User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Impact of VA Hematology/Oncology Clinical Pharmacy Practitioners in the Review of Community Prescriptions for Specialty Medications
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
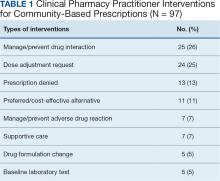
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
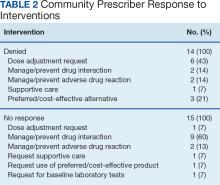
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
The value of a hematology/oncology clinical pharmacy practitioner (CPP) has been validated in several studies documenting their positive impact on patient outcomes, supportive care management, laboratory monitoring, medication error identification, and drug expenditure.1-6 With> 200 oncology-related US Food and Drug Administration approval notifications published from 2020 to 2023, it is no surprise that national trends in oncology drug clinic expenditures increased from $39.9 billion in 2020 to $44.1 billion in 2021.7,8 With the rapidly changing treatment landscape, new drug approvals, and risk of polypharmacy, oral anticancer agents carry a high risk for medication errors.4 Additional challenges include complex dosing regimens and instructions, adherence issues, drug interactions, adjustments for organ dysfunction, and extensive adverse effect (AE) profiles.
Because of the niche and complexity of oral anticancer agents, trained CPPs havehematology/oncology education and expertise that pharmacists without specialized training lack. A survey of 243 nonspecialized community pharmacists that assessed their knowledge of oral anticancer therapies revealed that only about half of the knowledge questions were answered correctly, illustrating an education gap among these pharmacists.9 The Hematology/Oncology Pharmacist Association's suggests that best practices for managing oral oncology therapy should include comprehensive medication review by an oncology-trained pharmacist for each prescription.10
The US Department of Veterans Affairs (VA) community care network, which was established by the MISSION Act, allows covered access for eligible veterans in the local community outside of the VA network. Unfortunately, this dual-system use of health care could increase the risk of poorly coordinated care and has been associated with the risk of inappropriate prescribing.11,12 It is unclear how many private practices enrolled in the community care program have access to oncology-trained pharmacists. Specialized pharmaceutical reviews of oral anticancer medication prescriptions from these practices are vital for veteran care. This study evaluates the clinical and financial interventions of hematology/oncology CPPs review of specialty hematology/oncology prescriptions from community care health care practitioners (HCPs) at the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas.
METHODS
This study is a retrospective review of Computerized Patient Record System (CPRS) records of patients at VANTHCS from January 1, 2015, to June 30, 2023. Patients included were aged ≥ 18 years, enrolled in the VA community care program, received a specialty hematology/oncology medication that was dispensed through VA pharmacies or VA-contracted pharmacies, and had an hematology/oncology CPP medication review documented in CPRS. The primary aim of this study was to assess the number and types of clinical interventions performed. A clinical intervention was defined as a documented communication attempt with a community care HCP or direct communication with a patient to address a specific medication-related issue noted during CPP review.
Review of specialty hematology/oncology medications by a hematology/oncology CPP included evaluation of therapy indication, such as whether the prescription meets clinical guidelines, VA criteria for use, or other clinical literature as judged appropriate by the CPP. In some cases, the CPP requested that the community care HCP prescribe a more cost-effective or formulary-preferred agent. Each prescription was reviewed for dosage and formulation appropriateness, drug interactions with available medication lists, baseline laboratory test completion, and recommended supportive care medicines. At times, patient counseling is completed as part of the clinical review. When necessary, CPPs could discuss patient cases with a VA-employed oncologist for further oversight regarding appropriateness and safety. Secondary outcomes included the number of interventions accepted or denied by the prescriber provider and cost savings.
Data collected included the type of malignancy, hematology/oncology specialty medication requested, number and type of interventions sent to the community care prescriber, number of interventions accepted or denied by the community care prescriber, and whether the CPP conducted patient counseling or dispensed or denied the product. Cost savings were calculated for medications that were denied or changed to a formulary preferred or cost-effective agent using pricing data from the National Acquisition Center Contract Catalog or Federal Supply Schedule Service as of April 2024.
RESULTS
A total of 221 hematology/oncology prescriptions met inclusion criteria. Among patients receiving these prescriptions, the median age was 70 years and 91% were male. The most common malignancies included 31 instances of multiple myeloma (14%), 26 for chronic lymphocytic leukemia (12%), 24 for prostate cancer (11%), 23 for glioblastoma/brain cancer (10%), 18 for renal cell carcinoma (8%), 17 for colorectal cancer (8%), and 15 for acute myeloid leukemia (7%). Clinical interventions by the hematology/oncology CPP were completed for 82 (37%) of the 221 prescriptions. One clinical intervention was communicated directly to the patient, and attempts were made to communicate with the community care HCP for the remaining 81 prescriptions. The CPP documented 97 clinical interventions for the 82 prescriptions (Table 1). The most commonly documented clinical interventions included: 25 for managing/preventing a drug interaction (26%), 24 for dose adjustment request (25%), 13 for prescription denial (13%), and 11 for requesting the use of a preferred or more cost-effective product (11%). Of note, 16 patients (7%) received counseling from the hematology/oncology CPP. Ten patients (5%) received counseling alone with no other intervention and did not meet the definition of a clinical intervention.
The most frequent prescriptions requiring intervention included 8 for enzalutamide, 7 for venetoclax, 6 for ibrutinib, and 5 each for lenalidomide, cabozantinib, and temozolomide. Among the 97 interventions, 68 were approved (70%), 15 received no response (16%), and 14 were denied by the community care HCP (14%). Despite obtaining no response or intervention denial from the community care HCP, hematology/oncology CPPs could approve these prescriptions if clinically appropriate, and their reasoning was documented. Table 2 further describes the types of interventions that were denied or obtained no response by the community care practitioner. Among the prescriptions denied by the hematology/oncology CPP, 11 were rejected for off-label indications and/or did not have support through primary literature, national guidelines, or VA criteria for use. Only 2 prescriptions were denied for safety concerns.
These documented clinical interventions had financial implications. For drugs with available cost data, requesting the use of a preferred/cost-effective product led to estimated savings of at least $263,536 over the study period with some ongoing cost savings. Prescription denials led to further estimated savings of $186,275 per month, although this is limited by the lack of known costs of alternative therapies the community care physicians chose.
DISCUSSION
More than one-third of prescriptions required clinical interventions, and 70% of these interventions were accepted by the community care prescriber, demonstrating the CPP’s essential role. Results indicate that most CPP clinical interventions involved clarifying and correcting doses, managing pertinent drug interactions, and ensuring appropriate use of medications according to clinical and national VA guidelines. Other studies have examined the impact of CPPs on patient care and cancer treatment.5,6 The randomized, multicenter AMBORA trial found that clinical pharmacist support reduced severe AEs and medication errors related to oral anticancer agents.5 The per-patient mean number of medication errors found by pharmacist review was 1.7 (range, 0 to 9), with most medication errors noted at the prescribing stage.5 Suzuki and colleagues analyzed data from 35,062 chemotherapy regimens and found that 53.1% of the chemotherapy prescriptions were modified because of pharmacist interventions.6 The most common reason for prescription modifications was prescription error.
Most of the clinical interventions in this study were accepted by community HCPs, indicating that these prescribers are receptive to hematology/oncology CPP input. Among those with no response, most were in relation to recommendations regarding drug interactions. In most of these cases, the drug interaction was not clinically concerning enough to require a response before the CPP approved the prescription. Therefore, it is unknown whether the outside HCP implemented the clinical recommendations. The most common types of clinical interventions the community care HCP declined were dose adjustment requests or requests to switch to a more cost-effective/formulary-preferred agent. In these cases, the prescriber’s preference was documented and, if clinically appropriate, approved by the CPP.
Although the financial implications of CPP clinical interventions were only marginally evaluated in this review, results suggest that cost savings by requests to switch to a cost-effective/formulary preferred agent or prescription denials are substantial. Because of changes in prescription costs over time, it is possible that savings from CPP intervention were greater than calculations using current Federal Supply Schedule Service pricing. The total impact of CPP prescription interventions on reducing or preventing hospitalizations or AEs is not known from this review, but other data suggest that cost savings may benefit the system.13,14
Limitations
This study's retrospective design is a limitation because practice patterns at the VANTHCS involving multiple hematology/oncology CPPs review of community care prescriptions might have evolved over time. The total financial implications of CPP interventions cannot fully be elucidated. The cost of alternative therapies used for patients who received a prescription denial is not factored into this review.
Conclusions
VANTHCS CPPs played an essential role in reviewing anticancer medication prescriptions from community care prescribers. In this study, CPP clinical interventions were completed for more than one-third of the prescriptions and the community-based HCP approved most of these interventions. These changes also resulted in financial benefits.
These findings add to the body of literature emphasizing the need for hematology/oncology-trained CPPs to review anticancer prescriptions and treatment plans. Our review could be used to justify CPP involvement in community care specialty medication review at VA facilities that do not currently have CPP involvement.
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
1. Shah NN, Casella E, Capozzi D, et al. Improving the safety of oral chemotherapy at an academic medical center. J Oncol Pract. 2016;12(1):e71-e76. doi:10.1200/JOP.2015.007260
2. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557. doi:10.2146/ajhp160475
3. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384. doi:10.18553/jmcp.2021.27.3.379
4. Schlichtig K, Dürr P, Dörje F, Fromm MF. Medication errors during treatment with new oral anticancer agents: consequences for clinical practice based on the AMBORA Study. Clin Pharmacol Ther. 2021;110(4):1075-1086. doi:10.1002/cpt.2338
5. Dürr P, Schlichtig K, Kelz C, et al. The randomized AMBORA Trial: impact of pharmacological/pharmaceutical care on medication safety and patient-reported outcomes during treatment with new oral anticancer agents. J Clin Oncol. 2021;39(18):1983-1994. doi:10.1200/JCO.20.03088
6. Suzuki S, Chan A, Nomura H, Johnson PE, Endo K, Saito S. Chemotherapy regimen checks performed by pharmacists contribute to safe administration of chemotherapy. J Oncol Pharm Pract. 2017;23(1):18-25. doi:10.1177/1078155215614998
7. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2022. Am J Health Syst Pharm. 2022;79(14):1158-1172. doi:10.1093/ajhp/zxac102
8. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. 2023.
9. O’Bryant CL, Crandell BC. Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. J Am Pharm Assoc (2003). 2008;48(5):632-639. doi:10.1331/JAPhA.2008.07082
10. Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 hematology/oncology pharmacist association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355. doi:10.1200/JOP.18.00581
11. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788
12. Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
13. Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866-872. doi:10.1177/1078155219875806
14. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
Circulating Tumor DNA Hints at BC Recurrence Risk
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
FROM ASCO 2024
Is Immunotherapy Best for Unresectable HCC with Moderate Liver Dysfunction?
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
In the last 10 years, clinical outcomes have improved for patients with unresectable hepatocellular carcinoma (uHCC). The cancer generally comes with chronic liver inflammation, and liver cirrhosis is present in up to 80% of cases.
Clinical trials that have tested systemic immunotherapies have excluded patients who don’t fall into the Child-Pugh class A criteria (CP-A) for liver disease, which is the least severe of the Child-Pugh classes A-C. Therefore, there has been much debate about whether patients who have more liver disease (moderate liver dysfunction) and fit under CP-B criteria, instead of CP-A, should be treated with immune checkpoint inhibitor (ICI) therapy or best supportive care (BSC).
A new study, led by Claudia Angela Maria Fulgenzi, MD, with the Department of Surgery and Cancer at the Imperial College London, England, published in JAMA Oncology on July 18, uses an alternative way to compare outcomes following two different paths of care for uHCC patients with moderate liver dysfunction.
How was the study done and what did the investigators find?
Researchers performed a retrospective, multicenter, international clinical case series of patients treated in routine practice in tertiary care centers across Europe, the United States, and Asia. They compared data from uHCC patients with CP-B who were receiving first-line ICI-based treatment regimens (n = 187) with a cohort of matched patients with CP-B receiving BSC (n = 156). The first-line immunotherapies were the monotherapy nivolumab or the combination (atezolizumab plus bevacizumab).
Immunotherapy was linked with significantly lower risk of death, compared with best supportive care.
ICI exposure was associated with a reduction of about 50% in the risk of death (hazard ratio, 0.55; 95% CI, 0.35-0.86; P < .001).
Is immunotherapy or best supportive care the superior treatment?
The authors wrote that the results point to “improved survival in association with ICI treatment, compared with BSC in patients with uHCC with CP-B liver dysfunction.”
According to the study’s senior author David Pinato, MD, PhD, “this is the first study to suggest that there might be an advantage [of treatment with immunotherapy] in a proportion of people with Child-Pugh B liver dysfunction and particularly so in those patients with more limited disease and portal vein tumor thrombosis.”
Will the findings of this study make treatment allocation for patients with uHCC and moderate liver dysfunction (CP-B) less controversial?
Because it is a retrospective study, Dr. Pinato said in an interview, that the findings are not definitive, but can be used to inform future randomized controlled trials.
Dr. Pinato, who is also with the Imperial College London, added that the findings may also introduce a new question.
Although the study was not powered to look at survival differences across the two immunotherapy options given to the patients, there did not seem to be a striking difference between using one immunotherapy (nivolumab) or a combination (atezolizumab plus bevacizumab), he said.
“This is quite important because we know that combinations are significantly superior to monotherapy in patients with normal liver function but based on our study we might say that this provides preliminary evidence that [superiority of combination therapy] might not be true if the liver function is worse.”
What do these findings add to the literature about how best to treat patients with uHCC and suboptimal liver function?
Without evidence of efficacy and safety for the group in previous studies, the widespread recommendation for those with moderate dysfunction has been BSC.
These findings “pave the way to select potential patient subgroups in clinical practice,” Dr. Pinato said. It also suggests that the safety level of immunotherapy treatments is acceptable in this patient population, so they are not necessarily disadvantaged compared to patients with more preserved liver function.
“This is the best level of evidence currently available to guide treatment decisions in patients with Child-Pugh B who have been universally excluded by prospective clinical trials and for whom there is no randomized comparison,” Dr. Pinato said.
Dr. Pinato reported personal fees from Roche, AstraZeneca, Eisai, Mina Therapeutics, Starpharma, Lift Biosciences, Boston Scientific, and Avammune, and grants from GSK, MSD, and BMS outside the submitted work. Dr. Fulgenzi has no disclosures. Other authors of the new research have multiple ties with pharmaceutical companies. Complete disclosures are available with the full text of the journal article.
FROM JAMA ONCOLOGY
‘Chemoresistance Can Be Reversed’: Toughest Cancers Targeted
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.
In the war against cancer, doctors and patients have long reached for three main weapons to target diseased cells: chemotherapy, radiation, and surgery.
But new research published this month in the journal Nature Materials suggests that manipulating the tissue around those cells — a strategy known as
“Our study shows the importance of the tumor microenvironment and its properties in dictating how cancer progresses and responds to drug treatment,” said first author Bauer LeSavage, PhD, who conducted the study as a postdoctoral researcher in the Bioengineering Department at Stanford University, Stanford, California. “It also demonstrates that chemoresistance can be reversed.”
Each year, about 66,000 people are diagnosed with pancreatic cancer, and 52,000 die from it. It is a particularly lethal type of cancer, with 5-year survival rates hovering around 7% — a rate that has not improved much since 1996 when the first-line chemotherapy drug gemcitabine was approved.
It looks different from many cancers, said Lynn Matrisian, PhD, chief science officer for the nonprofit Pancreatic Cancer Action Network. Instead of a tumorous mass, it is made of islands of cancer cells surrounded by unusually dense fibrous tissue known as the extracellular matrix, which can collapse blood vessels and prevent drugs from reaching the tumor.
For the study, Dr. LeSavage and his team engineered synthetic but lifelike three-dimensional pancreas tissue with varying degrees of stiffness and different biochemical properties. Then they inserted bits of real tumors from patients with pancreatic cancer, watched them grow, and tried to kill them with drugs.
They found that cells growing in a stiff matrix were more resistant to chemotherapy than those growing in a softer matrix. But the story didn’t end there.
They also found that high amounts of the tissue-strengthening protein hyaluronic acid in stiff tissue seemed to signal the cancer cells to develop tiny pumps on their surface which shuttled out the drugs before they could take effect.
When the researchers moved the cancer cells into either a softer matrix or a stiff matrix in which the hyaluronic acid receptor, called CD44, was blocked, the chemotherapy drugs started working again.
“This suggests that if we can disrupt the stiffness signaling that’s happening through the CD44 receptor, we could make patients’ pancreatic cancer treatable again by normal chemotherapy,” said senior study author Sarah Heilshorn, PhD, a professor of materials science and engineering at Stanford. “These results suggest an exciting new direction for new drug development.”
Targeting Nearby Tissue: A Novel Approach to Fighting Chemoresistance
The study is not the first to suggest that chemically targeting the microenvironment surrounding a tumor can influence how patients respond to treatment.
In one recent clinical trial, patients with metastatic pancreatic cancer were given an experimental drug to inhibit a protein called connective tissue growth factor, reduce fibrous tissue, and make pancreatic tumors easier to surgically remove. Results have not been published yet.
Other research suggests that the generic blood pressure drug losartan, when given in combination with chemotherapy and radiation, can boost survival in patients with advanced pancreatic cancer by, in part, improving the health of blood vessels that carry drugs to the tumor.
But other studies of such mechanotherapeutics have yielded inconclusive results, said Dr. Matrisian.
“This paper points to another reason why we should not give up on this approach,” she said.
Ning Wang, PhD, director of the new Institute for Mechanobiology at Northeastern University College of Engineering, Boston, said there is no question that the composition of a tumor’s environment can influence how cancer progresses or responds to drugs. The new paper, he said, adds an important new chapter to the evolving story.
“But it’s very complicated. It’s not as simple as saying make it softer or stiffer and you can change the outcome for the patient,” Dr. Wang said.
In fact, some research has shown that tissue becomes stiffer when cancer arises so it can contain it from spreading.
In one animal study of pancreatic cancer that had spread to the liver, administering drugs to soften the surrounding tissue, or stroma, actually had the opposite effect — accelerating tumor growth and reducing survival rates.
Dr. Wang also noted that any drug designed to influence the extracellular matrix would need to be extremely localized, to prevent damage to other tissues, like bone or heart muscle.
Dr. LeSavage said he sees the paper as a case study in how important the extracellular matrix is and an example of how artificially grown organs or tissues can play a key role in testing how drugs work or don’t work.
He imagines a day when doctors could personalize treatments by taking a bit of a patient’s tumor, growing it in artificial tissue, and seeing how different tissue-altering drugs affect different therapies.
“This isn’t something that is just unique to pancreatic cancer,” he said, noting that the extracellular matrix throughout the body interacts with different cancers. “If we could take someone who has a chemoresistant tumor and convert it into something that is sensitive to existing therapies again, we could give them a second chance.”
A version of this article appeared on Medscape.com.
Targeted Pancreatic Cancer Screening May Save Lives
TOPLINE:
METHODOLOGY:
- Pancreatic ductal adenocarcinoma has poor 5-year survival rates and is often detected at later stages. General population screening is not recommended, but high-risk individuals, such as those with familial or genetic predispositions, may benefit from regular surveillance.
- The Cancer of the Pancreas Screening (CAPS) program, initiated in 1998, has been evaluating the effectiveness of such targeted surveillance for over two decades, but whether targeted surveillance confers a survival benefit remains unclear.
- The current study evaluated 26 high-risk individuals in the CAPS program who were ultimately diagnosed with pancreatic ductal adenocarcinoma. These high-risk individuals had undergone surveillance with annual endoscopic ultrasonography or MRI prior to diagnosis.
- The researchers compared these 26 individuals with 1504 matched control patients with pancreatic ductal adenocarcinoma from the Surveillance, Epidemiology, and End Results (SEER) database. The high-risk individuals and SEER control patients were matched on age, sex, and year of diagnosis.
- The primary outcomes were tumor stage at diagnosis, overall survival, and pancreatic cancer-specific mortality.
TAKEAWAY:
- High-risk individuals were significantly more likely to be diagnosed with early-stage pancreatic cancer: 38.5% were diagnosed at stage I vs 10.3% in the general US population, and 30.8% were diagnosed at stage II vs 25.1% in the general US population (P < .001).
- The median tumor size at diagnosis was smaller in high-risk individuals than in control patients (2.5 vs 3.6 cm; P < .001), and significantly fewer high-risk individuals had distant metastases at diagnosis (M1 stage) vs control patients (26.9% vs 53.8%; P = .01).
- Overall, high-risk individuals lived about 4.5 years longer — median of 61.7 months vs 8 months for control patients (hazard ratio [HR], 4.19; P < .001). In the 20 high-risk patients with screen-detected cancer, median overall survival was even higher at 144 months.
- The probability of surviving 5 years was significantly better in the high-risk group (50%) than in the control group (9%). And at 5 years, high-risk individuals had a significantly lower probability of dying from pancreatic cancer (HR, 3.58; P < .001).
IN PRACTICE:
Surveillance of high-risk individuals led to detection of “smaller pancreatic cancers, a greater number of patients with stage I disease,” as well as “a much higher likelihood of long-term survival than unscreened patients in the general population,” the authors concluded. “These findings suggest that selective surveillance of individuals at high risk for pancreatic cancer may improve clinical outcomes.”
SOURCE:
This study, with first author Amanda L. Blackford, from Johns Hopkins Medical Institutions, Baltimore, was published online July 3 in JAMA Oncology.
LIMITATIONS:
The findings might have limited generalizability due to enrollment at academic referral centers, limited racial and ethnic diversity, and a small number of high-risk individuals progressing to pancreatic cancer. The study also lacked a control group of unscreened high-risk individuals.
DISCLOSURES:
This study was supported by the National Institutes of Health, Susan Wojcicki and Dennis Troper, and others. Several authors reported financial ties outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Pancreatic ductal adenocarcinoma has poor 5-year survival rates and is often detected at later stages. General population screening is not recommended, but high-risk individuals, such as those with familial or genetic predispositions, may benefit from regular surveillance.
- The Cancer of the Pancreas Screening (CAPS) program, initiated in 1998, has been evaluating the effectiveness of such targeted surveillance for over two decades, but whether targeted surveillance confers a survival benefit remains unclear.
- The current study evaluated 26 high-risk individuals in the CAPS program who were ultimately diagnosed with pancreatic ductal adenocarcinoma. These high-risk individuals had undergone surveillance with annual endoscopic ultrasonography or MRI prior to diagnosis.
- The researchers compared these 26 individuals with 1504 matched control patients with pancreatic ductal adenocarcinoma from the Surveillance, Epidemiology, and End Results (SEER) database. The high-risk individuals and SEER control patients were matched on age, sex, and year of diagnosis.
- The primary outcomes were tumor stage at diagnosis, overall survival, and pancreatic cancer-specific mortality.
TAKEAWAY:
- High-risk individuals were significantly more likely to be diagnosed with early-stage pancreatic cancer: 38.5% were diagnosed at stage I vs 10.3% in the general US population, and 30.8% were diagnosed at stage II vs 25.1% in the general US population (P < .001).
- The median tumor size at diagnosis was smaller in high-risk individuals than in control patients (2.5 vs 3.6 cm; P < .001), and significantly fewer high-risk individuals had distant metastases at diagnosis (M1 stage) vs control patients (26.9% vs 53.8%; P = .01).
- Overall, high-risk individuals lived about 4.5 years longer — median of 61.7 months vs 8 months for control patients (hazard ratio [HR], 4.19; P < .001). In the 20 high-risk patients with screen-detected cancer, median overall survival was even higher at 144 months.
- The probability of surviving 5 years was significantly better in the high-risk group (50%) than in the control group (9%). And at 5 years, high-risk individuals had a significantly lower probability of dying from pancreatic cancer (HR, 3.58; P < .001).
IN PRACTICE:
Surveillance of high-risk individuals led to detection of “smaller pancreatic cancers, a greater number of patients with stage I disease,” as well as “a much higher likelihood of long-term survival than unscreened patients in the general population,” the authors concluded. “These findings suggest that selective surveillance of individuals at high risk for pancreatic cancer may improve clinical outcomes.”
SOURCE:
This study, with first author Amanda L. Blackford, from Johns Hopkins Medical Institutions, Baltimore, was published online July 3 in JAMA Oncology.
LIMITATIONS:
The findings might have limited generalizability due to enrollment at academic referral centers, limited racial and ethnic diversity, and a small number of high-risk individuals progressing to pancreatic cancer. The study also lacked a control group of unscreened high-risk individuals.
DISCLOSURES:
This study was supported by the National Institutes of Health, Susan Wojcicki and Dennis Troper, and others. Several authors reported financial ties outside this work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Pancreatic ductal adenocarcinoma has poor 5-year survival rates and is often detected at later stages. General population screening is not recommended, but high-risk individuals, such as those with familial or genetic predispositions, may benefit from regular surveillance.
- The Cancer of the Pancreas Screening (CAPS) program, initiated in 1998, has been evaluating the effectiveness of such targeted surveillance for over two decades, but whether targeted surveillance confers a survival benefit remains unclear.
- The current study evaluated 26 high-risk individuals in the CAPS program who were ultimately diagnosed with pancreatic ductal adenocarcinoma. These high-risk individuals had undergone surveillance with annual endoscopic ultrasonography or MRI prior to diagnosis.
- The researchers compared these 26 individuals with 1504 matched control patients with pancreatic ductal adenocarcinoma from the Surveillance, Epidemiology, and End Results (SEER) database. The high-risk individuals and SEER control patients were matched on age, sex, and year of diagnosis.
- The primary outcomes were tumor stage at diagnosis, overall survival, and pancreatic cancer-specific mortality.
TAKEAWAY:
- High-risk individuals were significantly more likely to be diagnosed with early-stage pancreatic cancer: 38.5% were diagnosed at stage I vs 10.3% in the general US population, and 30.8% were diagnosed at stage II vs 25.1% in the general US population (P < .001).
- The median tumor size at diagnosis was smaller in high-risk individuals than in control patients (2.5 vs 3.6 cm; P < .001), and significantly fewer high-risk individuals had distant metastases at diagnosis (M1 stage) vs control patients (26.9% vs 53.8%; P = .01).
- Overall, high-risk individuals lived about 4.5 years longer — median of 61.7 months vs 8 months for control patients (hazard ratio [HR], 4.19; P < .001). In the 20 high-risk patients with screen-detected cancer, median overall survival was even higher at 144 months.
- The probability of surviving 5 years was significantly better in the high-risk group (50%) than in the control group (9%). And at 5 years, high-risk individuals had a significantly lower probability of dying from pancreatic cancer (HR, 3.58; P < .001).
IN PRACTICE:
Surveillance of high-risk individuals led to detection of “smaller pancreatic cancers, a greater number of patients with stage I disease,” as well as “a much higher likelihood of long-term survival than unscreened patients in the general population,” the authors concluded. “These findings suggest that selective surveillance of individuals at high risk for pancreatic cancer may improve clinical outcomes.”
SOURCE:
This study, with first author Amanda L. Blackford, from Johns Hopkins Medical Institutions, Baltimore, was published online July 3 in JAMA Oncology.
LIMITATIONS:
The findings might have limited generalizability due to enrollment at academic referral centers, limited racial and ethnic diversity, and a small number of high-risk individuals progressing to pancreatic cancer. The study also lacked a control group of unscreened high-risk individuals.
DISCLOSURES:
This study was supported by the National Institutes of Health, Susan Wojcicki and Dennis Troper, and others. Several authors reported financial ties outside this work.
A version of this article appeared on Medscape.com.
Could an EHR Nudge Reduce Unnecessary Biopsies?
Participating surgeons noted that the reminder system added minimal friction to their workflow, as it did not require additional clicks or actions on the day of the patient visit, reported lead author Neil Carleton, PhD, of UPMC Hillman Cancer Center, Pittsburgh, and colleagues in JAMA Surgery (JAMA Surg. 2024 Jul 17. doi: 10.1001/jamasurg.2024.2407).
This effort to reduce the rate of SLNB stems from the Choosing Wisely campaign, which recommends against axillary staging in women 70 years and older with early-stage, clinically node-negative (cN0), hormone receptor–positive (HR+) breast cancer, the investigators said.
“These recommendations were developed because axillary staging did not impact survival, and rates of SLN positivity were low because of the tumor’s biological phenotype,” they wrote. “Even in older patients with tumors that exhibit concerning clinicopathologic features, limited nodal involvement does not often alter receipt of chemotherapy independently from genomic testing. Despite these recommendations, most women still receive axillary surgery.”
How Did the Nudge System Aim to Reduce the Rate of SLNB?
The nudge intervention involved adding a new column to the Epic schedule view, which flagged eligible patients during their first outpatient surgical consultation. The flag appeared as a caution sign or red clipboard icon. When surgeons hovered over the icon, a text box appeared, reminding them to consider omitting SLNB after a detailed review of core biopsy pathology and ultrasonographic imaging.
The intervention was evaluated at eight outpatient clinics within an integrated healthcare system that included seven breast surgical oncologists.
The study began with a 12-month preintervention period to serve as a control, during which time SLNB rate was determined via 194 patients in the target demographic. SLNB rate was again collected during the 12-month intervention period, which involved 193 patients meeting enrollment criteria. Between these periods, the investigators conducted a brief session lasting less than 30 minutes to introduce the surgeons to the rationale and design of the nudge column.
How Effective Was the Nudge System?
The intervention reduced the SLNB rate from 46.9% to 23.8%, representing a 49.3% decrease in use of SLNB. Efficacy was further supported by a significant reduction in SLNB according to an interrupted time series model (adjusted odds ratio, 0.26; 95% confidence interval, 0.07 to 0.90; P = .03). Extended follow-up showed that this effect was durable beyond the intervention period, with a 6-month mean reduction in SLNB of 15.6%.
Omission of SLNB led to higher rates of pathological node positivity during the intervention period (15.2% vs 8.8%), with all positive cases staged as pN1. Adjuvant therapy recommendations were similar between groups and driven by genomic testing, not nodal status. The intervention period also saw a decrease in referrals for lymphedema evaluation (3.6% vs. 6.2%).
How Might the Nudge System Be Implemented in Other Practices?
Although the SLNB nudge system was effective in the present study, likelihood of uptake among practices could vary widely, according to Anne M. Wallace, MD, professor of clinical surgery at UC San Diego Health and director of the Moores Comprehensive Breast Health Program.
On a fundamental level, not all centers use Epic software, which could present issues with compatibility, Dr. Wallace said in an interview. More importantly, she added, many institutions already have EHR-based alerts and reminders in place, so it is not always feasible to add a new nudge for every possible clinical scenario.
“Already there are so many little icons that we have to go through now when we close a note,” she said. “That’s why electronic medical records are becoming one of the leading stressors in medicine.”
This presents a more complex challenge, Dr. Wallace said, particularly as potentially practice-changing data are becoming available, and physicians may not have time to learn about them and integrate them into routine practice. She suggested that the present system may be most appropriate for oncologists in solo practice, or in small group practices where it is more challenging to have routine conversations about changing standards of care.
What Are the Risks of Using the Nudge System?
One of those conversations may surround the validity of the recommendation implemented in the present study.
Although the Society of Surgical Oncology recommends against SLNB in the described demographic, other experts, including Dr. Wallace, take a more nuanced view of the decision.
She noted that some patients with a chronological age of 70 may have a lower biological age, casting doubt on the legitimacy of the age threshold, and those near the threshold may wish to make the decision about staging for themselves.
Beyond these concerns, Dr. Wallace described two potential risks involved in forgoing SLNB.
First, there’s the potential for underestimating the tumor’s severity, she said, as this could mean a trip back to the operating room. A tumor initially thought to be low-grade might later be found to be high-grade, necessitating further surgery. Some patients might refuse additional surgery, leaving the more aggressive tumor untreated.
Second, the nudge system could complicate radiation treatment decisions, Dr. Wallace said. Without full nodal status, some radiation oncologists might push for additional radiation therapy, which incurs a greater treatment burden than SNLB.
What Are Some Alternatives to the Nudge System?
After discussing the strengths and weaknesses of the present EHR-based nudge system, and others like it, Dr. Wallace returned to the importance of ongoing communication among colleagues managing complex cases.
At UC San Diego Health, where oncologists meet weekly for a 2-hour breast cancer conference, “we nudge each other,” she said.
This study was supported by the Shear Family Foundation, UPMC eRecord Ambulatory Decision Support and Analytics, UPMC Hillman Cancer Center Biostatistics Facility, and National Institutes of Health. The investigators disclosed relationships with Pfizer, Amgen, the Lewin Group, and Milestone Pennsylvania, and others.
Participating surgeons noted that the reminder system added minimal friction to their workflow, as it did not require additional clicks or actions on the day of the patient visit, reported lead author Neil Carleton, PhD, of UPMC Hillman Cancer Center, Pittsburgh, and colleagues in JAMA Surgery (JAMA Surg. 2024 Jul 17. doi: 10.1001/jamasurg.2024.2407).
This effort to reduce the rate of SLNB stems from the Choosing Wisely campaign, which recommends against axillary staging in women 70 years and older with early-stage, clinically node-negative (cN0), hormone receptor–positive (HR+) breast cancer, the investigators said.
“These recommendations were developed because axillary staging did not impact survival, and rates of SLN positivity were low because of the tumor’s biological phenotype,” they wrote. “Even in older patients with tumors that exhibit concerning clinicopathologic features, limited nodal involvement does not often alter receipt of chemotherapy independently from genomic testing. Despite these recommendations, most women still receive axillary surgery.”
How Did the Nudge System Aim to Reduce the Rate of SLNB?
The nudge intervention involved adding a new column to the Epic schedule view, which flagged eligible patients during their first outpatient surgical consultation. The flag appeared as a caution sign or red clipboard icon. When surgeons hovered over the icon, a text box appeared, reminding them to consider omitting SLNB after a detailed review of core biopsy pathology and ultrasonographic imaging.
The intervention was evaluated at eight outpatient clinics within an integrated healthcare system that included seven breast surgical oncologists.
The study began with a 12-month preintervention period to serve as a control, during which time SLNB rate was determined via 194 patients in the target demographic. SLNB rate was again collected during the 12-month intervention period, which involved 193 patients meeting enrollment criteria. Between these periods, the investigators conducted a brief session lasting less than 30 minutes to introduce the surgeons to the rationale and design of the nudge column.
How Effective Was the Nudge System?
The intervention reduced the SLNB rate from 46.9% to 23.8%, representing a 49.3% decrease in use of SLNB. Efficacy was further supported by a significant reduction in SLNB according to an interrupted time series model (adjusted odds ratio, 0.26; 95% confidence interval, 0.07 to 0.90; P = .03). Extended follow-up showed that this effect was durable beyond the intervention period, with a 6-month mean reduction in SLNB of 15.6%.
Omission of SLNB led to higher rates of pathological node positivity during the intervention period (15.2% vs 8.8%), with all positive cases staged as pN1. Adjuvant therapy recommendations were similar between groups and driven by genomic testing, not nodal status. The intervention period also saw a decrease in referrals for lymphedema evaluation (3.6% vs. 6.2%).
How Might the Nudge System Be Implemented in Other Practices?
Although the SLNB nudge system was effective in the present study, likelihood of uptake among practices could vary widely, according to Anne M. Wallace, MD, professor of clinical surgery at UC San Diego Health and director of the Moores Comprehensive Breast Health Program.
On a fundamental level, not all centers use Epic software, which could present issues with compatibility, Dr. Wallace said in an interview. More importantly, she added, many institutions already have EHR-based alerts and reminders in place, so it is not always feasible to add a new nudge for every possible clinical scenario.
“Already there are so many little icons that we have to go through now when we close a note,” she said. “That’s why electronic medical records are becoming one of the leading stressors in medicine.”
This presents a more complex challenge, Dr. Wallace said, particularly as potentially practice-changing data are becoming available, and physicians may not have time to learn about them and integrate them into routine practice. She suggested that the present system may be most appropriate for oncologists in solo practice, or in small group practices where it is more challenging to have routine conversations about changing standards of care.
What Are the Risks of Using the Nudge System?
One of those conversations may surround the validity of the recommendation implemented in the present study.
Although the Society of Surgical Oncology recommends against SLNB in the described demographic, other experts, including Dr. Wallace, take a more nuanced view of the decision.
She noted that some patients with a chronological age of 70 may have a lower biological age, casting doubt on the legitimacy of the age threshold, and those near the threshold may wish to make the decision about staging for themselves.
Beyond these concerns, Dr. Wallace described two potential risks involved in forgoing SLNB.
First, there’s the potential for underestimating the tumor’s severity, she said, as this could mean a trip back to the operating room. A tumor initially thought to be low-grade might later be found to be high-grade, necessitating further surgery. Some patients might refuse additional surgery, leaving the more aggressive tumor untreated.
Second, the nudge system could complicate radiation treatment decisions, Dr. Wallace said. Without full nodal status, some radiation oncologists might push for additional radiation therapy, which incurs a greater treatment burden than SNLB.
What Are Some Alternatives to the Nudge System?
After discussing the strengths and weaknesses of the present EHR-based nudge system, and others like it, Dr. Wallace returned to the importance of ongoing communication among colleagues managing complex cases.
At UC San Diego Health, where oncologists meet weekly for a 2-hour breast cancer conference, “we nudge each other,” she said.
This study was supported by the Shear Family Foundation, UPMC eRecord Ambulatory Decision Support and Analytics, UPMC Hillman Cancer Center Biostatistics Facility, and National Institutes of Health. The investigators disclosed relationships with Pfizer, Amgen, the Lewin Group, and Milestone Pennsylvania, and others.
Participating surgeons noted that the reminder system added minimal friction to their workflow, as it did not require additional clicks or actions on the day of the patient visit, reported lead author Neil Carleton, PhD, of UPMC Hillman Cancer Center, Pittsburgh, and colleagues in JAMA Surgery (JAMA Surg. 2024 Jul 17. doi: 10.1001/jamasurg.2024.2407).
This effort to reduce the rate of SLNB stems from the Choosing Wisely campaign, which recommends against axillary staging in women 70 years and older with early-stage, clinically node-negative (cN0), hormone receptor–positive (HR+) breast cancer, the investigators said.
“These recommendations were developed because axillary staging did not impact survival, and rates of SLN positivity were low because of the tumor’s biological phenotype,” they wrote. “Even in older patients with tumors that exhibit concerning clinicopathologic features, limited nodal involvement does not often alter receipt of chemotherapy independently from genomic testing. Despite these recommendations, most women still receive axillary surgery.”
How Did the Nudge System Aim to Reduce the Rate of SLNB?
The nudge intervention involved adding a new column to the Epic schedule view, which flagged eligible patients during their first outpatient surgical consultation. The flag appeared as a caution sign or red clipboard icon. When surgeons hovered over the icon, a text box appeared, reminding them to consider omitting SLNB after a detailed review of core biopsy pathology and ultrasonographic imaging.
The intervention was evaluated at eight outpatient clinics within an integrated healthcare system that included seven breast surgical oncologists.
The study began with a 12-month preintervention period to serve as a control, during which time SLNB rate was determined via 194 patients in the target demographic. SLNB rate was again collected during the 12-month intervention period, which involved 193 patients meeting enrollment criteria. Between these periods, the investigators conducted a brief session lasting less than 30 minutes to introduce the surgeons to the rationale and design of the nudge column.
How Effective Was the Nudge System?
The intervention reduced the SLNB rate from 46.9% to 23.8%, representing a 49.3% decrease in use of SLNB. Efficacy was further supported by a significant reduction in SLNB according to an interrupted time series model (adjusted odds ratio, 0.26; 95% confidence interval, 0.07 to 0.90; P = .03). Extended follow-up showed that this effect was durable beyond the intervention period, with a 6-month mean reduction in SLNB of 15.6%.
Omission of SLNB led to higher rates of pathological node positivity during the intervention period (15.2% vs 8.8%), with all positive cases staged as pN1. Adjuvant therapy recommendations were similar between groups and driven by genomic testing, not nodal status. The intervention period also saw a decrease in referrals for lymphedema evaluation (3.6% vs. 6.2%).
How Might the Nudge System Be Implemented in Other Practices?
Although the SLNB nudge system was effective in the present study, likelihood of uptake among practices could vary widely, according to Anne M. Wallace, MD, professor of clinical surgery at UC San Diego Health and director of the Moores Comprehensive Breast Health Program.
On a fundamental level, not all centers use Epic software, which could present issues with compatibility, Dr. Wallace said in an interview. More importantly, she added, many institutions already have EHR-based alerts and reminders in place, so it is not always feasible to add a new nudge for every possible clinical scenario.
“Already there are so many little icons that we have to go through now when we close a note,” she said. “That’s why electronic medical records are becoming one of the leading stressors in medicine.”
This presents a more complex challenge, Dr. Wallace said, particularly as potentially practice-changing data are becoming available, and physicians may not have time to learn about them and integrate them into routine practice. She suggested that the present system may be most appropriate for oncologists in solo practice, or in small group practices where it is more challenging to have routine conversations about changing standards of care.
What Are the Risks of Using the Nudge System?
One of those conversations may surround the validity of the recommendation implemented in the present study.
Although the Society of Surgical Oncology recommends against SLNB in the described demographic, other experts, including Dr. Wallace, take a more nuanced view of the decision.
She noted that some patients with a chronological age of 70 may have a lower biological age, casting doubt on the legitimacy of the age threshold, and those near the threshold may wish to make the decision about staging for themselves.
Beyond these concerns, Dr. Wallace described two potential risks involved in forgoing SLNB.
First, there’s the potential for underestimating the tumor’s severity, she said, as this could mean a trip back to the operating room. A tumor initially thought to be low-grade might later be found to be high-grade, necessitating further surgery. Some patients might refuse additional surgery, leaving the more aggressive tumor untreated.
Second, the nudge system could complicate radiation treatment decisions, Dr. Wallace said. Without full nodal status, some radiation oncologists might push for additional radiation therapy, which incurs a greater treatment burden than SNLB.
What Are Some Alternatives to the Nudge System?
After discussing the strengths and weaknesses of the present EHR-based nudge system, and others like it, Dr. Wallace returned to the importance of ongoing communication among colleagues managing complex cases.
At UC San Diego Health, where oncologists meet weekly for a 2-hour breast cancer conference, “we nudge each other,” she said.
This study was supported by the Shear Family Foundation, UPMC eRecord Ambulatory Decision Support and Analytics, UPMC Hillman Cancer Center Biostatistics Facility, and National Institutes of Health. The investigators disclosed relationships with Pfizer, Amgen, the Lewin Group, and Milestone Pennsylvania, and others.
FROM JAMA SURGERY
Does Extended Postop Follow-Up Improve Survival in Gastric Cancer?
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Factors Linked to Complete Response, Survival in Pancreatic Cancer
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
How Aspirin May Lower Risk for Colorectal Cancer
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
Genetics and Lifestyle Choices Can Affect Early Prostate Cancer Deaths
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.