User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Breakthrough Blood Test for Colorectal Cancer Gets Green Light
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
FDA Calls AstraZeneca’s NSCLC Trial Design Into Question
The trial in question, AEGEAN, investigated perioperative durvalumab for resectable NSCLC tumors across 802 patients. Patients without EGFR or ALK mutations were randomly assigned to receive durvalumab before surgery alongside platinum-containing chemotherapy and after surgery for a year as monotherapy or to receive chemotherapy and surgery alone.
Patients receiving durvalumab demonstrated better event-free survival at 1 year (73.4% vs 64.5% without durvalumab) and a better pathologic complete response rate (17.2% vs 4.3% without). Currently, AstraZeneca is seeking to add the indication for durvalumab to those the agent already has.
However, at the July 25 ODAC meeting, the committee explained that the AEGEAN trial design makes it impossible to tell whether patients benefited from durvalumab before surgery, after it, or at both points.
Mounting evidence, including from AstraZeneca’s own studies, suggests that the benefit of immune checkpoint inhibitors, such as durvalumab, comes before surgery. That means prescribing durvalumab after surgery could be exposing patients to serious side effects and financial toxicity, with potentially no clinical benefit, “magnifying the risk of potential overtreatment,” the committee cautioned.
When AEGEAN was being designed in 2018, FDA requested that AstraZeneca address the uncertainty surrounding when to use durvalumab by including separate neoadjuvant and adjuvant arms, or at least an arm where patients were treated with neoadjuvant durvalumab alone to compare with treatment both before and after surgery.
The company didn’t follow through and, during the July 25 meeting, the committee wanted answers. “Why did you not comply with this?” asked ODAC committee acting chair Daniel Spratt, MD, a radiation oncologist at Case Western Reserve University in Cleveland, Ohio.
AstraZeneca personnel explained that doing so would have required many more subjects, made the trial more expensive, and added about 2 years to AEGEAN.
One speaker noted that the company, which makes more than $4 billion a year on durvalumab, would have taken about 2 days to recoup that added cost. Others wondered whether the motive was to sell durvalumab for as long as possible across a patient’s course of treatment.
Perhaps the biggest reason the company ignored the request is that “it wasn’t our understanding at that time that this was a barrier to approval,” an AstraZeneca regulatory affairs specialist said.
To this end, the agency asked its advisory panel to vote on whether it should require — instead of simply request, as it did with AstraZeneca — companies to prove that patients need immunotherapy both before and after surgery in resectable NSCLC.
The 11-member panel voted unanimously that it should make this a requirement, and several members said it should do so in other cancers as well.
However, when the agency asked whether durvalumab’s resectable NSCLC approval should be delayed until AstraZeneca conducts a trial to answer the neoadjuvant vs adjuvant question, the panel members didn’t think so.
The consensus was that because AEGEAN showed a decent benefit, patients and physicians should have it as an option, and approval shouldn’t be delayed. The panel said that the bigger question about the benefit of maintenance therapy should be left to future studies.
FDA usually follows the advice of its advisory panels.
A version of this article appeared on Medscape.com.
The trial in question, AEGEAN, investigated perioperative durvalumab for resectable NSCLC tumors across 802 patients. Patients without EGFR or ALK mutations were randomly assigned to receive durvalumab before surgery alongside platinum-containing chemotherapy and after surgery for a year as monotherapy or to receive chemotherapy and surgery alone.
Patients receiving durvalumab demonstrated better event-free survival at 1 year (73.4% vs 64.5% without durvalumab) and a better pathologic complete response rate (17.2% vs 4.3% without). Currently, AstraZeneca is seeking to add the indication for durvalumab to those the agent already has.
However, at the July 25 ODAC meeting, the committee explained that the AEGEAN trial design makes it impossible to tell whether patients benefited from durvalumab before surgery, after it, or at both points.
Mounting evidence, including from AstraZeneca’s own studies, suggests that the benefit of immune checkpoint inhibitors, such as durvalumab, comes before surgery. That means prescribing durvalumab after surgery could be exposing patients to serious side effects and financial toxicity, with potentially no clinical benefit, “magnifying the risk of potential overtreatment,” the committee cautioned.
When AEGEAN was being designed in 2018, FDA requested that AstraZeneca address the uncertainty surrounding when to use durvalumab by including separate neoadjuvant and adjuvant arms, or at least an arm where patients were treated with neoadjuvant durvalumab alone to compare with treatment both before and after surgery.
The company didn’t follow through and, during the July 25 meeting, the committee wanted answers. “Why did you not comply with this?” asked ODAC committee acting chair Daniel Spratt, MD, a radiation oncologist at Case Western Reserve University in Cleveland, Ohio.
AstraZeneca personnel explained that doing so would have required many more subjects, made the trial more expensive, and added about 2 years to AEGEAN.
One speaker noted that the company, which makes more than $4 billion a year on durvalumab, would have taken about 2 days to recoup that added cost. Others wondered whether the motive was to sell durvalumab for as long as possible across a patient’s course of treatment.
Perhaps the biggest reason the company ignored the request is that “it wasn’t our understanding at that time that this was a barrier to approval,” an AstraZeneca regulatory affairs specialist said.
To this end, the agency asked its advisory panel to vote on whether it should require — instead of simply request, as it did with AstraZeneca — companies to prove that patients need immunotherapy both before and after surgery in resectable NSCLC.
The 11-member panel voted unanimously that it should make this a requirement, and several members said it should do so in other cancers as well.
However, when the agency asked whether durvalumab’s resectable NSCLC approval should be delayed until AstraZeneca conducts a trial to answer the neoadjuvant vs adjuvant question, the panel members didn’t think so.
The consensus was that because AEGEAN showed a decent benefit, patients and physicians should have it as an option, and approval shouldn’t be delayed. The panel said that the bigger question about the benefit of maintenance therapy should be left to future studies.
FDA usually follows the advice of its advisory panels.
A version of this article appeared on Medscape.com.
The trial in question, AEGEAN, investigated perioperative durvalumab for resectable NSCLC tumors across 802 patients. Patients without EGFR or ALK mutations were randomly assigned to receive durvalumab before surgery alongside platinum-containing chemotherapy and after surgery for a year as monotherapy or to receive chemotherapy and surgery alone.
Patients receiving durvalumab demonstrated better event-free survival at 1 year (73.4% vs 64.5% without durvalumab) and a better pathologic complete response rate (17.2% vs 4.3% without). Currently, AstraZeneca is seeking to add the indication for durvalumab to those the agent already has.
However, at the July 25 ODAC meeting, the committee explained that the AEGEAN trial design makes it impossible to tell whether patients benefited from durvalumab before surgery, after it, or at both points.
Mounting evidence, including from AstraZeneca’s own studies, suggests that the benefit of immune checkpoint inhibitors, such as durvalumab, comes before surgery. That means prescribing durvalumab after surgery could be exposing patients to serious side effects and financial toxicity, with potentially no clinical benefit, “magnifying the risk of potential overtreatment,” the committee cautioned.
When AEGEAN was being designed in 2018, FDA requested that AstraZeneca address the uncertainty surrounding when to use durvalumab by including separate neoadjuvant and adjuvant arms, or at least an arm where patients were treated with neoadjuvant durvalumab alone to compare with treatment both before and after surgery.
The company didn’t follow through and, during the July 25 meeting, the committee wanted answers. “Why did you not comply with this?” asked ODAC committee acting chair Daniel Spratt, MD, a radiation oncologist at Case Western Reserve University in Cleveland, Ohio.
AstraZeneca personnel explained that doing so would have required many more subjects, made the trial more expensive, and added about 2 years to AEGEAN.
One speaker noted that the company, which makes more than $4 billion a year on durvalumab, would have taken about 2 days to recoup that added cost. Others wondered whether the motive was to sell durvalumab for as long as possible across a patient’s course of treatment.
Perhaps the biggest reason the company ignored the request is that “it wasn’t our understanding at that time that this was a barrier to approval,” an AstraZeneca regulatory affairs specialist said.
To this end, the agency asked its advisory panel to vote on whether it should require — instead of simply request, as it did with AstraZeneca — companies to prove that patients need immunotherapy both before and after surgery in resectable NSCLC.
The 11-member panel voted unanimously that it should make this a requirement, and several members said it should do so in other cancers as well.
However, when the agency asked whether durvalumab’s resectable NSCLC approval should be delayed until AstraZeneca conducts a trial to answer the neoadjuvant vs adjuvant question, the panel members didn’t think so.
The consensus was that because AEGEAN showed a decent benefit, patients and physicians should have it as an option, and approval shouldn’t be delayed. The panel said that the bigger question about the benefit of maintenance therapy should be left to future studies.
FDA usually follows the advice of its advisory panels.
A version of this article appeared on Medscape.com.
Which Patients With Early TNBC Can Avoid Chemotherapy?
TOPLINE:
which suggest that stromal TILs could be a useful biomarker to optimize treatment decisions in this patient population.
METHODOLOGY:
- The absolute benefit of chemotherapy remains unclear among patients with stage I TNBC. High levels of stromal TILs, a promising biomarker, have been linked to better survival in patients with TNBC, but data focused on stage I disease are lacking.
- In the current analysis, researchers identified a cohort of 1041 women (mean age at diagnosis, 64.4 years) from the Netherlands Cancer Registry with stage I TNBC who had an available TIL score and had undergone a lumpectomy or a mastectomy but had not received neoadjuvant or adjuvant chemotherapy.
- Patients’ clinical data were matched to their corresponding pathologic data provided by the Dutch Pathology Registry, and a pathologist blinded to outcomes scored stromal TIL levels according to the International Immuno-Oncology Biomarker Working Group guidelines.
- The primary endpoint was breast cancer–specific survival at prespecified stromal TIL cutoffs of 30%, 50%, and 75%. Secondary outcomes included specific survival by pathologic tumor stage and overall survival.
TAKEAWAY:
- Overall, 8.6% of women had a pT1a tumor, 38.7% had a pT1b tumor, and 52.6% had a pT1c tumor. In the cohort, 25.6% of patients had stromal TIL levels of 30% or higher, 19.5% had levels of 50% or higher, and 13.5% had levels of 75% or higher.
- Over a median follow-up of 11.4 years, 335 patients died, 107 (32%) of whom died from breast cancer. Patients with smaller tumors (pT1abNO) had better survival outcomes than those with larger tumors (pT1cNO) — a 10-year breast cancer–specific survival of 92% vs 86%, respectively.
- In the overall cohort, stromal TIL levels of 30% or higher were associated with better breast cancer–specific survival than those with stromal TIL levels below 30% (96% vs 87%; hazard ratio [HR], 0.45). Stromal TIL levels of 50% or greater were also associated with better 10-year breast cancer–specific survival than those with levels below 50% (92% vs 88%; HR, 0.59). A similar pattern was observed for stromal TIL levels and overall survival.
- In patients with pT1c tumors, the 10-year breast cancer–specific survival among those with stromal TIL levels of 30% or higher was 95% vs 83% for levels below the 30% cutoff (HR, 0.24). Similarly, the 10-year breast cancer–specific survival for those in the 50% or higher group was 95% vs 84% for levels below that cutoff (HR, 0.27). The 10-year breast cancer–specific survival improved to 98% among patients with stromal TIL levels of 75% or higher (HR, 0.09).
IN PRACTICE:
The results supported the establishment of “treatment-optimization clinical trials in patients with stage I TNBC, using [stromal] TIL level as an integral biomarker to prospectively confirm the observed excellent survival when neoadjuvant or adjuvant chemotherapy is not administered,” the authors wrote. Assessing stromal TILs is also “inexpensive,” the authors added.
SOURCE:
The research, conducted by Marleen Kok, MD, PhD, Department of Medical Oncology, the Netherlands Cancer Institute, Amsterdam, and colleagues, was published online in JAMA Oncology.
LIMITATIONS:
The authors noted that the study was limited by its observational nature. The patients were drawn from a larger cohort, about half of whom received adjuvant chemotherapy, and the patients who did not receive chemotherapy may have had favorable tumor characteristics. There were also no data on BRCA1 or BRCA2 germline mutation status and recurrences and/or distant metastases. The database did not include data on patient ethnicity because most Dutch patients were White.
DISCLOSURES:
Research at the Netherlands Cancer Institute was supported by institutional grants from the Dutch Cancer Society and the Dutch Ministry of Health, Welfare and Sport. Dr. Kok declared financial relationships with several organizations including Gilead and Domain Therapeutics, as well as institutional grants from AstraZeneca, BMS, and Roche. Other authors also declared numerous financial relationships for themselves and their institutions with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
which suggest that stromal TILs could be a useful biomarker to optimize treatment decisions in this patient population.
METHODOLOGY:
- The absolute benefit of chemotherapy remains unclear among patients with stage I TNBC. High levels of stromal TILs, a promising biomarker, have been linked to better survival in patients with TNBC, but data focused on stage I disease are lacking.
- In the current analysis, researchers identified a cohort of 1041 women (mean age at diagnosis, 64.4 years) from the Netherlands Cancer Registry with stage I TNBC who had an available TIL score and had undergone a lumpectomy or a mastectomy but had not received neoadjuvant or adjuvant chemotherapy.
- Patients’ clinical data were matched to their corresponding pathologic data provided by the Dutch Pathology Registry, and a pathologist blinded to outcomes scored stromal TIL levels according to the International Immuno-Oncology Biomarker Working Group guidelines.
- The primary endpoint was breast cancer–specific survival at prespecified stromal TIL cutoffs of 30%, 50%, and 75%. Secondary outcomes included specific survival by pathologic tumor stage and overall survival.
TAKEAWAY:
- Overall, 8.6% of women had a pT1a tumor, 38.7% had a pT1b tumor, and 52.6% had a pT1c tumor. In the cohort, 25.6% of patients had stromal TIL levels of 30% or higher, 19.5% had levels of 50% or higher, and 13.5% had levels of 75% or higher.
- Over a median follow-up of 11.4 years, 335 patients died, 107 (32%) of whom died from breast cancer. Patients with smaller tumors (pT1abNO) had better survival outcomes than those with larger tumors (pT1cNO) — a 10-year breast cancer–specific survival of 92% vs 86%, respectively.
- In the overall cohort, stromal TIL levels of 30% or higher were associated with better breast cancer–specific survival than those with stromal TIL levels below 30% (96% vs 87%; hazard ratio [HR], 0.45). Stromal TIL levels of 50% or greater were also associated with better 10-year breast cancer–specific survival than those with levels below 50% (92% vs 88%; HR, 0.59). A similar pattern was observed for stromal TIL levels and overall survival.
- In patients with pT1c tumors, the 10-year breast cancer–specific survival among those with stromal TIL levels of 30% or higher was 95% vs 83% for levels below the 30% cutoff (HR, 0.24). Similarly, the 10-year breast cancer–specific survival for those in the 50% or higher group was 95% vs 84% for levels below that cutoff (HR, 0.27). The 10-year breast cancer–specific survival improved to 98% among patients with stromal TIL levels of 75% or higher (HR, 0.09).
IN PRACTICE:
The results supported the establishment of “treatment-optimization clinical trials in patients with stage I TNBC, using [stromal] TIL level as an integral biomarker to prospectively confirm the observed excellent survival when neoadjuvant or adjuvant chemotherapy is not administered,” the authors wrote. Assessing stromal TILs is also “inexpensive,” the authors added.
SOURCE:
The research, conducted by Marleen Kok, MD, PhD, Department of Medical Oncology, the Netherlands Cancer Institute, Amsterdam, and colleagues, was published online in JAMA Oncology.
LIMITATIONS:
The authors noted that the study was limited by its observational nature. The patients were drawn from a larger cohort, about half of whom received adjuvant chemotherapy, and the patients who did not receive chemotherapy may have had favorable tumor characteristics. There were also no data on BRCA1 or BRCA2 germline mutation status and recurrences and/or distant metastases. The database did not include data on patient ethnicity because most Dutch patients were White.
DISCLOSURES:
Research at the Netherlands Cancer Institute was supported by institutional grants from the Dutch Cancer Society and the Dutch Ministry of Health, Welfare and Sport. Dr. Kok declared financial relationships with several organizations including Gilead and Domain Therapeutics, as well as institutional grants from AstraZeneca, BMS, and Roche. Other authors also declared numerous financial relationships for themselves and their institutions with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
which suggest that stromal TILs could be a useful biomarker to optimize treatment decisions in this patient population.
METHODOLOGY:
- The absolute benefit of chemotherapy remains unclear among patients with stage I TNBC. High levels of stromal TILs, a promising biomarker, have been linked to better survival in patients with TNBC, but data focused on stage I disease are lacking.
- In the current analysis, researchers identified a cohort of 1041 women (mean age at diagnosis, 64.4 years) from the Netherlands Cancer Registry with stage I TNBC who had an available TIL score and had undergone a lumpectomy or a mastectomy but had not received neoadjuvant or adjuvant chemotherapy.
- Patients’ clinical data were matched to their corresponding pathologic data provided by the Dutch Pathology Registry, and a pathologist blinded to outcomes scored stromal TIL levels according to the International Immuno-Oncology Biomarker Working Group guidelines.
- The primary endpoint was breast cancer–specific survival at prespecified stromal TIL cutoffs of 30%, 50%, and 75%. Secondary outcomes included specific survival by pathologic tumor stage and overall survival.
TAKEAWAY:
- Overall, 8.6% of women had a pT1a tumor, 38.7% had a pT1b tumor, and 52.6% had a pT1c tumor. In the cohort, 25.6% of patients had stromal TIL levels of 30% or higher, 19.5% had levels of 50% or higher, and 13.5% had levels of 75% or higher.
- Over a median follow-up of 11.4 years, 335 patients died, 107 (32%) of whom died from breast cancer. Patients with smaller tumors (pT1abNO) had better survival outcomes than those with larger tumors (pT1cNO) — a 10-year breast cancer–specific survival of 92% vs 86%, respectively.
- In the overall cohort, stromal TIL levels of 30% or higher were associated with better breast cancer–specific survival than those with stromal TIL levels below 30% (96% vs 87%; hazard ratio [HR], 0.45). Stromal TIL levels of 50% or greater were also associated with better 10-year breast cancer–specific survival than those with levels below 50% (92% vs 88%; HR, 0.59). A similar pattern was observed for stromal TIL levels and overall survival.
- In patients with pT1c tumors, the 10-year breast cancer–specific survival among those with stromal TIL levels of 30% or higher was 95% vs 83% for levels below the 30% cutoff (HR, 0.24). Similarly, the 10-year breast cancer–specific survival for those in the 50% or higher group was 95% vs 84% for levels below that cutoff (HR, 0.27). The 10-year breast cancer–specific survival improved to 98% among patients with stromal TIL levels of 75% or higher (HR, 0.09).
IN PRACTICE:
The results supported the establishment of “treatment-optimization clinical trials in patients with stage I TNBC, using [stromal] TIL level as an integral biomarker to prospectively confirm the observed excellent survival when neoadjuvant or adjuvant chemotherapy is not administered,” the authors wrote. Assessing stromal TILs is also “inexpensive,” the authors added.
SOURCE:
The research, conducted by Marleen Kok, MD, PhD, Department of Medical Oncology, the Netherlands Cancer Institute, Amsterdam, and colleagues, was published online in JAMA Oncology.
LIMITATIONS:
The authors noted that the study was limited by its observational nature. The patients were drawn from a larger cohort, about half of whom received adjuvant chemotherapy, and the patients who did not receive chemotherapy may have had favorable tumor characteristics. There were also no data on BRCA1 or BRCA2 germline mutation status and recurrences and/or distant metastases. The database did not include data on patient ethnicity because most Dutch patients were White.
DISCLOSURES:
Research at the Netherlands Cancer Institute was supported by institutional grants from the Dutch Cancer Society and the Dutch Ministry of Health, Welfare and Sport. Dr. Kok declared financial relationships with several organizations including Gilead and Domain Therapeutics, as well as institutional grants from AstraZeneca, BMS, and Roche. Other authors also declared numerous financial relationships for themselves and their institutions with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Baseline Bone Pain Predicts Survival in Metastatic Hormone-Sensitive Prostate Cancer
TOPLINE:
METHODOLOGY:
- Prostate cancer often metastasizes to the bones, leading to pain and a reduced quality of life. While the relationship between bone pain and overall survival in metastatic, castration-resistant prostate cancer is well-documented, its impact in metastatic hormone-sensitive prostate cancer is less clear.
- Researchers conducted a post hoc secondary analysis using data from the SWOG-1216 phase 3 randomized clinical trial, which included 1279 men diagnosed with metastatic hormone-sensitive prostate cancer from 248 centers across the United States. Patients had received androgen deprivation therapy either with orteronel or bicalutamide.
- Among the 1197 patients (median age, 67.6 years) with data on bone pain included in the secondary analysis, 301 (23.5%) reported bone pain at baseline.
- The primary outcome was overall survival; secondary outcomes included progression-free survival and prostate-specific antigen response.
TAKEAWAY:
- The median overall survival for patients with baseline bone pain was 3.9 years compared with not reached (95% CI, 6.6 years to not reached) for those without bone pain at a median follow-up of 4 years (adjusted hazard ratio [aHR], 1.66; P < .001).
- Similarly, patients with bone pain had a shorter progression-free survival vs those without bone pain (median, 1.3 years vs 3.7 years; aHR, 1.46; P < .001).
- The complete prostate-specific antigen response rate at 7 months was also lower for patients with baseline bone pain (46.3% vs 66.3%; P < .001).
IN PRACTICE:
Patients with metastatic hormone-sensitive prostate cancer “with baseline bone pain had worse survival outcomes than those without baseline bone pain,” the authors wrote. “These results highlight the need to consider bone pain in prognostic modeling, treatment selection, patient monitoring, and follow-up and suggest prioritizing these patients for clinical trials and immediate systemic treatment initiation.”
SOURCE:
The study, led by Georges Gebrael, MD, Huntsman Cancer Institute at the University of Utah, Salt Lake City, Utah, was published online in JAMA Network Open.
LIMITATIONS:
The post hoc design may introduce bias. Orteronel failed to receive regulatory approval, which may affect the generalizability of the findings. In addition, the study did not account for synchronous vs metachronous disease status, a known established prognostic factor.
DISCLOSURES:
The study was funded by the National Institutes of Health/National Cancer Institute and Millennium Pharmaceuticals (Takeda Oncology Company). Several authors declared ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prostate cancer often metastasizes to the bones, leading to pain and a reduced quality of life. While the relationship between bone pain and overall survival in metastatic, castration-resistant prostate cancer is well-documented, its impact in metastatic hormone-sensitive prostate cancer is less clear.
- Researchers conducted a post hoc secondary analysis using data from the SWOG-1216 phase 3 randomized clinical trial, which included 1279 men diagnosed with metastatic hormone-sensitive prostate cancer from 248 centers across the United States. Patients had received androgen deprivation therapy either with orteronel or bicalutamide.
- Among the 1197 patients (median age, 67.6 years) with data on bone pain included in the secondary analysis, 301 (23.5%) reported bone pain at baseline.
- The primary outcome was overall survival; secondary outcomes included progression-free survival and prostate-specific antigen response.
TAKEAWAY:
- The median overall survival for patients with baseline bone pain was 3.9 years compared with not reached (95% CI, 6.6 years to not reached) for those without bone pain at a median follow-up of 4 years (adjusted hazard ratio [aHR], 1.66; P < .001).
- Similarly, patients with bone pain had a shorter progression-free survival vs those without bone pain (median, 1.3 years vs 3.7 years; aHR, 1.46; P < .001).
- The complete prostate-specific antigen response rate at 7 months was also lower for patients with baseline bone pain (46.3% vs 66.3%; P < .001).
IN PRACTICE:
Patients with metastatic hormone-sensitive prostate cancer “with baseline bone pain had worse survival outcomes than those without baseline bone pain,” the authors wrote. “These results highlight the need to consider bone pain in prognostic modeling, treatment selection, patient monitoring, and follow-up and suggest prioritizing these patients for clinical trials and immediate systemic treatment initiation.”
SOURCE:
The study, led by Georges Gebrael, MD, Huntsman Cancer Institute at the University of Utah, Salt Lake City, Utah, was published online in JAMA Network Open.
LIMITATIONS:
The post hoc design may introduce bias. Orteronel failed to receive regulatory approval, which may affect the generalizability of the findings. In addition, the study did not account for synchronous vs metachronous disease status, a known established prognostic factor.
DISCLOSURES:
The study was funded by the National Institutes of Health/National Cancer Institute and Millennium Pharmaceuticals (Takeda Oncology Company). Several authors declared ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prostate cancer often metastasizes to the bones, leading to pain and a reduced quality of life. While the relationship between bone pain and overall survival in metastatic, castration-resistant prostate cancer is well-documented, its impact in metastatic hormone-sensitive prostate cancer is less clear.
- Researchers conducted a post hoc secondary analysis using data from the SWOG-1216 phase 3 randomized clinical trial, which included 1279 men diagnosed with metastatic hormone-sensitive prostate cancer from 248 centers across the United States. Patients had received androgen deprivation therapy either with orteronel or bicalutamide.
- Among the 1197 patients (median age, 67.6 years) with data on bone pain included in the secondary analysis, 301 (23.5%) reported bone pain at baseline.
- The primary outcome was overall survival; secondary outcomes included progression-free survival and prostate-specific antigen response.
TAKEAWAY:
- The median overall survival for patients with baseline bone pain was 3.9 years compared with not reached (95% CI, 6.6 years to not reached) for those without bone pain at a median follow-up of 4 years (adjusted hazard ratio [aHR], 1.66; P < .001).
- Similarly, patients with bone pain had a shorter progression-free survival vs those without bone pain (median, 1.3 years vs 3.7 years; aHR, 1.46; P < .001).
- The complete prostate-specific antigen response rate at 7 months was also lower for patients with baseline bone pain (46.3% vs 66.3%; P < .001).
IN PRACTICE:
Patients with metastatic hormone-sensitive prostate cancer “with baseline bone pain had worse survival outcomes than those without baseline bone pain,” the authors wrote. “These results highlight the need to consider bone pain in prognostic modeling, treatment selection, patient monitoring, and follow-up and suggest prioritizing these patients for clinical trials and immediate systemic treatment initiation.”
SOURCE:
The study, led by Georges Gebrael, MD, Huntsman Cancer Institute at the University of Utah, Salt Lake City, Utah, was published online in JAMA Network Open.
LIMITATIONS:
The post hoc design may introduce bias. Orteronel failed to receive regulatory approval, which may affect the generalizability of the findings. In addition, the study did not account for synchronous vs metachronous disease status, a known established prognostic factor.
DISCLOSURES:
The study was funded by the National Institutes of Health/National Cancer Institute and Millennium Pharmaceuticals (Takeda Oncology Company). Several authors declared ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Fed Worker Health Plans Ban Maximizers and Copay Accumulators: Why Not for the Rest of the US?
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Paclitaxel Drug-Drug Interactions in the Military Health System
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
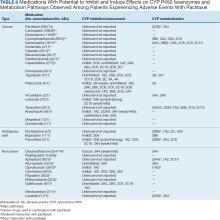
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
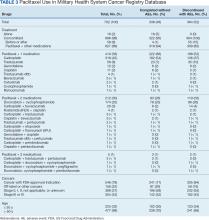
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
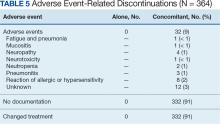
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Open Clinical Trials for Patients With Prostate Cancer
The clinical trials listed below are all open as of July 12, 2024; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for prostate cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making regarding precision oncology tests among veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out informed decision making due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention and implement the intervention. A decision support intervention may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; DoD
Location: San Francisco VAMC
DeADT - Living Well With Prostate Cancer
The goal of this pilot randomized implementation trial is to compare 2 strategies to reduce low-value androgen deprivation therapy (ADT) use for prostate cancer care. The aim of the study is to compare implementation of the 2 strategies: use of a clinical reminder order check intervention vs a clinician script/patient education approach, and their impacts on low-value ADT use after 6 months. The main goal of both interventions will be to decrease ADT overuse for patients with prostate cancer, but to do this in a way that is acceptable to the provider who treat these patients. Provider participants will engage with 1 of the interventions triggered in the electronic health record when their patients are deemed likely to receive low-value ADT. Provider participants receive only 1 intervention. The intervention is triggered for every clinic visit involving a patient deemed to be receiving low-value ADT, so provider participants may receive their assigned intervention multiple times. Researchers will compare provider use of both strategies to determine implementation outcomes and whether 1 was more effective in reducing low-value ADT use.
ID: NCT06199986
Sponsor; Collaborator: University of Michigan; VA, National Cancer Institute
Location: VA Ann Arbor Healthcare System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for Oligometastatic Prostate Cancer (VA STARPORT)
This is a prospective, open-label, multicenter, seamless phase II to phase III randomized clinical trial designed to compare somatostatin with or without positron emission tomography (PET)-directed local therapy in improving the castration-resistant prostate cancer-free survival for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1 to 10 sites of metastatic disease based on the clinical determination.
ID: NCT04787744
Sponsor; Investigators: VA Office of Research and Development; Abhishek Solani, MD, MS, Edward Hines Jr.
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VAMC, Baltimore VAMC, VA Boston Healthcare System, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VAMC, VA New Jersey Healthcare System, VA NY Harbor Healthcare System, Durham VAMC, Louis Stokes VAMC, Corporal Michael J. Crescenz VAMC, Michael E. DeBakey VAMC, Hunter Holmes McGuire VAMC, William S. Middleton Memorial Veterans Hospital, Clement J. Zablocki VAMC
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Investigator: VA Office of Research and Development; Jason L. Vassy, MD, MPH
Location: VA Boston Healthcare System
Prostate Active Surveillance Study (PASS)
This research study is for men who have chosen active surveillance as a management plan for their prostate cancer. Active surveillance is defined as close monitoring of prostate cancer with the offer of treatment if there are changes in test results. This study seeks to discover markers that will identify cancers that are more aggressive from those tumors that grow slowly.
ID: NCT00756665
Sponsor; Collaborators: University of Washington; Canary Foundation, Early Detection Research Network
Locations: VA San Francisco Health Care System, VA Puget Sound Health Care System
A Study of Checkpoint Inhibitors in Men With Progressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to prostate-specific antigen progression, maximal prostate-specific antigen response, time to initiation of alternative antineoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pretreatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Collaborator: VA Office of Research and Development; Merck Sharp & Dohme LLC
Locations: San Francisco VAMC, VA Greater Los Angeles Healthcare System, Washington DC VAMC, Bay Pines VA Healthcare System Jesse Brown VAMC, VA Ann Arbor Healthcare System, James J. Peter VAMC, VA NY Harbor Healthcare System, Durham VAMC, Corporal Michael J. Crescenz VAMC, Hunter Holmes McGuire VAMC, VA Puget Sound Health Care System
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolideand Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in the treatment of high-risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation therapy drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Investigator: VA Office of Research and Development; Ryan P. Kopp, MD
Locations: VA Greater Los Angeles Healthcare System, James J. Peters VAMC, VA Portland Health Care System, South Texas Veterans Health Care System, VA Puget Sound Health Care System
Active, Not Recruiting
Intramuscular Mechanisms of Androgen Deprivation-related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes androgen deprivation therapy (ADT), or the lowering of the levels of the hormone testosterone as much as possible. Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as “sarcopenia,” and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because the investigators do not understand the mechanisms that cause it. The mitochondria are part of the cells responsible for providing energy to muscles, but to this date, the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT.
ID: NCT03867357
Sponsor; Collaborators: Seattle Institute for Biomedical and Clinical Research; DoD, University of Washington
Location: VA Puget Sound Health Care System
Radiation Therapy With or Without Androgen-Deprivation Therapy in Treating Patients With Prostate Cancer
RATIONALE: Radiation therapy uses high-energy X-rays and other types of radiation to kill tumor cells and shrink tumors. Androgens can cause the growth of prostate cancer cells. Androgen deprivation therapy (ADT) may lessen the amount of androgens made by the body. It is not yet known whether radiation therapy is more effective with or without ADT in treating patients with prostate cancer.
PURPOSE: This randomized phase III trial is studying radiation therapy to see how well it works compared with radiation therapy given together with ADT in treating patients with prostate cancer.
ID: NCT00936390
Sponsor; Collaborators: Radiation Therapy Oncology Group; National Cancer Institute, NRG Oncology
Locations: 518 locations, James A. Haley VA Hospital
Enzalutamide With or Without Abiraterone and Prednisone in Treating Patients With Castration-Resistant Metastatic Prostate Cancer
This randomized phase III trial studies enzalutamide to see how well it works compared to enzalutamide, abiraterone, and prednisone in treating patients with castration-resistant metastatic prostate cancer. Androgens can cause the growth of prostate cancer cells. Drugs, such as enzalutamide, abiraterone acetate, and prednisone, may lessen the amount of androgens made by the body.
ID: NCT01949337
Sponsor; Collaborators: Alliance for Clinical Trials in Oncology; NCI, Astellas Pharma US, Inc., Medivation, Inc., Biologics, Inc.
Locations: 539 locations, including VA Connecticut Healthcare System
S1216, Phase III ADT+TAK-700 vs ADT+Bicalutamide for Metastatic Prostate Cancer (S1216)
The purpose of this study is to compare overall survival in newly diagnosed metastatic prostate cancer patients randomly assigned to ADT + TAK-700 vs androgen deprivation therapy (ADT) + bicalutamide.
ID: NCT01809691
Sponsor; Collaborators: SWOG Cancer Research Network; Millennium Pharmaceuticals, Inc., NCI
Locations: 560 locations, including VA New York Harbor Healthcare System
Androgen Ablation Therapy With or Without Chemotherapy in Treating Patients With Metastatic Prostate Cancer (CHAARTED)
RATIONALE: Androgens can cause the growth of prostate cancer cells. Androgen ablation therapy may stop the adrenal glands from making androgens. Drugs used in chemotherapy, such as docetaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether androgen-ablation therapy is more effective with or without docetaxel in treating metastatic prostate cancer.
PURPOSE: This randomized phase III trial is studying androgen ablation therapy and chemotherapy to see how well they work compared to androgen ablation therapy alone in treating patients with metastatic prostate cancer.
ID: NCT00309985
Sponsor; Collaborator: ECOG-ACRIN Cancer Research Group; NCI
Locations: 343 locations, including Mather VAMC
Not Yet Recruiting
biraterone, Enzalutamide, or Apalutamide in Castrate-Sensitive Prostate Cancer
The investigators have used national Veterans Health Administration (VHA) data to demonstrate real-world efficacy of abiraterone and enzalutamide in veterans with metastatic castration-resistant prostate cancer. In the real world that is the VHA, the investigators have successfully estimated g values that accurately predict overall survival, and the use of this metric in other settings should now be explored. In the egalitarian system that is the VHA, the treatment of prostate cancer is excellent, uniform across the US and indifferent to race. The choices made are clearly personalized, given not all men received all therapies, and younger veterans were treated more aggressively.
ID: NCT05422911
Sponsor: James J. Peters VAMC
Location: James J. Peters VAMC
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires, and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor, Investigator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD
Location: VA Greater Los Angeles Healthcare System
18F-DCFPyL PET-CT Scan and Prostate Cancer
The primary objective of this study is to assess the efficacy of 18F-DCFPyL PET-CT for initial staging of prostate cancer in veterans compared to conventional imaging (99mTc-MDP bone scan and diagnostic CT or MRI). The primary clinical endpoint of our study is the percentage of veterans with prostate cancer in which the 18F-DCFPyL PET-CT identifies M1 disease at initial staging. Secondary objectives included frequency of the change in primary treatment plan after initial staging.
ID: NCT03852654
Sponsor, Investigator: Lida Jafari, MD
Location: VA Greater Los Angeles Healthcare System
Neoadjuvant Therapy With Docetaxel and Ketoconazole in Patients With High-Risk Prostate Cancer: A Pilot Study (IST 16167)
Eligible patients with high-risk prostate cancer who are scheduled to undergo radical prostatectomy will receive 4 cycles of therapy with ketoconazole and docetaxel prior to surgery resection. A cycle of therapy is defined as 21 days (3 weeks). Pharmacokinetic analysis will be performed during the first and second cycles of therapy. All patients will be evaluated for toxicity, tumor response, and recurrence.
ID: NCT00870714
Sponsor, Collaborator: Kansas City VAMC; Sanofi
Location: Kansas City VAMC
A Study of Epirubicin With Estramustine Phosphate and Celecoxib for the Treatment of Prostate Cancer
The purpose of this clinical trial is to find out the effect of epirubicin with estramustine phosphate and celecoxib on PSA and objective response in patients with hormone-resistant prostate cancer, as well as to evaluate the toxicity and quality of life of this combination. Celecoxib is an FDA-approved drug that treats arthritis. Epirubicin, alone or with estramustine phosphate, has been used in the treatment of hormone-resistant prostate cancer. These drugs have demonstrated evidence of tumor blood vessel suppression and a combination of these 3 drugs could possibly arrest further tumor growth or even make the tumor decrease in size.
ID: NCT00218205
Sponsor, Collaborator; Investigator: VA New Jersey Health Care System; Pfizer; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Phase II Trial of Combination Therapy With Celecoxib and Taxotere for the Treatment of Stage D3 Prostate Cancer
The purpose of this clinical trial is to find out the safety and effectiveness as well as the patient’s quality of life while taking the combination of Taxotere and celecoxib on patients with hormone refractory prostate cancer. Celecoxib (Celebrex) is an FDA-approved drug that treats arthritis. Taxotere (Docetaxel) is an FDA-approved chemotherapy drug to treat certain forms of cancer. Both drugs have demonstrated evidences of tumor blood vessel suppression and combination of these 2 drugs could possibly arrest further tumor growth or make the tumor decrease in size.
ID: NCT00215345
Sponsor, Collaborator; Investigator: Department of Veterans Affairs, New Jersey; Pfizer, Sanofi; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Yoga Program for Patients Undergoing Prostate Cancer Surgery
Men with localized prostate cancer are often treated with surgery, a treatment that is associated with high rates of adverse effects such as erectile dysfunction (ED) and urinary incontinence (UI) which impact quality of life. Yoga may improve control of UI and improve ED by bringing awareness to and strengthening the pelvic floor musculature. The randomized controlled pilot study is to assess the feasibility of an innovative hybrid (in-person and virtual) twice-weekly yoga program that includes a prehabilitation component and to obtain preliminary data that will help assess its potential effectiveness in alleviating prostate cancer treatment symptom burden (primarily ED and UI). The long-term goal is to develop a scalable and sustainable yoga program that helps cancer survivors manage their treatment side effects.
ID: NCT05929300
Sponsor, Investigator: VA Office of Research and Development; Abigail Silva, PhD, MPH
Location: Edward Hines Jr. VA Hospital
The clinical trials listed below are all open as of July 12, 2024; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for prostate cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making regarding precision oncology tests among veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out informed decision making due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention and implement the intervention. A decision support intervention may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; DoD
Location: San Francisco VAMC
DeADT - Living Well With Prostate Cancer
The goal of this pilot randomized implementation trial is to compare 2 strategies to reduce low-value androgen deprivation therapy (ADT) use for prostate cancer care. The aim of the study is to compare implementation of the 2 strategies: use of a clinical reminder order check intervention vs a clinician script/patient education approach, and their impacts on low-value ADT use after 6 months. The main goal of both interventions will be to decrease ADT overuse for patients with prostate cancer, but to do this in a way that is acceptable to the provider who treat these patients. Provider participants will engage with 1 of the interventions triggered in the electronic health record when their patients are deemed likely to receive low-value ADT. Provider participants receive only 1 intervention. The intervention is triggered for every clinic visit involving a patient deemed to be receiving low-value ADT, so provider participants may receive their assigned intervention multiple times. Researchers will compare provider use of both strategies to determine implementation outcomes and whether 1 was more effective in reducing low-value ADT use.
ID: NCT06199986
Sponsor; Collaborator: University of Michigan; VA, National Cancer Institute
Location: VA Ann Arbor Healthcare System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for Oligometastatic Prostate Cancer (VA STARPORT)
This is a prospective, open-label, multicenter, seamless phase II to phase III randomized clinical trial designed to compare somatostatin with or without positron emission tomography (PET)-directed local therapy in improving the castration-resistant prostate cancer-free survival for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1 to 10 sites of metastatic disease based on the clinical determination.
ID: NCT04787744
Sponsor; Investigators: VA Office of Research and Development; Abhishek Solani, MD, MS, Edward Hines Jr.
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VAMC, Baltimore VAMC, VA Boston Healthcare System, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VAMC, VA New Jersey Healthcare System, VA NY Harbor Healthcare System, Durham VAMC, Louis Stokes VAMC, Corporal Michael J. Crescenz VAMC, Michael E. DeBakey VAMC, Hunter Holmes McGuire VAMC, William S. Middleton Memorial Veterans Hospital, Clement J. Zablocki VAMC
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Investigator: VA Office of Research and Development; Jason L. Vassy, MD, MPH
Location: VA Boston Healthcare System
Prostate Active Surveillance Study (PASS)
This research study is for men who have chosen active surveillance as a management plan for their prostate cancer. Active surveillance is defined as close monitoring of prostate cancer with the offer of treatment if there are changes in test results. This study seeks to discover markers that will identify cancers that are more aggressive from those tumors that grow slowly.
ID: NCT00756665
Sponsor; Collaborators: University of Washington; Canary Foundation, Early Detection Research Network
Locations: VA San Francisco Health Care System, VA Puget Sound Health Care System
A Study of Checkpoint Inhibitors in Men With Progressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to prostate-specific antigen progression, maximal prostate-specific antigen response, time to initiation of alternative antineoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pretreatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Collaborator: VA Office of Research and Development; Merck Sharp & Dohme LLC
Locations: San Francisco VAMC, VA Greater Los Angeles Healthcare System, Washington DC VAMC, Bay Pines VA Healthcare System Jesse Brown VAMC, VA Ann Arbor Healthcare System, James J. Peter VAMC, VA NY Harbor Healthcare System, Durham VAMC, Corporal Michael J. Crescenz VAMC, Hunter Holmes McGuire VAMC, VA Puget Sound Health Care System
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolideand Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in the treatment of high-risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation therapy drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Investigator: VA Office of Research and Development; Ryan P. Kopp, MD
Locations: VA Greater Los Angeles Healthcare System, James J. Peters VAMC, VA Portland Health Care System, South Texas Veterans Health Care System, VA Puget Sound Health Care System
Active, Not Recruiting
Intramuscular Mechanisms of Androgen Deprivation-related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes androgen deprivation therapy (ADT), or the lowering of the levels of the hormone testosterone as much as possible. Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as “sarcopenia,” and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because the investigators do not understand the mechanisms that cause it. The mitochondria are part of the cells responsible for providing energy to muscles, but to this date, the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT.
ID: NCT03867357
Sponsor; Collaborators: Seattle Institute for Biomedical and Clinical Research; DoD, University of Washington
Location: VA Puget Sound Health Care System
Radiation Therapy With or Without Androgen-Deprivation Therapy in Treating Patients With Prostate Cancer
RATIONALE: Radiation therapy uses high-energy X-rays and other types of radiation to kill tumor cells and shrink tumors. Androgens can cause the growth of prostate cancer cells. Androgen deprivation therapy (ADT) may lessen the amount of androgens made by the body. It is not yet known whether radiation therapy is more effective with or without ADT in treating patients with prostate cancer.
PURPOSE: This randomized phase III trial is studying radiation therapy to see how well it works compared with radiation therapy given together with ADT in treating patients with prostate cancer.
ID: NCT00936390
Sponsor; Collaborators: Radiation Therapy Oncology Group; National Cancer Institute, NRG Oncology
Locations: 518 locations, James A. Haley VA Hospital
Enzalutamide With or Without Abiraterone and Prednisone in Treating Patients With Castration-Resistant Metastatic Prostate Cancer
This randomized phase III trial studies enzalutamide to see how well it works compared to enzalutamide, abiraterone, and prednisone in treating patients with castration-resistant metastatic prostate cancer. Androgens can cause the growth of prostate cancer cells. Drugs, such as enzalutamide, abiraterone acetate, and prednisone, may lessen the amount of androgens made by the body.
ID: NCT01949337
Sponsor; Collaborators: Alliance for Clinical Trials in Oncology; NCI, Astellas Pharma US, Inc., Medivation, Inc., Biologics, Inc.
Locations: 539 locations, including VA Connecticut Healthcare System
S1216, Phase III ADT+TAK-700 vs ADT+Bicalutamide for Metastatic Prostate Cancer (S1216)
The purpose of this study is to compare overall survival in newly diagnosed metastatic prostate cancer patients randomly assigned to ADT + TAK-700 vs androgen deprivation therapy (ADT) + bicalutamide.
ID: NCT01809691
Sponsor; Collaborators: SWOG Cancer Research Network; Millennium Pharmaceuticals, Inc., NCI
Locations: 560 locations, including VA New York Harbor Healthcare System
Androgen Ablation Therapy With or Without Chemotherapy in Treating Patients With Metastatic Prostate Cancer (CHAARTED)
RATIONALE: Androgens can cause the growth of prostate cancer cells. Androgen ablation therapy may stop the adrenal glands from making androgens. Drugs used in chemotherapy, such as docetaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether androgen-ablation therapy is more effective with or without docetaxel in treating metastatic prostate cancer.
PURPOSE: This randomized phase III trial is studying androgen ablation therapy and chemotherapy to see how well they work compared to androgen ablation therapy alone in treating patients with metastatic prostate cancer.
ID: NCT00309985
Sponsor; Collaborator: ECOG-ACRIN Cancer Research Group; NCI
Locations: 343 locations, including Mather VAMC
Not Yet Recruiting
biraterone, Enzalutamide, or Apalutamide in Castrate-Sensitive Prostate Cancer
The investigators have used national Veterans Health Administration (VHA) data to demonstrate real-world efficacy of abiraterone and enzalutamide in veterans with metastatic castration-resistant prostate cancer. In the real world that is the VHA, the investigators have successfully estimated g values that accurately predict overall survival, and the use of this metric in other settings should now be explored. In the egalitarian system that is the VHA, the treatment of prostate cancer is excellent, uniform across the US and indifferent to race. The choices made are clearly personalized, given not all men received all therapies, and younger veterans were treated more aggressively.
ID: NCT05422911
Sponsor: James J. Peters VAMC
Location: James J. Peters VAMC
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires, and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor, Investigator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD
Location: VA Greater Los Angeles Healthcare System
18F-DCFPyL PET-CT Scan and Prostate Cancer
The primary objective of this study is to assess the efficacy of 18F-DCFPyL PET-CT for initial staging of prostate cancer in veterans compared to conventional imaging (99mTc-MDP bone scan and diagnostic CT or MRI). The primary clinical endpoint of our study is the percentage of veterans with prostate cancer in which the 18F-DCFPyL PET-CT identifies M1 disease at initial staging. Secondary objectives included frequency of the change in primary treatment plan after initial staging.
ID: NCT03852654
Sponsor, Investigator: Lida Jafari, MD
Location: VA Greater Los Angeles Healthcare System
Neoadjuvant Therapy With Docetaxel and Ketoconazole in Patients With High-Risk Prostate Cancer: A Pilot Study (IST 16167)
Eligible patients with high-risk prostate cancer who are scheduled to undergo radical prostatectomy will receive 4 cycles of therapy with ketoconazole and docetaxel prior to surgery resection. A cycle of therapy is defined as 21 days (3 weeks). Pharmacokinetic analysis will be performed during the first and second cycles of therapy. All patients will be evaluated for toxicity, tumor response, and recurrence.
ID: NCT00870714
Sponsor, Collaborator: Kansas City VAMC; Sanofi
Location: Kansas City VAMC
A Study of Epirubicin With Estramustine Phosphate and Celecoxib for the Treatment of Prostate Cancer
The purpose of this clinical trial is to find out the effect of epirubicin with estramustine phosphate and celecoxib on PSA and objective response in patients with hormone-resistant prostate cancer, as well as to evaluate the toxicity and quality of life of this combination. Celecoxib is an FDA-approved drug that treats arthritis. Epirubicin, alone or with estramustine phosphate, has been used in the treatment of hormone-resistant prostate cancer. These drugs have demonstrated evidence of tumor blood vessel suppression and a combination of these 3 drugs could possibly arrest further tumor growth or even make the tumor decrease in size.
ID: NCT00218205
Sponsor, Collaborator; Investigator: VA New Jersey Health Care System; Pfizer; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Phase II Trial of Combination Therapy With Celecoxib and Taxotere for the Treatment of Stage D3 Prostate Cancer
The purpose of this clinical trial is to find out the safety and effectiveness as well as the patient’s quality of life while taking the combination of Taxotere and celecoxib on patients with hormone refractory prostate cancer. Celecoxib (Celebrex) is an FDA-approved drug that treats arthritis. Taxotere (Docetaxel) is an FDA-approved chemotherapy drug to treat certain forms of cancer. Both drugs have demonstrated evidences of tumor blood vessel suppression and combination of these 2 drugs could possibly arrest further tumor growth or make the tumor decrease in size.
ID: NCT00215345
Sponsor, Collaborator; Investigator: Department of Veterans Affairs, New Jersey; Pfizer, Sanofi; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Yoga Program for Patients Undergoing Prostate Cancer Surgery
Men with localized prostate cancer are often treated with surgery, a treatment that is associated with high rates of adverse effects such as erectile dysfunction (ED) and urinary incontinence (UI) which impact quality of life. Yoga may improve control of UI and improve ED by bringing awareness to and strengthening the pelvic floor musculature. The randomized controlled pilot study is to assess the feasibility of an innovative hybrid (in-person and virtual) twice-weekly yoga program that includes a prehabilitation component and to obtain preliminary data that will help assess its potential effectiveness in alleviating prostate cancer treatment symptom burden (primarily ED and UI). The long-term goal is to develop a scalable and sustainable yoga program that helps cancer survivors manage their treatment side effects.
ID: NCT05929300
Sponsor, Investigator: VA Office of Research and Development; Abigail Silva, PhD, MPH
Location: Edward Hines Jr. VA Hospital
The clinical trials listed below are all open as of July 12, 2024; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for prostate cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
Patient Decision Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This clinical trial explores and implements methods to improve informed decision making regarding precision oncology tests among veterans with prostate cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Precision oncology, the use of germline genetic testing and tumor-based molecular assays to inform cancer care, has become an important aspect of evidence-based care for men with advanced prostate cancer. Veterans with metastatic castrate-resistant prostate cancer may not be carrying out informed decision making due to unmet decisional needs. An informed decision is a choice based on complete and accurate information. The information gained from this study will help researchers develop a decision support intervention and implement the intervention. A decision support intervention may serve as a valuable tool to reduce ongoing racial disparities in genetic testing and encourage enrollment to precision oncology trials.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; DoD
Location: San Francisco VAMC
DeADT - Living Well With Prostate Cancer
The goal of this pilot randomized implementation trial is to compare 2 strategies to reduce low-value androgen deprivation therapy (ADT) use for prostate cancer care. The aim of the study is to compare implementation of the 2 strategies: use of a clinical reminder order check intervention vs a clinician script/patient education approach, and their impacts on low-value ADT use after 6 months. The main goal of both interventions will be to decrease ADT overuse for patients with prostate cancer, but to do this in a way that is acceptable to the provider who treat these patients. Provider participants will engage with 1 of the interventions triggered in the electronic health record when their patients are deemed likely to receive low-value ADT. Provider participants receive only 1 intervention. The intervention is triggered for every clinic visit involving a patient deemed to be receiving low-value ADT, so provider participants may receive their assigned intervention multiple times. Researchers will compare provider use of both strategies to determine implementation outcomes and whether 1 was more effective in reducing low-value ADT use.
ID: NCT06199986
Sponsor; Collaborator: University of Michigan; VA, National Cancer Institute
Location: VA Ann Arbor Healthcare System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for Oligometastatic Prostate Cancer (VA STARPORT)
This is a prospective, open-label, multicenter, seamless phase II to phase III randomized clinical trial designed to compare somatostatin with or without positron emission tomography (PET)-directed local therapy in improving the castration-resistant prostate cancer-free survival for veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1 to 10 sites of metastatic disease based on the clinical determination.
ID: NCT04787744
Sponsor; Investigators: VA Office of Research and Development; Abhishek Solani, MD, MS, Edward Hines Jr.
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VAMC, Baltimore VAMC, VA Boston Healthcare System, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VAMC, VA New Jersey Healthcare System, VA NY Harbor Healthcare System, Durham VAMC, Louis Stokes VAMC, Corporal Michael J. Crescenz VAMC, Michael E. DeBakey VAMC, Hunter Holmes McGuire VAMC, William S. Middleton Memorial Veterans Hospital, Clement J. Zablocki VAMC
The Prostate Cancer, Genetic Risk, and Equitable Screening Study (ProGRESS)
Prostate cancer is the most common non-skin cancer among veterans and the second leading cause of male cancer death. Current methods of screening men for prostate cancer are inaccurate and cannot identify which men do not have prostate cancer or have low-grade cases that will not cause harm and which men have significant prostate cancer needing treatment. False-positive screening tests can result in unnecessary prostate biopsies for men who do not need them. However, new genetic testing might help identify which men are at highest risk for prostate cancer. This study will examine whether a genetic test helps identify men at risk for significant prostate cancer while helping men who are at low risk for prostate cancer avoid unnecessary biopsies. If this genetic test proves beneficial, it will improve the way that health care providers screen male veterans for prostate cancer.
ID: NCT05926102
Sponsor; Investigator: VA Office of Research and Development; Jason L. Vassy, MD, MPH
Location: VA Boston Healthcare System
Prostate Active Surveillance Study (PASS)
This research study is for men who have chosen active surveillance as a management plan for their prostate cancer. Active surveillance is defined as close monitoring of prostate cancer with the offer of treatment if there are changes in test results. This study seeks to discover markers that will identify cancers that are more aggressive from those tumors that grow slowly.
ID: NCT00756665
Sponsor; Collaborators: University of Washington; Canary Foundation, Early Detection Research Network
Locations: VA San Francisco Health Care System, VA Puget Sound Health Care System
A Study of Checkpoint Inhibitors in Men With Progressive Metastatic Castrate Resistant Prostate Cancer Characterized by a Mismatch Repair Deficiency or Biallelic CDK12 Inactivation (CHOMP)
The primary objective is to assess the activity and efficacy of pembrolizumab, a checkpoint inhibitor, in veterans with metastatic castration-resistant prostate cancer characterized by either mismatch repair deficiency (dMMR) or biallelic inactivation of CDK12 (CDK12-/-). The secondary objectives involve determining the frequency with which dMMR and CDK12-/- occur in this patient population, as well as the effects of pembrolizumab on various clinical endpoints (time to prostate-specific antigen progression, maximal prostate-specific antigen response, time to initiation of alternative antineoplastic therapy, time to radiographic progression, overall survival, and safety and tolerability). Lastly, the study will compare the pretreatment and at-progression metastatic tumor biopsies to investigate the molecular correlates of resistance and sensitivity to pembrolizumab via RNA-sequencing, exome-sequencing, selected protein analyses, and multiplexed immunofluorescence.
ID: NCT04104893
Sponsor; Collaborator: VA Office of Research and Development; Merck Sharp & Dohme LLC
Locations: San Francisco VAMC, VA Greater Los Angeles Healthcare System, Washington DC VAMC, Bay Pines VA Healthcare System Jesse Brown VAMC, VA Ann Arbor Healthcare System, James J. Peter VAMC, VA NY Harbor Healthcare System, Durham VAMC, Corporal Michael J. Crescenz VAMC, Hunter Holmes McGuire VAMC, VA Puget Sound Health Care System
A Single-Arm Phase II Study of Neoadjuvant Intensified Androgen Deprivation (Leuprolideand Abiraterone Acetate) in Combination With AKT Inhibition (Capivasertib) for High-Risk Localized Prostate Cancer With PTEN Loss (SNARE)
The purpose of this study is to learn about how an investigational drug intervention completed before doing prostate surgery (specifically, radical prostatectomy with lymph node dissection) may help in the treatment of high-risk localized prostate cancers that are most resistant to standard treatments. This is a phase II research study. For this study, capivasertib, the study drug, will be taken with intensified androgen deprivation therapy drugs (iADT; abiraterone and leuprolide) prior to radical prostatectomy. This study drug treatment will be evaluated to see if it is effective in shrinking and destroying prostate cancer tumors prior to surgery and to further evaluate its safety prior to prostate cancer surgery.
ID: NCT05593497
Sponsor; Investigator: VA Office of Research and Development; Ryan P. Kopp, MD
Locations: VA Greater Los Angeles Healthcare System, James J. Peters VAMC, VA Portland Health Care System, South Texas Veterans Health Care System, VA Puget Sound Health Care System
Active, Not Recruiting
Intramuscular Mechanisms of Androgen Deprivation-related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes androgen deprivation therapy (ADT), or the lowering of the levels of the hormone testosterone as much as possible. Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as “sarcopenia,” and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because the investigators do not understand the mechanisms that cause it. The mitochondria are part of the cells responsible for providing energy to muscles, but to this date, the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT.
ID: NCT03867357
Sponsor; Collaborators: Seattle Institute for Biomedical and Clinical Research; DoD, University of Washington
Location: VA Puget Sound Health Care System
Radiation Therapy With or Without Androgen-Deprivation Therapy in Treating Patients With Prostate Cancer
RATIONALE: Radiation therapy uses high-energy X-rays and other types of radiation to kill tumor cells and shrink tumors. Androgens can cause the growth of prostate cancer cells. Androgen deprivation therapy (ADT) may lessen the amount of androgens made by the body. It is not yet known whether radiation therapy is more effective with or without ADT in treating patients with prostate cancer.
PURPOSE: This randomized phase III trial is studying radiation therapy to see how well it works compared with radiation therapy given together with ADT in treating patients with prostate cancer.
ID: NCT00936390
Sponsor; Collaborators: Radiation Therapy Oncology Group; National Cancer Institute, NRG Oncology
Locations: 518 locations, James A. Haley VA Hospital
Enzalutamide With or Without Abiraterone and Prednisone in Treating Patients With Castration-Resistant Metastatic Prostate Cancer
This randomized phase III trial studies enzalutamide to see how well it works compared to enzalutamide, abiraterone, and prednisone in treating patients with castration-resistant metastatic prostate cancer. Androgens can cause the growth of prostate cancer cells. Drugs, such as enzalutamide, abiraterone acetate, and prednisone, may lessen the amount of androgens made by the body.
ID: NCT01949337
Sponsor; Collaborators: Alliance for Clinical Trials in Oncology; NCI, Astellas Pharma US, Inc., Medivation, Inc., Biologics, Inc.
Locations: 539 locations, including VA Connecticut Healthcare System
S1216, Phase III ADT+TAK-700 vs ADT+Bicalutamide for Metastatic Prostate Cancer (S1216)
The purpose of this study is to compare overall survival in newly diagnosed metastatic prostate cancer patients randomly assigned to ADT + TAK-700 vs androgen deprivation therapy (ADT) + bicalutamide.
ID: NCT01809691
Sponsor; Collaborators: SWOG Cancer Research Network; Millennium Pharmaceuticals, Inc., NCI
Locations: 560 locations, including VA New York Harbor Healthcare System
Androgen Ablation Therapy With or Without Chemotherapy in Treating Patients With Metastatic Prostate Cancer (CHAARTED)
RATIONALE: Androgens can cause the growth of prostate cancer cells. Androgen ablation therapy may stop the adrenal glands from making androgens. Drugs used in chemotherapy, such as docetaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether androgen-ablation therapy is more effective with or without docetaxel in treating metastatic prostate cancer.
PURPOSE: This randomized phase III trial is studying androgen ablation therapy and chemotherapy to see how well they work compared to androgen ablation therapy alone in treating patients with metastatic prostate cancer.
ID: NCT00309985
Sponsor; Collaborator: ECOG-ACRIN Cancer Research Group; NCI
Locations: 343 locations, including Mather VAMC
Not Yet Recruiting
biraterone, Enzalutamide, or Apalutamide in Castrate-Sensitive Prostate Cancer
The investigators have used national Veterans Health Administration (VHA) data to demonstrate real-world efficacy of abiraterone and enzalutamide in veterans with metastatic castration-resistant prostate cancer. In the real world that is the VHA, the investigators have successfully estimated g values that accurately predict overall survival, and the use of this metric in other settings should now be explored. In the egalitarian system that is the VHA, the treatment of prostate cancer is excellent, uniform across the US and indifferent to race. The choices made are clearly personalized, given not all men received all therapies, and younger veterans were treated more aggressively.
ID: NCT05422911
Sponsor: James J. Peters VAMC
Location: James J. Peters VAMC
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires, and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor, Investigator: VA Greater Los Angeles Healthcare System; Gholam Berenji, MD
Location: VA Greater Los Angeles Healthcare System
18F-DCFPyL PET-CT Scan and Prostate Cancer
The primary objective of this study is to assess the efficacy of 18F-DCFPyL PET-CT for initial staging of prostate cancer in veterans compared to conventional imaging (99mTc-MDP bone scan and diagnostic CT or MRI). The primary clinical endpoint of our study is the percentage of veterans with prostate cancer in which the 18F-DCFPyL PET-CT identifies M1 disease at initial staging. Secondary objectives included frequency of the change in primary treatment plan after initial staging.
ID: NCT03852654
Sponsor, Investigator: Lida Jafari, MD
Location: VA Greater Los Angeles Healthcare System
Neoadjuvant Therapy With Docetaxel and Ketoconazole in Patients With High-Risk Prostate Cancer: A Pilot Study (IST 16167)
Eligible patients with high-risk prostate cancer who are scheduled to undergo radical prostatectomy will receive 4 cycles of therapy with ketoconazole and docetaxel prior to surgery resection. A cycle of therapy is defined as 21 days (3 weeks). Pharmacokinetic analysis will be performed during the first and second cycles of therapy. All patients will be evaluated for toxicity, tumor response, and recurrence.
ID: NCT00870714
Sponsor, Collaborator: Kansas City VAMC; Sanofi
Location: Kansas City VAMC
A Study of Epirubicin With Estramustine Phosphate and Celecoxib for the Treatment of Prostate Cancer
The purpose of this clinical trial is to find out the effect of epirubicin with estramustine phosphate and celecoxib on PSA and objective response in patients with hormone-resistant prostate cancer, as well as to evaluate the toxicity and quality of life of this combination. Celecoxib is an FDA-approved drug that treats arthritis. Epirubicin, alone or with estramustine phosphate, has been used in the treatment of hormone-resistant prostate cancer. These drugs have demonstrated evidence of tumor blood vessel suppression and a combination of these 3 drugs could possibly arrest further tumor growth or even make the tumor decrease in size.
ID: NCT00218205
Sponsor, Collaborator; Investigator: VA New Jersey Health Care System; Pfizer; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Phase II Trial of Combination Therapy With Celecoxib and Taxotere for the Treatment of Stage D3 Prostate Cancer
The purpose of this clinical trial is to find out the safety and effectiveness as well as the patient’s quality of life while taking the combination of Taxotere and celecoxib on patients with hormone refractory prostate cancer. Celecoxib (Celebrex) is an FDA-approved drug that treats arthritis. Taxotere (Docetaxel) is an FDA-approved chemotherapy drug to treat certain forms of cancer. Both drugs have demonstrated evidences of tumor blood vessel suppression and combination of these 2 drugs could possibly arrest further tumor growth or make the tumor decrease in size.
ID: NCT00215345
Sponsor, Collaborator; Investigator: Department of Veterans Affairs, New Jersey; Pfizer, Sanofi; Basil Kasimis, MD
Location: VA New Jersey Health Care System
A Yoga Program for Patients Undergoing Prostate Cancer Surgery
Men with localized prostate cancer are often treated with surgery, a treatment that is associated with high rates of adverse effects such as erectile dysfunction (ED) and urinary incontinence (UI) which impact quality of life. Yoga may improve control of UI and improve ED by bringing awareness to and strengthening the pelvic floor musculature. The randomized controlled pilot study is to assess the feasibility of an innovative hybrid (in-person and virtual) twice-weekly yoga program that includes a prehabilitation component and to obtain preliminary data that will help assess its potential effectiveness in alleviating prostate cancer treatment symptom burden (primarily ED and UI). The long-term goal is to develop a scalable and sustainable yoga program that helps cancer survivors manage their treatment side effects.
ID: NCT05929300
Sponsor, Investigator: VA Office of Research and Development; Abigail Silva, PhD, MPH
Location: Edward Hines Jr. VA Hospital
Acquired Factor VIII Deficiency Presenting as Compartment Syndrome
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Using Telehealth to Increase Lung Cancer Screening Referrals for At-Risk Veterans in Rural Communities
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
Annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) of the chest has been shown to reduce mortality rates for individuals at risk for lung cancer.1 Despite the benefits, < 5% of those who were eligible for LCS in the United States were screened in 2022.2 Implementation of a LCS program in rural communities is especially challenging because they are sparsely populated, medically underserved, and located far from urban centers.2-7 It is estimated that 1 in 5 people live in rural areas. Rates of tobacco smoking and cancer are higher in rural communities when compared with urban communities.8,9 The scarcity of physicians in rural areas who are familiar with LCS may further impede individuals who are at risk from accessing this life saving service.5,6 As a result, these individuals may not regularly undergo LCS as recommended.9
Telehealth, or the remote delivery of health care services via telecommunications, is an emerging approach for addressing unmet medical needs in rural communities and is being utilized widely by the US Department of Veterans Affairs (VA).4,10-15 The Veterans Integrated Service Network 12 (Great Lakes Network) has established the Clinical Resource Hub (CRH), a telehealth network comprising of licensed independent physicians, nurse practitioners, registered nurses, and ancillary staff. The CRH offers regular, remote health care services to several community-based outpatient clinics (CBOC) primary care clinics located in rural northern Wisconsin and the Upper Peninsula of Michigan.10,14
The utility of telehealth in promoting LCS among at-risk veterans living in rural communities has not been firmly established.4-6 To address this issue, we conducted a proof-of-principle quality improvement project to determine whether a telehealth intervention would increase referrals among at-risk veterans who reside in rural northern Wisconsin and the Upper Peninsula of Michigan who are self-enrolled in a CBOC smoking cessation program in Green Bay, Wisconsin. The CBOC provides primary health care to veterans residing in rural northern Wisconsin and the Upper Peninsula of Michigan as defined by US Department of Agriculture rural-urban commuting area codes.16 The intervention aimed to refer these individuals to the closest available and centralized LCS program, which is located at the Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin.
METHODS
We reviewed electronic health records (EHR) of LCS-eligible veterans treated by 2 authors (SH and TB) who were self-enrolled in the smoking cessation program at the Green Bay CBOC between October 1, 2020, and September 30, 2021. The program provides comprehensive evidence-based tobacco use treatment, online self-help resources, behavioral counseling, and medicines for smoking cessation.17 Veterans aged 50 to 80 years with a smoking history of ≥ 20 pack-years, who currently smoke cigarettes or quit within the past 15 years, were considered at risk for lung cancer and eligible for LCS. After confirming eligibility, pertinent demographic data were abstracted from each EHR.
Telehealth Intervention
The CJZVAMC centralized LCS program manages all delivery processes and has been previously shown to increase uptake of LCS and improve patient outcomes among veterans as compared to a decentralized approach.18,19 In the centralized approach, eligible veterans were referred by a CBOC primary care practitioner (PCP) to a designated centralized LCS program. The centralized LCS program provides further evaluation and disposition, which includes structured and shared decision making, ordering LDCT of the chest, reporting LDCT results to the patient and PCP, devising a goal-directed care plan, and managing follow-up LDCTs as indicated (Figure 1).18,19
This intervention was initiated before other measures aimed to increase the LCS enrollment for at-risk rural veterans at the CBOC, (eg, mailing LCS education fact sheet to veterans).20 After reviewing prospective veterans’ EHRs, 1 author (TB) contacted LCS-eligible veterans by telephone and left a voicemail if contact could not be established. A second telephone call was placed within 2 months of the initial call if no call back was documented in the EHR. When verbal contact was established, the goals of the centralized LCS program were described and the veteran was invited to participate.21
Veterans were seen at CBOCs affiliated with CJZVAMC. The CJZVAMC LCS coordinator was notified whenever a veteran agreed to enroll into LCS and then ordered LDCT, which was performed and read at CJZVAMC. Once LDCT has been ordered, 1 author (TB) reviewed the veteran’s EHR for LDCT completion over the next 4 months.Upon conclusion of the intervention period, the number of veterans referred for LDCT and the number of LDCTs performed were recorded. Each LDCT was reviewed and coded by medical imaging clinicians according to Lung CT Screening Reporting and Data System (Lung-RADS) version 1.1 and coded as 0, 1, 2, 3, or 4 based on the nodule with the highest degree of suspicion.22 The LDCT and reports were also reviewed by pulmonary physicians at the CJZVAMC Lung Nodule Clinic with recommendations issued and reported to the PCP treating the veteran, such as annual follow-up with LDCT or referral to specialty care for further evaluation as indicated.
RESULTS
Of 117 veterans enrolled in the smoking cessation program at the CBOC during the intervention period, 74 (63%) were eligible to undergo LCS, and 68 (58%) were contacted by telephone (Figure 2). Eligible patients were primarily White male veterans; their mean (SD) age was 65.0 years (7.6). Participation in LCS was discussed with 41 (60%) veterans either during the initial or second telephone call of which 29 (71%) agreed to enroll and 12 (29%) declined. Veterans did not provide reasons for declining participation at the time of the telephone call.
Among the 74 eligible veterans who attended the smoking cessation program, only 3 had LDCT performed before initiation of this project (4%). At the conclusion of the telehealth intervention period, 19 veterans had LDCT performed (26%). Ten LDCTs were coded Lung-RADS 1, 7 Lung-RADS 2, 1 Lung-RADS 3, and 1 Lung-RADS 4B. In each case, annual follow-up LDCT or referral to a LCS clinician was pursued as indicated.22
DISCUSSION
This proof-of-principle quality improvement project found that a high percentage (66%) of individuals in rural communities who were contacted via telehealth agreed to participate in a regional LCS program. The program reviewed LDCT results, ordered follow-up LDCTs, and recommended further evaluations.18,19 Whether this centralized LCS process could also promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians, if abnormal imaging findings are detected, remains unclear.
It has been well established LDCT LCS reduces lung cancer-specific and overall mortality rates among eligible current and former smokers.1,9,23 The 5-year relative survival rate of veterans diagnosed with localized non-small cell lung cancer is 63%; that number drops to 7% in those with advanced disease attesting to the utility of LCS in detecting early stage lung cancer.2 Despite these favorable observations, however, screening rates with free LDCT remains low in rural communities.3-7
This proof-of-principle quality improvement project found that telehealth intervention may increase referrals of at-risk veterans who reside in rural communities to the closest centralized LCS program located at aregional VAMC. This program is responsible for reviewing the results of the initial LDCT, ordering follow-up LDCT, and recommending further evaluation as indicated.18,19 Whether this centralized LCS process would promote adherence with subsequent annual LDCT and/or scheduled clinic appointments with designated clinicians if abnormal imaging findings are detected is yet to be determined.
We found that among 74 LCS-eligible rural veterans attending a CBOC-based smoking cessation program, only 3 (4%) underwent LDCT screening before this telehealth intervention was launched. This low LCS rate among veterans attempting to quit smoking may have been related, in part, to a lack of awareness of this intervention and/or barriers to LCS access.7,10,21,24 Deploying a telehealth intervention targeting LCS could address this life threatening and unmet medical need in rural communities.25 The results of this proof-of-principle quality improvement project support this contention with the reported increased referrals to and completion of initial LDCT within 4 months of the telehealth encounter.
Limitations
This was a small, single site project composed of predominantly White male rural veterans participating in a smoking cessation program associated with a VA facility.26,27 It is not clear whether similar outcomes would be observed in at-risk veterans who do not participate in a smoking cessation program or in more diverse communities. We were unable to contact 40% of LCS-eligible rural veterans by telephone. Twelve veterans reached by telephone declined to participate in LCS without providing a reason, and only 19 of 68 eligible veterans (28%) underwent LDCT screening during the 4-month telehealth intervention. The reasons underlying this overall low accrual rate and whether rural veterans prefer other means of personal communication regarding LCS were not determined. Lastly, generalizability of our initial observations to other veterans living in rural communities is limited because the project was conducted only in rural northern Wisconsin and the Upper Peninsula of Michigan.
Conclusions
At-risk rural veterans may be willing to participate in a centralized LCS program at a regional VA medical facility when contacted and coordinated using telehealth modalities. These findings offer support for future prospective, multisite, VA telehealth-based studies to be conducted in rural areas. The results of this project also suggest that telehealth intervention could increase referrals of at-risk rural veterans to the closest centralized LCS program located at a regional VA medical facility.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
2. State of Lung Cancer: 2023 Report. American Lung Association. November 14, 2023. Accessed June 4, 2024. https://www.lung.org/getmedia/186786b6-18c3-46a9-a7e7-810f3ce4deda/SOLC-2023-Print-Report.pdf
3. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis. 2019;11(3):873-881. doi:10.21037/jtd.2019.01.105
4. Petraglia AF, Olazagasti JM, Strong A, Dunn B, Anderson RT, Hanley M. Establishing satellite lung cancer screening sites with telehealth to address disparities between high-risk smokers and American College of Radiology-approved Centers of Designation. J Thorac Imaging. 2021;36(1):2-5. doi:10.1097/RTI.0000000000000520
5. Odahowski CL, Zahnd WE, Eberth JM. Challenges and opportunities for lung cancer screening in rural America. J Am Coll Radiol. 2019;16(4 Pt B):590-595. doi:10.1016/j.jacr.2019.01.001
6. Rohatgi KW, Marx CM, Lewis-Thames MW, Liu J, Colditz GA, James AS. Urban-rural disparities in access to low-dose computed tomography lung cancer screening in Missouri and Illinois. Prev Chronic Dis. 2020;17:E140. doi:10.5888/pcd17.200202
7. Boudreau JH, Miller DR, Qian S, Nunez ER, Caverly TJ, Wiener RS. Access to lung cancer screening in the Veterans Health Administration: does geographic distribution match need in the population? Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
8. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute, US Dept of Health and Human Services; April 15, 2020. Accessed June 4, 2024. https://seer.cancer.gov/archive/csr/1975_2017/index.html
9. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
10. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17(5):582-587. doi:10.1016/j.nurpra.2021.02.020
11. Yacoub JH, Swanson CE, Jay AK, Cooper C, Spies J, Krishnan P. The radiology virtual reading room: during and beyond the COVID-19 pandemic. J Digit Imaging. 2021;34(2):308-319. doi:10.1007/s10278-021-00427-4
12. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38(6):925-929. doi:10.1002/hed.24386
13. Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23(7):e26759. doi:10.2196/26759
14. Raza T, Joshi M, Schapira RM, Agha Z. Pulmonary telemedicine - a model to access the subspecialist services in underserved rural areas. Int J Med Inform. 2009;78(1):53-59. doi:10.1016/j.ijmedinf.2008.07.010
15. Chen A, Ayub MH, Mishuris RG, et al. Telehealth policy, practice, and education: a position statement of the Society of General Internal Medicine. J Gen Intern Med. 2023;38(11):2613-2620. doi:10.1007/s11606-023-08190-8
16. Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed June 4, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
17. VHA Directive 1056: National Smoking and Tobacco Use Cessation Program. Veterans Health Administration, US Dept of Veterans Affairs; September 5, 2019. Accessed June 4, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8488
18. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818-825. doi:10.1016/j.chest.2021.09.002
19. Lewis JA, Samuels LR, Denton J, et al. The association of health care system resources with lung cancer screening implementation: a cohort study. Chest. 2022;162(3):701-711. doi:10.1016/j.chest.2022.03.050
20. US Dept of Veterans Affairs. Lung cancer screening: patient education fact sheet. Accessed July 8, 2024. https://www.cancer.va.gov/assets/pdf/survey/LCSflyer.pdf
21. Melzer AC, Golden SE, Ono SS, Datta S, Crothers K, Slatore CG. What exactly is shared decision-making? A qualitative study of shared decision-making in lung cancer screening. J Gen Intern Med. 2020;35(2):546-553. doi:10.1007/s11606-019-05516-3
22. Chelala L, Hossain R, Kazerooni EA, Christensen JD, Dyer DS, White CS. Lung-RADS Version 1.1: challenges and a look ahead, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216(6):1411-1422. doi:10.2214/AJR.20.24807
23. Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open. 2021;4(10):e2128176. doi:10.1001/jamanetworkopen.2021.28176
24. Golden SE, Ono SS, Thakurta SG, et al. “I’m putting my trust in their hands”: a qualitative study of patients’ views on clinician initial communication about lung cancer screening. Chest. 2020;158(3):1260-1267. doi:10.1016/j.chest.2020.02.072
25. Park ER, Chiles C, Cinciripini PM, et al. Impact of the COVID-19 pandemic on telehealth research in cancer prevention and care: a call to sustain telehealth advances. Cancer. 2021;127(3):334-338. doi:10.1002/cncr.33227
26. Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14(9):1528-1537. doi:10.1016/j.jtho.2019.04.024
27. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi:10.1016/j.cct.2021.106586
Prognostication in Hospice Care: Challenges, Opportunities, and the Importance of Functional Status
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer, and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer, and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
Predicting life expectancy and providing an end-of-life diagnosis in hospice and palliative care is a challenge for most clinicians. Lack of training, limited communication skills, and relationships with patients are all contributing factors. These skills can improve with the use of functional scoring tools in conjunction with the patient’s comorbidities and physical/psychological symptoms. The Palliative Performance Scale (PPS), Karnofsky Performance Scale (KPS), and Eastern Cooperative Oncology Group Performance Status Scale (ECOG) are commonly used functional scoring tools.
The PPS measures 5 functional dimensions including ambulation, activity level, ability to administer self-care, oral intake, and level of consciousness.1 It has been shown to be valid for a broad range of palliative care patients, including those with advanced cancer or life-threatening noncancer diagnoses in hospitals or hospice care.2 The scale, measured in 10% increments, runs from 100% (completely functional) to 0% (dead). A PPS ≤ 70% helps meet hospice eligibility criteria.
The KPS evaluates functional impairment and helps with prognostication. Developed in 1948, it evaluates a patient’s functional ability to tolerate chemotherapy, specifically in lung cancer, and has since been validated to predict mortality across older adults and in chronic disease populations.3,4 The KPS is also measured in 10% increments ranging from 100% (completely functional without assistance) to 0% (dead). A KPS ≤ 70% assists with hospice eligibility criteria (Table 1).5
Developed in 1974, the ECOG has been identified as one of the most important functional status tools in adult cancer care.6 It describes a cancer patient’s functional ability, evaluating their ability to care for oneself and participate in daily activities.7 The ECOG is a 6-point scale; patients can receive scores ranging from 0 (fully active) to 5 (dead). An ECOG score of 4 (sometimes 3) is generally supportive of meeting hospice eligibility (Table 2).6
CASE Presentation
An 80-year-old patient was admitted to the hospice service at the Veterans Affairs Puget Sound Health Care System (VAPSHCS) community living center (CLC) in Tacoma, Washington, from a community-based acute care hospital. His medical history included prostate cancer with metastasis to his pelvis and type 2 diabetes mellitus, which was stable with treatment with oral medication. Six weeks earlier the patient reported a severe frontal headache that was not responding to over-the-counter analgesics. After 2 days with these symptoms, including a ground-level fall without injuries, he presented to the VAPSHCS emergency department (ED) where a complete neurological examination, including magnetic resonance imaging, revealed a left frontoparietal brain lesion that was 4.2 cm × 3.4 cm × 4.2 cm.
The patient experienced a seizure during his ED evaluation and was admitted for treatment. He underwent a craniotomy where most, but not all the lesions were successfully removed. Postoperatively, the patient exhibited right-sided neglect, gait instability, emotional lability, and cognitive communication disorder. The patient completed 15 of 20 planned radiation treatments but declined further radiation or chemotherapy. The patient decided to halt radiation treatments after being informed by the oncology service that the treatments would likely only add 1 to 2 months to his overall survival, which was < 6 months. The patient elected to focus his goals of care on comfort, dignity, and respect at the end of life and accepted recommendations to be placed into end-of-life hospice care. He was then transferred to the VAPSHCS CLC in Tacoma, Washington, for hospice care.
Upon admission, the patient weighed 94 kg, his vital signs were within reference range, and he reported no pain or headaches. His initial laboratory results revealed a 13.2 g/dL hemoglobin, 3.6 g/dL serum albumin, and a 5.5% hemoglobin A1c, all of which fall into a normal reference range. He had a reported ECOG score of 3 and a KPS score of 50% by the transferring medical team. The patient’s medications included scheduled dexamethasone, metformin, senna, levetiracetam, and as-needed midazolam nasal spray for breakthrough seizures. He also had as-needed acetaminophen for pain. He was alert, oriented ×3, and fully ambulatory but continuously used a 4-wheeled walker for safety and gait instability.
After the patient’s first night, the hospice team met with him to discuss his understanding of his health issues. The patient appeared to have low health literacy but told the team, “I know I am dying.” He had completed written advance directives and a Portable Order for Life-Sustaining Treatment indicating that life-sustaining treatments, including cardiopulmonary resuscitation, supplemental mechanical feeding, or intubation, were not to be used to keep him alive.
At his first 90-day recertification, the patient had gained 8 kg and laboratory results revealed a 14.6 g/dL hemoglobin, 3.8 g/dL serum albumin, and a 6.1% hemoglobin A1c. His ECOG score remained at 3, but his KPS score had increased to 60%. The patient exhibited no new neurologic symptoms or seizures and reported no headaches but had 2 ground-level falls without injury. On both occasions the patient chose not to use his walker to go to the bathroom because it was “too far from my bed.” Per VA policy, after discussions with the hospice team, he was recertified for 90 more days of hospice care. At the end of 6 months in CLC, the patient’s weight remained stable, as did his complete blood count and comprehensive medical panel. He had 1 additional noninjurious ground-level fall and again reported no pain and no use of as-needed acetaminophen. His only medical complication was testing positive for COVID-19, but he remained asymptomatic. The patient was graduated from hospice care and referred to a nearby non-VA adult family home in the community after 180 days. At that time his ECOG score was 2 and his KPS score had increased to 70%.
DISCUSSION
Primary brain tumors account for about 2% of all malignant neoplasms in adults. About half of them represent gliomas. Glioblastoma multiforme derived from neuroepithelial cells is the most frequent and deadly primary malignant central nervous system tumor in adults.8 About 50% of patients with glioblastomas are aged ≥ 65 years at diagnosis.9 A retrospective study of Centers for Medicare and Medicaid Services claims data paired with the Surveillance, Epidemiology, and End Results database indicated a median survival of 4 months for patients with glioblastoma multiforme aged > 65 years, including all treatment modalities.10 Surgical resection combined with radiation and chemotherapy offers the best prognosis for the preservation of neurologic function.11 However, comorbidities, adverse drug effects, and the potential for postoperative complications pose significant risks, especially for older patients. Ultimately, goals of care conversations and advance directives play a very important role in evaluating benefits vs risks with this malignancy.
Our patient was aged 80 years and had previously been diagnosed with metastatic prostate malignancy. His goals of care focused on spending time with his friends, leaving his room to eat in the facility dining area, and continuing his daily walks. He remained clear that he did not want his care team to institute life-sustaining treatments to be kept alive and felt the information regarding the risks vs benefits of accepting chemotherapy was not aligned with his goals of care. Over the 6 months that he received hospice care, he gained weight, improved his hemoglobin and serum albumin levels, and ambulated with the use of a 4-wheeled walker. As the patient exhibited no functional decline or new comorbidities and his functional status improved, the clinical staff felt he no longer needed hospice services. The patient had an ECOG score of 2 and a KPS score of 70% at his hospice graduation.
Medical prognostication is one of the biggest challenges clinicians face. Clinicians are generally “over prognosticators,” and their thoughts tend to be based on the patient relationship, overall experiences in health care, and desire to treat and cure patients.12 In hospice we are asked to define the usual, normal, or expected course of a disease, but what does that mean? Although metastatic malignancies usually have a predictable course in comparison to diagnoses such as dementia, chronic obstructive pulmonary disease, or congestive heart failure, the challenges to improve prognostic ability andpredict disease course continue.13-15 Focusing on functional status, goals of care, and comorbidities are keys to helping with prognosis. Given the challenge, we find the PPS, KPS, and ECOG scales important tools.
When prognosticating, we attempt to define quantity and quality of life (which our patients must define independently or from the voice of their surrogate) and their ability to perform daily activities. Quality of life in patients with glioblastoma is progressively and significantly impacted due to the emergence of debilitating neurologic symptoms arising from infiltrative tumor growth into functionally intact brain tissue that restricts and disrupts normal day-to-day activities. However, functional status plays a significant role in helping the hospice team improve its overall prognosis.
Conclusions
This case study illustrates the difficulty that comes with prognostication(s) despite a patient's severely morbid disease, history of metastatic prostate cancer, and advanced age. Although a diagnosis may be concerning, documenting a patient’s status using functional scales prior to hospice admission and during the recertification process is helpful in prognostication. Doing so will allow health care professionals to have an accepted medical standard to use regardless how distinct the patient's diagnosis. The expression, “as the disease does not read the textbook,” may serve as a helpful reminder in talking with patients and their families. This is important as most patient’s clinical disease courses are different and having the opportunity to use performance status scales may help improve prognostic skills.
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/
1. Cleary TA. The Palliative Performance Scale (PPSv2) Version 2. In: Downing GM, ed. Medical Care of the Dying. 4th ed. Victoria Hospice Society, Learning Centre for Palliative Care; 2006:120.
2. Palliative Performance Scale. ePrognosis, University of California San Francisco. Accessed June 14, 2024. https://eprognosis.ucsf.edu/pps.php
3. Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191-205.
4. Khalid MA, Achakzai IK, Ahmed Khan S, et al. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11(4):301-305.
5. Karnofsky Performance Scale. US Dept of Veterans Affairs. Accessed June 14, 2024. https://www.hiv.va.gov/provider/tools/karnofsky-performance-scale.asp
6. Mischel A-M, Rosielle DA. Eastern Cooperative Oncology Group Performance Status. Palliative Care Network of Wisconsin. December 10, 2021. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/eastern-cooperative-oncology-group-performance-status/
7. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
8. Nizamutdinov D, Stock EM, Dandashi JA, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67-e74. doi:10.1016/j.wneu.2017.09.104
9. Kita D Ciernik IFVaccarella S Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17-22. doi:10.1159/000210017
10. Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122(2):189-197. doi:10.1002/cnr.29742
11. Brown, NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;14(13):3161. doi:10.3390/cancers14133161
12. Christalakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. University of Chicago Press; 2000.
13. Weissman DE. Determining Prognosis in Advanced Cancer. Palliative Care Network of Wisconsin. January 28, 2019. Accessed June 14, 2014. https://www.mypcnow.org/fast-fact/determining-prognosis-in-advanced-cancer/
14. Childers JW, Arnold R, Curtis JR. Prognosis in End-Stage COPD. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognosis-in-end-stage-copd/
15. Reisfield GM, Wilson GR. Prognostication in Heart Failure. Palliative Care Network of Wisconsin. February 11, 2019. Accessed June 14, 2024. https://www.mypcnow.org/fast-fact/prognostication-in-heart-failure/