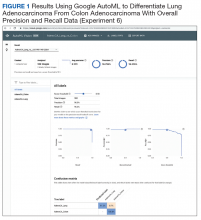
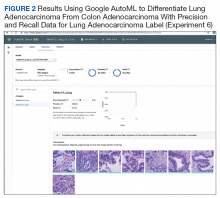
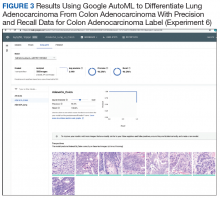
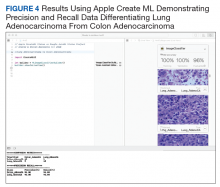
User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


ASCO to award $50,000 young investigator grant to study MCL
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Early-career researchers who are interested in studying
The young investigator grant is for a 1-year period and the award is used to fund a project focused on clinical or translational research on the clinical biology, natural history, prevention, screening, diagnosis, therapy, or epidemiology of MCL.
The purpose of this annual award, according to ASCO, is to fund physicians during the transition from a fellowship program to a faculty appointment.
Eligible applicants must be physicians currently in the last 2 years of final subspecialty training and within 10 years of having obtained his or her medical degree. Additionally, applicants must be planning a research career in clinical oncology, with a focus on MCL.
The grant selection committee’s primary criteria include the significance and originality of the proposed study and hypothesis, the feasibility of the experiment and methodology, whether it has an appropriate and detailed statistical analysis plan, and if the research is patient oriented.
The application deadline is Jan. 7, 2020, and the award term is July 1, 2020–June 30, 2021.
Application instructions are available on the ASCO website.
Trastuzumab benefit lasts long-term in HER2+ breast cancer
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
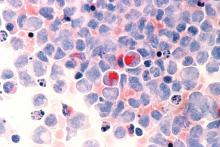
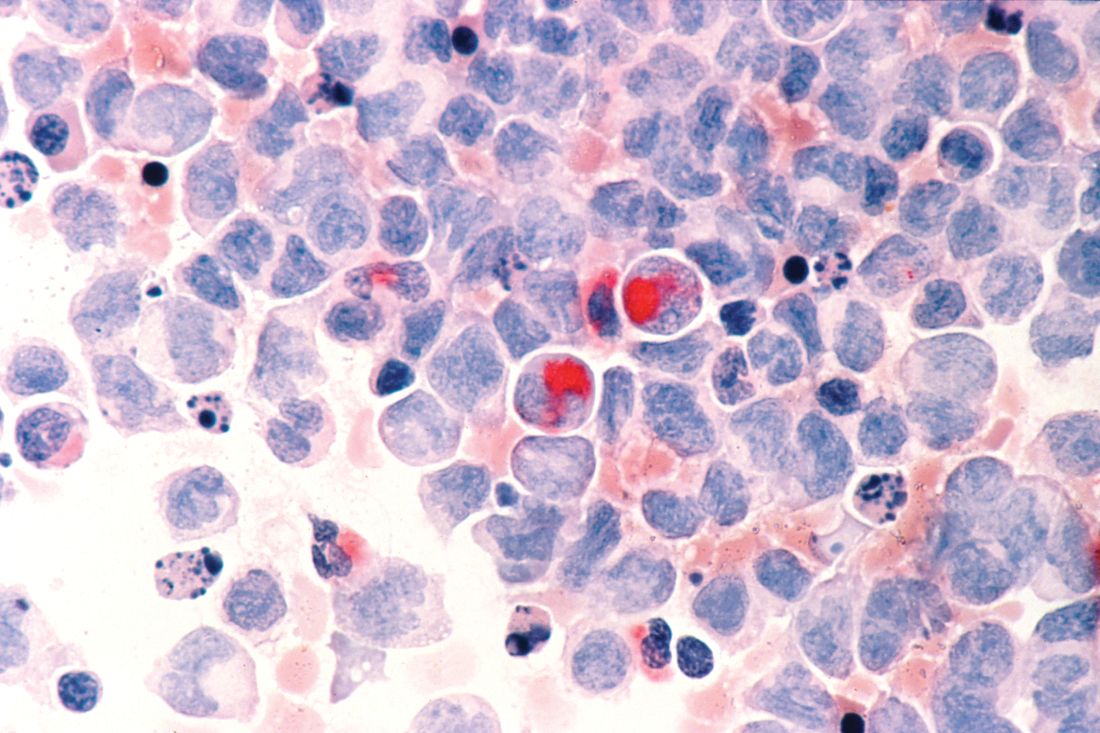
Adverse cytogenetics trump molecular risk in NPM1-mutated AML
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
REPORTING FROM THE JOURNAL OF CLINICAL ONCOLOGY
Nivolumab boosts overall survival in HCC
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
REPORTING FROM ESMO 2019
New practice guideline: CRC screening isn’t necessary for low-risk patients aged 50-75 years
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
FROM BMJ
Smoking Out the Truth About Pot and Cancer
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
MINNEAPOLIS -- Medical professionals within the US Department of Veterans Affairs (VA) can’t prescribe cannabis or certify patients to be able to get it. VA pharmacists can’t dispense it. Still, “we’re asked about it plenty,” a hospice and palliative care specialist told colleagues, at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
That brings up a big question, said Michael Stellini, MD, MS, FACP, FAAHPM, of Wayne State University, Karmanos Cancer Center, and the John D. Dingell VA Medical Center, in Detroit Michigan: “Should sick people be smoking pot?”
Even the question itself isn’t a simple one to answer since smoking isn’t the only way to consume cannabis for medical purposes. And figuring out the best advice is difficult. As Dr. Stellini said, there’s plenty of uncertainty about crucial cannabis topics like safety and benefits.
Dr. Stellini offered a number of facts and tips about cannabis in medicine.
Understand ‘qualifying conditions’ in your state
In states with legal medical marijuana, he said, physicians do not prescribe marijuana. However, they may certify that patients are eligible to get the drug for medical purposes if they meet certain qualifications.
A typical list of qualifying conditions includes diseases such as cancer, glaucoma, HIV/AIDS and Crohn’s disease. Qualifying conditions also tend to include treatments for severe diseases that produce wasting syndrome, severe and chronic pain, severe nausea, seizures and severe and persistent muscle spasm.
In Michigan, where Dr. Stellini practices, a panel in 2018 approved a long list of added qualifying conditions such as chronic pain, obsessive compulsive disorder and arthritis. But the panel rejected other conditions such as anxiety, asthma, panic attacks and schizophrenia.
Vaporizers are an alternative to joints, but...
Vaporizers are commonly used as an alternative to smoking marijuana joints, Dr. Stellini said, and they don’t significantly release tars or much if any carbon monoxide. While research is limited, he said, use of vaporizers hasn’t been linked to more lung cancer or chronic obstructive pulmonary disease.
“Vaping” is another option, but it’s been linked to dozens of deaths and hundreds of cases of illness in recent weeks. Many patients have reported using products that contain THC, a component of marijuana.
Other delivery methods exist
Marijuana can be ingested in liquid and solid food. “But edibles can have a slow onset of action compared to vaporizing or smoking,” Dr. Stellini said. “You might over-indulge. When users get to their steady state, they might have some adverse effects [AEs].”
Marijuana still has risks
Cannabis use has a long list of well-known AEs linked to the THC component. The most common are drowsiness, fatigue, dizziness, dry mouth, anxiety, cognitive effects, cough, and nausea, Dr. Stellini said. More serious AEs such as psychosis have been reported.
And, of course, users of cannabis with THC get high if they use enough.
A 2017 National Academies of Sciences, Engineering and Medicine report linked cannabis use to a higher risk of motor vehicle accidents. Still, Dr. Stellini said, “it’s relatively safe with respect to mortality, especially compared to opioids.”1
Risk of use in cancer may be low
Research suggest that patients with cancer use cannabis as much as other people and perhaps even more, Dr. Stellini said. But are they facing any extra risks? In general, he said, it doesn’t appear that way.
Cannabis seems to be safe when used with chemotherapy, he said, and drug-drug interactions in cancer appear to be rare. Some studies have suggested that cannabinoids—a component of marijuana—may be an effective treatment for chemotherapy-induced peripheral neuropathy.
However, he said, 1 study has raised a red flag about a possible interaction with cancer immunotherapy. Researchers found evidence that patients who used cannabis had lower tumor response rates to nivolomab for advanced melanoma, non-small cell lung cancer, and renal clear cell carcinoma. However, survival wasn’t affected.2
Meanwhile, he said, there’s no strong evidence that cannabis is a useful treatment for cancer, he said, although it’s worth investigating.
Cannabidiol is the hot new product
Cannabidiol, also known as CBD, has become hugely popular, Dr. Stellini said. It is derived from hemp and doesn’t cause a “buzz” like cannabis.
Due to lack of regulation, he said, buyers should beware. And, he said, CBD has multiple EAs. Standard doses can cause drowsiness, fatigue, dizziness, dry mouth, hypotension and lightheadedness.
Dr. Stellini reports no relevant disclosures.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549-554.
For Cancer Survivors, Nutrition Is Empowering
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
MINNEAPOLIS -- Ignore the big health claims about vitamin supplements, pork, and nitrate-free food products. Meet patients “where they are,” even if that means you focus first on helping a morbidly obese patient maintain her weight instead of losing pounds. And use nutrition to empower patients and reduce the risk of cancer recurrence.
Dianne Piepenburg, MS, RDN, CSO, a certified oncology nutritionist at the Malcolm Randall VA Medical Center in Gainesville, Florida, offered these tips and more in a presentation about nutrition for cancer survivors. She spoke at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
According to the National Institutes of Health, an estimated 17 million cancer survivors live in the US, accounting for 5% of the population. Nearly two-thirds are aged ≥ 65 years.1
Piepenburg highlighted the existence of certified specialists in oncology nutrition (CSOs). To be certified, registered dietitian nutritionists must have worked in that job for at least 2 years, have at least 2,000 hours of practice experience within the past 5 years and pass a board exam every 5 years.
Oncology nutritionists seek to empower cancer survivors to regain equilibrium in their lives, she said. “When a patient is told what scan to have next, what blood work they have to have, what treatment they need to be on, they feel they’re losing control,” she said. “Nutrition gives the power back to them, and they feel like there’s something they can do that’s in their control.”
Piepenburg urged colleagues to “meet patients where they are.” She gave the example of a patient with breast cancer whose body mass index is in the 50s, making her morbidly obese. “Our discussion wasn’t, ‘Let’s start [losing weight] today.’ Instead, I said, ‘Can we at least prevent you from gaining any more weight?’ She thought she could at least do that, try to recuperate a bit, and then start looking at a healthy weight loss. We’ll start there and circle back in a few months and see where we’re at.”
Piepenburg urged colleagues to bring exercise into the discussion. “We need people to be physically active no matter what phase of their survivorship journey they are in,” she said.
What about people who say, “I’ve never exercised a day in my life”? Her response: “I tell folks that we need them to move more. Maybe they’re walking to the mailbox or 3 laps around the house that day.”
Oncology patients should also watch sugar, meat, and processed foods. Refined sugar, fast food and processed food should be limited, Piepenburg said, along with red meats, such as beef, pork and lamb.
“Pork is not the ‘other white meat.’ How many of you grew up seeing and hearing that in the 1970s and 1980s? It’s a red meat, and it’s metabolized like a red meat.”
Advise patients to limit bacon, sausage, and lunch meat, she said, “even if they say, ‘I bought the nitrate-free and it’s really healthy for me.’”
It’s okay to eat some red meat, she said, “but there’s a tipping point. Tell them they can have some red meat but have it as a treat and please focus more on plant-based proteins—nuts, beans, legumes. But it’s tough for a lot of our veterans who grew up on meat and potatoes, and the only vegetable they eat is corn.”
It’s tough to limit grilling in a place like Minnesota, Piepenburg said, where the prime grilling season is short, and locals go a bit nuts when it’s nice enough outside. “I tell them to at least marinate the meat and put it on indirect heat.”
Finally, she encouraged oncology care providers to not fall for vitamin hype. Don’t rely on supplements for cancer prevention, she said. With some exceptions, she said, research has suggested they don’t work, and a 1990s study of beta-carotene and retinyl palmitate (vitamin A) in lung cancer was halted because patients actually fared worse on the regimen, although the effects didn’t seem to persist.2
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
1. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Office of Cancer Survivorship. Statistics. Updated February 8, 2019. Accessed October 7, 2019.
2. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743-1750.
Targeted agents vs. chemoimmunotherapy as first-line treatment of CLL
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
SAN FRANCISCO – Should targeted agents replace chemoimmunotherapy (CIT) as first-line treatment for chronic lymphocytic leukemia (CLL)? A recent debate suggests there’s no consensus.
William G. Wierda, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, debated the topic at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Wierda argued that CLL patients should receive a BTK inhibitor or BCL2 inhibitor, with or without obinutuzumab, as first-line therapy because these targeted agents have been shown to provide better progression-free survival (PFS) than CIT, and the targeted therapies may prolong overall survival (OS) as well.
Dr. Brown countered that targeted agents don’t improve PFS for all CLL patients, improved PFS doesn’t always translate to improved OS, and targeted agents cost more than CIT.
No role for CIT as first-line treatment
“We have two approaches right now, with nonchemoimmunotherapy-based treatment,” Dr. Wierda said. “One approach, with small-molecule inhibitors, is to have a sustained and durable period of disease control, particularly with BTK inhibitors. The other strategy that has emerged is deep remissions with fixed-duration treatment with BCL2 small-molecule inhibitor-based therapy, which, I would argue, is better than being exposed to genotoxic chemoimmunotherapy.”
Dr. Wierda went on to explain that the BTK inhibitor ibrutinib has been shown to improve PFS, compared with CIT, in phase 3 trials.
In the iLLUMINATE trial, researchers compared ibrutinib plus obinutuzumab to chlorambucil plus obinutuzumab as first-line treatment in CLL. At a median follow-up of 31.3 months, the median PFS was not reached in the ibrutinib arm and was 19 months in the chlorambucil arm (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:43-56).
In the A041202 study, researchers compared ibrutinib alone (Ib) or in combination with rituximab (Ib-R) to bendamustine plus rituximab (BR) in untreated, older patients with CLL. The 2-year PFS estimates were 74% in the BR arm, 87% in the Ib arm, and 88% in the Ib-R arm (P less than .001 for BR vs. Ib or Ib-R; N Engl J Med. 2018; 379:2517-28).
In the E1912 trial, researchers compared Ib-R to fludarabine, cyclophosphamide, and rituximab (FCR) in younger, untreated CLL patients. The 3-year PFS was 89.4% with Ib-R and 72.9% with FCR (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43).
Dr. Wierda noted that the E1912 trial also showed superior OS with Ib-R. The 3-year OS rate was 98.8% with Ib-R and 91.5% with FCR (P less than .001). However, there was no significant difference in OS between the treatment arms in the A041202 trial or the iLLUMINATE trial.
“But I would argue that is, in part, because of short follow-up,” Dr. Wierda said. “The trials were all designed to look at progression-free survival, not overall survival. With longer follow-up, we may see differences in overall survival emerging.”
Dr. Wierda went on to say that fixed‐duration treatment with the BCL2 inhibitor venetoclax can improve PFS over CIT.
In the phase 3 CLL14 trial, researchers compared fixed-duration treatment with venetoclax plus obinutuzumab to chlorambucil plus obinutuzumab in previously untreated CLL patients with comorbidities. The estimated PFS at 2 years was 88.2% in the venetoclax group and 64.1% in the chlorambucil group (P less than .001; N Engl J Med. 2019; 380:2225-36).
“[There was] no difference in overall survival,” Dr. Wierda noted. “But, again, I would argue ... that follow-up is relatively limited. We may ultimately see a difference in overall survival.”
Based on these findings, Dr. Wierda made the following treatment recommendations:
- Any CLL patient with del(17p) or TP53 mutation, and older, unfit patients with unmutated IGHV should receive a BTK inhibitor, with or without obinutuzumab.
- All young, fit patients, and older, unfit patients with mutated IGHV should receive a BCL2 inhibitor plus obinutuzumab.
Dr. Wierda also noted that ibrutinib and venetoclax in combination have shown early promise for patients with previously untreated CLL (N Engl J Med. 2019; 380:2095-2103).
CIT still has a role as first-line treatment
Dr. Brown suggested that a PFS benefit may not be enough to recommend targeted agents over CIT. For one thing, the PFS benefit doesn’t apply to all patients, as the IGHV-mutated subgroup does equally well with CIT and targeted agents.
In the IGHV-mutated group from the E1912 trial, the 3-year PFS was 88% for patients who received Ib-R and those who received FCR (N Engl J Med. 2019 Aug 1;381:432-43). In the A041202 study, the 2-year PFS among IGHV-mutated patients was 87% in the BR arm, 86% in the Ib arm, and 88% in the Ib-R arm (N Engl J Med. 2018; 379:2517-28).
In the CLL14 trial, PFS rates were similar among IGHV-mutated patients who received chlorambucil plus obinutuzumab and IGHV-mutated or unmutated patients who received venetoclax and obinutuzumab (N Engl J Med. 2019; 380:2225-36).
Dr. Brown also noted that the overall improvement in PFS observed with ibrutinib and venetoclax doesn’t always translate to improved OS.
In the A041202 study, there was no significant difference in OS between the Ib, Ib-R, and BR arms (N Engl J Med. 2018; 379:2517-28). There was no significant difference in OS between the ibrutinib and chlorambucil arms in the iLLUMINATE trial (Lancet Oncol. 2019 Jan;20[1]:43-56). And there was no significant difference in OS between the venetoclax and chlorambucil arms in the CLL14 trial (N Engl J Med. 2019; 380:2225-36).
However, in the RESONATE-2 trial, ibrutinib provided an OS benefit over chlorambucil. The 2-year OS was 95% and 84%, respectively (P = .0145; Haematologica. Sept 2018;103:1502-10). Dr. Brown said the OS advantage in this study was due to the “very poor comparator of chlorambucil and very limited crossover.”
As Dr. Wierda mentioned, the OS rate was higher with Ib-R than with FCR in the E1912 trial. The 3-year OS rate was 98.8% and 91.5%, respectively (P less than .001; N Engl J Med. 2019 Aug 1;381:432-43). Dr. Brown noted, however, that there were few deaths in this study, and many of them “were not clearly related to the disease or its treatment.”
Dr. Brown also pointed out that FCR has been shown to have curative potential in IGHV-mutated CLL in both the FCR300 trial (Blood. 2016 127:303-9) and the CLL8 trial (Blood. 2016 127:208-15).
Another factor to consider is the greater cost of targeted agents. One analysis suggested the per-patient lifetime cost of CLL treatment in the United States will increase from $147,000 to $604,000 as targeted therapies overtake CIT as first-line treatment (J Clin Oncol. 2017 Jan 10;35[2]:166-174).
“Given all of the above, chemoimmunotherapy is going to remain part of the treatment repertoire for CLL,” Dr. Brown said. “It’s our only known potential cure for the fit, mutated patients ... and can also result in prolonged treatment-free intervals for patients who are older. As we manage CLL as a chronic disease over a lifetime, we need to continue to have this in our armamentarium.”
Specifically, Dr. Brown said CIT is appropriate for patients who don’t have del(17p) or mutated TP53. FCR should be given to young, fit patients with IGHV-mutated CLL, and FCR or BR should be given to older patients and young, fit patients with IGHV-unmutated CLL.
Dr. Brown and Dr. Wierda reported financial ties to multiple pharmaceutical companies, including makers of CLL treatments.
REPORTING FROM NCCN HEMATOLOGIC MALIGNANCIES
Comparing Artificial Intelligence Platforms for Histopathologic Cancer Diagnosis
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
Gene recurrence score helps predict successful combination therapy for early breast cancer
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
FROM JAMA ONCOLOGY