed precise enumeration of the CAR T cells in the blood of the patient.”
The R square (coefficient of determination) for ddPCR and flow cytometry was 0.9995 and 0.9997 for tisa-cel and axi-cel, respectively (P < .0001 for each). This is particularly useful for assessing whether low CAR T-cell levels on flow cytometry are background signals resulting from nonspecific binding of the antibodies or true low levels, and the findings therefore have implications for improving clinical decision-making and outcomes in CAR T-cell therapy recipients, he said.
A novel quantitative assay used with flow cytometry helps to precisely measure chimeric antigen receptor (CAR) T-cell engraftment and in vivo expansion to predict patient outcomes after CAR T-cell infusion, according to researchers at the Fondazione IRCCS Istituto Nazionale Tumorion in Milan.
Higher frequencies of CAR-positive T cells at day 9 after infusion, as measured using the polymerase chain reaction (PCR)-based assay, accurately distinguished responders from nonresponders, Paolo Corradini, MD, said at the 3rd European CAR T-cell Meeting.
The findings, first presented in December at the American Society of Hematology annual conference, suggest the assay could improve treatment decision-making, Dr. Corradini of the University of Milan said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association
He and his colleagues prospectively collected samples from 16 patients with diffuse large B-cell lymphoma, 5 with transformed follicular lymphoma, and 7 with primary mediastinal B-cell lymphoma who were treated with either axicabtagene ciloleucel (axi-cel; Yescarta) or tisagenlecleucel (tisa-cal; Kymriah) between November 2019 and July 2020. CAR T cells were monitored using flow cytometry.
Pivotal trial data and subsequent findings with respect to tisa-cel and axi-cel have demonstrated that CAR T-cell engraftment and in vivo expansion have a crucial impact on disease response and toxicity: a cut-off value of CAR+ cells at day 9 greater than 24.5/microliters distinguished responders from nonresponders with a sensitivity of 87.5% and specificity of 81%, Dr. Corradini noted.
“But we have also devised a methodology by digital droplet PCR (ddPCR) recently that correlates perfectly with the flow cytometry data,” he said, adding that the assay is “easy and allowed precise enumeration of the CAR T cells in the blood of the patient.”
The R square (coefficient of determination) for ddPCR and flow cytometry was 0.9995 and 0.9997 for tisa-cel and axi-cel, respectively (P < .0001 for each). This is particularly useful for assessing whether low CAR T-cell levels on flow cytometry are background signals resulting from nonspecific binding of the antibodies or true low levels, and the findings therefore have implications for improving clinical decision-making and outcomes in CAR T-cell therapy recipients, he said.
REPORTING FROM CART21
Steroid complications in GVHD common, boost costs of care
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
FROM TCT 2021