User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Osimertinib improves survival in advanced NSCLC
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
REPORTING FROM ESMO 2019
Building Blocks: AVAHO Past President Looks Back
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
Mortality after breast cancer diagnosis found higher for men
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
FROM JAMA ONCOLOGY
New 2020 Priorities: Expanding AVAHO Outreach and Influence
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
In Informed Consent, Capacity Is Crucial
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
Lung Cancer in the VA at a National Level
In Bladder Cancer, New Systemic Treatments Arise
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
Clinical Trials Should Be Standard of Care
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”
FDA issues warning for CDK 4/6 inhibitors
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
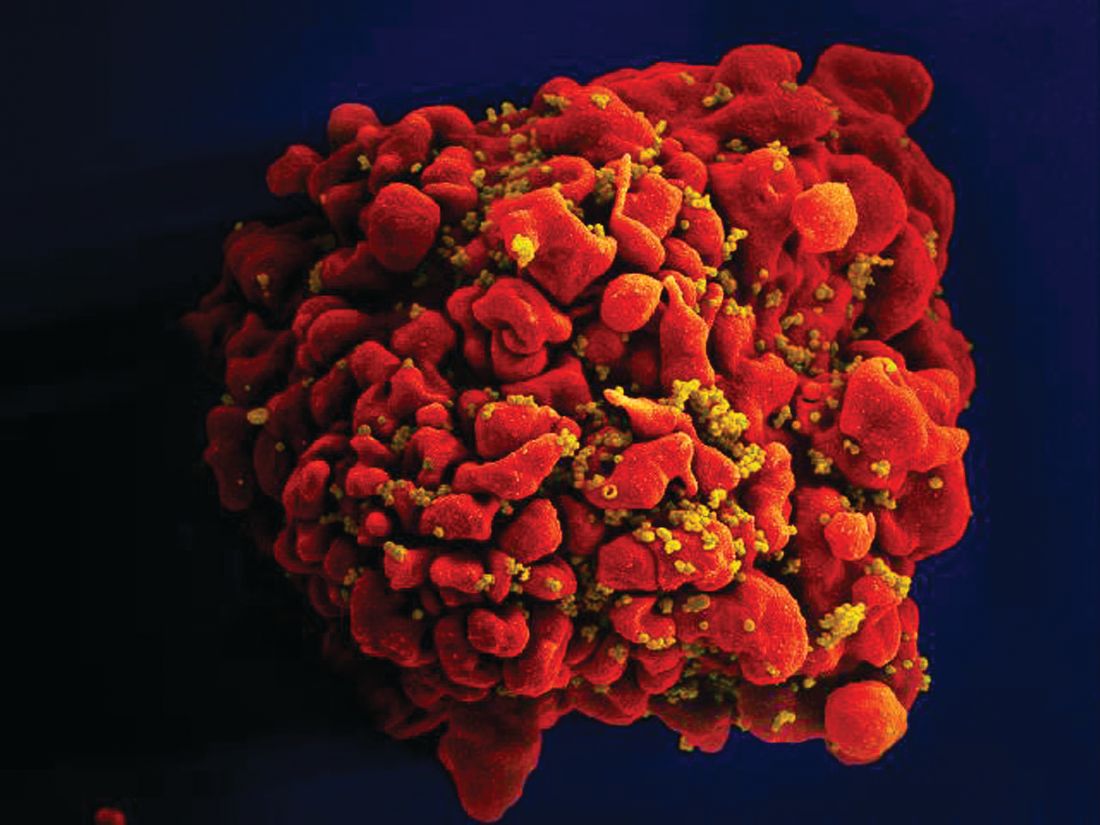
Stem cells gene edited to be HIV resistant treat ALL, but not HIV
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Donor cells depleted of the HIV coreceptor CCR5 effectively treated ALL, but not HIV.
Major finding: The patient had a sustained complete remission of ALL, but HIV persisted after transplantation.
Study details: Case report of a 27-year-old man with ALL and HIV.
Disclosures: The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
Source: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.