User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Women experience more chemoradiotherapy toxicity in rectal cancer
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
FROM JAMA ONCOLOGY
Key clinical point: Women show significantly higher rates of toxic effects from rectal cancer chemoradiotherapy than men.
Major finding: Women experience significantly higher rates of leukopenia and diarrhea from rectal cancer chemoradiotherapy.
Study details: A pooled analysis of data from two phase 3, randomized, controlled trials in 1,016 patients.
Disclosures: The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
Source: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Sequential CRT, immunotherapy nets high PFS in node-positive cervical cancer
Sequential chemoradiotherapy (CRT) and immunotherapy is safe, well tolerated, and efficacious among patients with locally advanced cervical cancer being treated with curative intent, a multicenter phase 1 trial suggests.
Less than 10% of patients treated with this sequence experienced a grade 3 toxicity. Meanwhile, more than 80% were alive and free of disease progression at 1 year.
“Despite standard CRT, most women with lymph node–positive cervical cancer experience disease recurrence,” note the investigators, led by Jyoti S. Mayadev, MD, associate professor in the department of radiation medicine and applied sciences, University of California, San Diego, in La Jolla. “Our study is potentially transformative in the standard treatment schema of locally advanced cervical cancer, with the prospect for immuno-oncology to add durable survival in patients with node-positive disease, a current unmet oncologic need.”
The investigators enrolled in the trial 32 women from Gynecology Oncology Cooperative Group member institutions who had stage IB2 to IVA cervical cancer with positive pelvic and/or para-aortic lymph nodes. Treatment consisted of six weekly doses of cisplatin, 40 mg/m2, concurrent with extended-field, 3-dimensional conformal radiotherapy, followed by the immune checkpoint inhibitor ipilimumab (Yervoy) every 21 days for four cycles.
Results reported in JAMA Oncology showed that all 32 patients completed CRT and 21 patients went on to receive ipilimumab. Among the latter, 86% completed all four planned cycles and the rest completed two cycles.
In the group receiving sequential CRT and ipilimumab, 9.5% experienced grade 3 toxicity (lipase increase in one case and dermatitis in another case). Both toxicities were self-limited.
With a 14.8-month median follow-up, the patients treated with CRT-ipilimumab had a 12-month overall survival rate of 90%, and a 12-month progression-free survival rate of 81% (median durations were not reached). Neither human papillomavirus genotype nor HLA subtype was associated with these outcomes.
Translational analyses showed that patients experienced an increase in peripheral blood T cells expressing programmed cell death 1 (PD-1) after CRT that was then sustained with ipilimumab therapy. “[T]he use of an immune checkpoint inhibitor could stimulate the antitumor activity of tumor-specific cytotoxic T cells and augment radiation-induced neoantigen load,” the investigators proposed.
“To our knowledge, this phase 1 study is the first to show tolerability with a signal of efficacy of an immune check-point inhibitor ... as a part of the definitive treatment of locally advanced cervical cancer,” they concluded. “Our findings show promise for the use of immunotherapy in the definitive setting of locally advanced, node-positive cervical cancer; patients with this cancer historically have a poor prognosis with standard therapy alone.”
Dr. Mayadev disclosed receiving a grant from the National Cancer Institute during the conduct of the study, personal fees from AstraZeneca, grants from NRG Oncology, and personal fees and nonfinancial support from the Gynecology Oncology Group Foundation outside the submitted work; receiving compensation for serving on the advisory board of Varian Medical Systems in 2018; and being a speaker for Samsung Medical Systems in 2017. The study was supported by the National Cancer Institute and by institutional funds.
SOURCE: Mayadev JS et al. JAMA Oncol. 2019 Nov 27. doi: 10.1001/jamaoncol.2019.3857.
Sequential chemoradiotherapy (CRT) and immunotherapy is safe, well tolerated, and efficacious among patients with locally advanced cervical cancer being treated with curative intent, a multicenter phase 1 trial suggests.
Less than 10% of patients treated with this sequence experienced a grade 3 toxicity. Meanwhile, more than 80% were alive and free of disease progression at 1 year.
“Despite standard CRT, most women with lymph node–positive cervical cancer experience disease recurrence,” note the investigators, led by Jyoti S. Mayadev, MD, associate professor in the department of radiation medicine and applied sciences, University of California, San Diego, in La Jolla. “Our study is potentially transformative in the standard treatment schema of locally advanced cervical cancer, with the prospect for immuno-oncology to add durable survival in patients with node-positive disease, a current unmet oncologic need.”
The investigators enrolled in the trial 32 women from Gynecology Oncology Cooperative Group member institutions who had stage IB2 to IVA cervical cancer with positive pelvic and/or para-aortic lymph nodes. Treatment consisted of six weekly doses of cisplatin, 40 mg/m2, concurrent with extended-field, 3-dimensional conformal radiotherapy, followed by the immune checkpoint inhibitor ipilimumab (Yervoy) every 21 days for four cycles.
Results reported in JAMA Oncology showed that all 32 patients completed CRT and 21 patients went on to receive ipilimumab. Among the latter, 86% completed all four planned cycles and the rest completed two cycles.
In the group receiving sequential CRT and ipilimumab, 9.5% experienced grade 3 toxicity (lipase increase in one case and dermatitis in another case). Both toxicities were self-limited.
With a 14.8-month median follow-up, the patients treated with CRT-ipilimumab had a 12-month overall survival rate of 90%, and a 12-month progression-free survival rate of 81% (median durations were not reached). Neither human papillomavirus genotype nor HLA subtype was associated with these outcomes.
Translational analyses showed that patients experienced an increase in peripheral blood T cells expressing programmed cell death 1 (PD-1) after CRT that was then sustained with ipilimumab therapy. “[T]he use of an immune checkpoint inhibitor could stimulate the antitumor activity of tumor-specific cytotoxic T cells and augment radiation-induced neoantigen load,” the investigators proposed.
“To our knowledge, this phase 1 study is the first to show tolerability with a signal of efficacy of an immune check-point inhibitor ... as a part of the definitive treatment of locally advanced cervical cancer,” they concluded. “Our findings show promise for the use of immunotherapy in the definitive setting of locally advanced, node-positive cervical cancer; patients with this cancer historically have a poor prognosis with standard therapy alone.”
Dr. Mayadev disclosed receiving a grant from the National Cancer Institute during the conduct of the study, personal fees from AstraZeneca, grants from NRG Oncology, and personal fees and nonfinancial support from the Gynecology Oncology Group Foundation outside the submitted work; receiving compensation for serving on the advisory board of Varian Medical Systems in 2018; and being a speaker for Samsung Medical Systems in 2017. The study was supported by the National Cancer Institute and by institutional funds.
SOURCE: Mayadev JS et al. JAMA Oncol. 2019 Nov 27. doi: 10.1001/jamaoncol.2019.3857.
Sequential chemoradiotherapy (CRT) and immunotherapy is safe, well tolerated, and efficacious among patients with locally advanced cervical cancer being treated with curative intent, a multicenter phase 1 trial suggests.
Less than 10% of patients treated with this sequence experienced a grade 3 toxicity. Meanwhile, more than 80% were alive and free of disease progression at 1 year.
“Despite standard CRT, most women with lymph node–positive cervical cancer experience disease recurrence,” note the investigators, led by Jyoti S. Mayadev, MD, associate professor in the department of radiation medicine and applied sciences, University of California, San Diego, in La Jolla. “Our study is potentially transformative in the standard treatment schema of locally advanced cervical cancer, with the prospect for immuno-oncology to add durable survival in patients with node-positive disease, a current unmet oncologic need.”
The investigators enrolled in the trial 32 women from Gynecology Oncology Cooperative Group member institutions who had stage IB2 to IVA cervical cancer with positive pelvic and/or para-aortic lymph nodes. Treatment consisted of six weekly doses of cisplatin, 40 mg/m2, concurrent with extended-field, 3-dimensional conformal radiotherapy, followed by the immune checkpoint inhibitor ipilimumab (Yervoy) every 21 days for four cycles.
Results reported in JAMA Oncology showed that all 32 patients completed CRT and 21 patients went on to receive ipilimumab. Among the latter, 86% completed all four planned cycles and the rest completed two cycles.
In the group receiving sequential CRT and ipilimumab, 9.5% experienced grade 3 toxicity (lipase increase in one case and dermatitis in another case). Both toxicities were self-limited.
With a 14.8-month median follow-up, the patients treated with CRT-ipilimumab had a 12-month overall survival rate of 90%, and a 12-month progression-free survival rate of 81% (median durations were not reached). Neither human papillomavirus genotype nor HLA subtype was associated with these outcomes.
Translational analyses showed that patients experienced an increase in peripheral blood T cells expressing programmed cell death 1 (PD-1) after CRT that was then sustained with ipilimumab therapy. “[T]he use of an immune checkpoint inhibitor could stimulate the antitumor activity of tumor-specific cytotoxic T cells and augment radiation-induced neoantigen load,” the investigators proposed.
“To our knowledge, this phase 1 study is the first to show tolerability with a signal of efficacy of an immune check-point inhibitor ... as a part of the definitive treatment of locally advanced cervical cancer,” they concluded. “Our findings show promise for the use of immunotherapy in the definitive setting of locally advanced, node-positive cervical cancer; patients with this cancer historically have a poor prognosis with standard therapy alone.”
Dr. Mayadev disclosed receiving a grant from the National Cancer Institute during the conduct of the study, personal fees from AstraZeneca, grants from NRG Oncology, and personal fees and nonfinancial support from the Gynecology Oncology Group Foundation outside the submitted work; receiving compensation for serving on the advisory board of Varian Medical Systems in 2018; and being a speaker for Samsung Medical Systems in 2017. The study was supported by the National Cancer Institute and by institutional funds.
SOURCE: Mayadev JS et al. JAMA Oncol. 2019 Nov 27. doi: 10.1001/jamaoncol.2019.3857.
FROM JAMA ONCOLOGY
Large state disparities seen for lung cancer screening
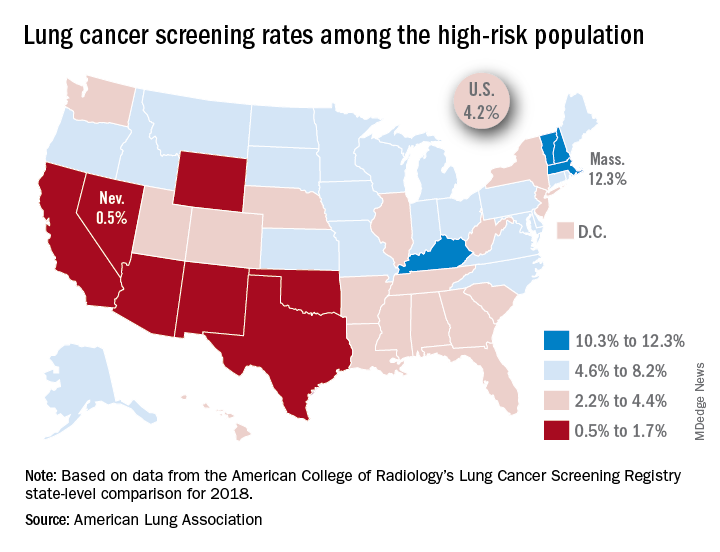
That disparity might suggest that Massachusetts has an exceptionally high rate, but it’s only 12.3%. And that means Nevada’s rate is very low, which it is: Only 0.5% of those at high risk are getting screened with annual low-dose CT scans, the ALA said in its 2019 State of Lung Cancer report.
“[The low rate of screening] may be because of a lack of access or low awareness and knowledge among patients and providers. As rates vary tremendously between states, it is clear that more can be done to increase screening rates,” the ALA stated.
Nationally, the screening rate is 4.2% among those at high risk for lung cancer, but “if everyone currently eligible were screened, close to 48,000 lives could be saved,” the ALA noted in its report.
Six states other than Nevada are below the 2% mark: Arizona, California, New Mexico, Oklahoma, Texas, and Wyoming. Besides Massachusetts, the three other states above 10% are Kentucky, New Hampshire, and Vermont, according to data from the American College of Radiology’s Lung Cancer Screening Registry state-level comparison for 2018.
For individuals at high risk for lung cancer – those aged 55-80 years who have at least a 30 pack-year history and either still smoke or have quit within 15 years – “screening with annual low-dose CT scans can reduce the lung cancer death rate by up to 20% by detecting tumors at early stages when the cancer is more likely to be curable,” the ALA wrote.

That disparity might suggest that Massachusetts has an exceptionally high rate, but it’s only 12.3%. And that means Nevada’s rate is very low, which it is: Only 0.5% of those at high risk are getting screened with annual low-dose CT scans, the ALA said in its 2019 State of Lung Cancer report.
“[The low rate of screening] may be because of a lack of access or low awareness and knowledge among patients and providers. As rates vary tremendously between states, it is clear that more can be done to increase screening rates,” the ALA stated.
Nationally, the screening rate is 4.2% among those at high risk for lung cancer, but “if everyone currently eligible were screened, close to 48,000 lives could be saved,” the ALA noted in its report.
Six states other than Nevada are below the 2% mark: Arizona, California, New Mexico, Oklahoma, Texas, and Wyoming. Besides Massachusetts, the three other states above 10% are Kentucky, New Hampshire, and Vermont, according to data from the American College of Radiology’s Lung Cancer Screening Registry state-level comparison for 2018.
For individuals at high risk for lung cancer – those aged 55-80 years who have at least a 30 pack-year history and either still smoke or have quit within 15 years – “screening with annual low-dose CT scans can reduce the lung cancer death rate by up to 20% by detecting tumors at early stages when the cancer is more likely to be curable,” the ALA wrote.

That disparity might suggest that Massachusetts has an exceptionally high rate, but it’s only 12.3%. And that means Nevada’s rate is very low, which it is: Only 0.5% of those at high risk are getting screened with annual low-dose CT scans, the ALA said in its 2019 State of Lung Cancer report.
“[The low rate of screening] may be because of a lack of access or low awareness and knowledge among patients and providers. As rates vary tremendously between states, it is clear that more can be done to increase screening rates,” the ALA stated.
Nationally, the screening rate is 4.2% among those at high risk for lung cancer, but “if everyone currently eligible were screened, close to 48,000 lives could be saved,” the ALA noted in its report.
Six states other than Nevada are below the 2% mark: Arizona, California, New Mexico, Oklahoma, Texas, and Wyoming. Besides Massachusetts, the three other states above 10% are Kentucky, New Hampshire, and Vermont, according to data from the American College of Radiology’s Lung Cancer Screening Registry state-level comparison for 2018.
For individuals at high risk for lung cancer – those aged 55-80 years who have at least a 30 pack-year history and either still smoke or have quit within 15 years – “screening with annual low-dose CT scans can reduce the lung cancer death rate by up to 20% by detecting tumors at early stages when the cancer is more likely to be curable,” the ALA wrote.
How should we monitor for ovarian cancer recurrence?
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.
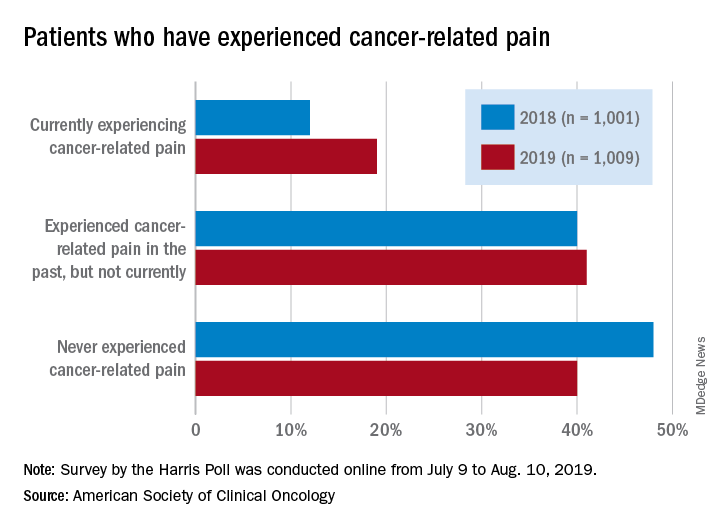
Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
FDA approves Givlaari for treatment of acute hepatic porphyria
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
Multimodal therapies almost double survival in anaplastic thyroid cancer
CHICAGO –
Median survival for participants in a specialized program who have been able to benefit from targeted therapy and immunotherapy now stands at 16 months, with 43% of patients surviving 2 years or more, said Anastasios Maniakas, MD, at the annual meeting of the American Thyroid Association.
Median survival was 8 months during 2000-2013, before the program, dubbed FAST (Facilitating Anaplastic Thyroid Cancer Specialized Treatment), was initiated at the University of Texas MD Anderson Cancer Center, Houston.
These increased survival rates were driven primarily by better targeting of mutation-specific therapy and by immunotherapy, said Dr. Maniakas, a fellow in head and neck surgery at the center. This targeting, in turn, was facilitated by timely staging and genetic work-up, as well as appropriate clinical trial enrollment.
As word has spread about the program, referrals went up by 44%, said Dr. Maniakas. Members of the FAST team include representatives from oncologic endocrinology, head and neck surgery, radiation oncology, pathology, and basic science.
Historically, anaplastic thyroid cancer (ATC) has had a 12-month overall survival rate of less than 30% for patients who have advanced disease, said Dr. Maniakas, citing a recent analysis showing that, in 1,567 ATC cases, the median survival was just 4 months, and the 6-month survival rate was 35%.
The FAST team’s engagement starts with rapid intake whereby patients see a physician within 3-5 days of initial contact with the center, explained Dr. Maniakas. A prescheduled work-up is completed within another 3-7 days. It includes basic lab work, cell-free DNA testing, BRAF immunohistochemistry, and molecular testing. Additional consults and appropriate medical imaging for staging are also included in the initial work-up.
With these data in hand, physicians meet again with patients in a treatment-planning clinic to assess eligibility for participation in a clinical trial. Patients will otherwise receive standard-of-care therapy that may include surgery or BRAF-directed therapy. However, said Dr. Maniakas, the FAST approach has resulted in a boost of more than 30% in clinical trial participation by ATC patients. Adjunctive therapies are also tailored to patients under the care of the FAST team, which may include stereotactic body-radiation therapy, surgery, and immunotherapy.
The team is tracking a cohort of patients who received surgery with or without radiation therapy, preceded by neoadjuvant BRAF/MEK inhibitor therapy – an approach used since 2017. Of 20 patients who were positive for BRAF-V600E, 16 are still alive at a median 1.21 years of follow-up since diagnosis, said Dr. Maniakas. The median survival time for those who did not receive surgery is 0.8 years, whereas the median survival has not been reached for those who also had surgery.
Molecular testing and initial screening of ATC patients is an essential component of the cancer center’s precision medicine approach, said Dr. Maniakas. “Genetic profiling has become a key player in ATC management and survival.”
In looking at outcomes at the cancer center, Dr. Maniakas and his collaborators divided the patients into three groups. The first included 227 patients seen during 2000-2013, before the program was initiated. The 100 participants in the second group initiated treatment sometime during 2014-2016, after the program was launched but before the targeted therapy and immunotherapy trial was fully implemented. Since 2017, 152 participants in the third group have had the opportunity to participate in the clinical trial, as well as receiving surgery with or without radiation therapy after neoadjuvant immunotherapy.
Since 2017, 97% of ATC patients have had genetic profiling done. Most patients are receiving rapid determination of BRAF-V600E status with immunohistochemistry, with results available in a few days, followed by liquid biopsy (available in about 2 weeks), and then next-generation sequencing. Results for the latter, considered the gold standard, can take up to 3 weeks.
Patients participating in the program were aged a mean 65 years at diagnosis, and just over half were men. The number of patients receiving targeted therapy has continued to rise, said Dr. Maniakas. From 2000 to 2013, just 9% of patients received targeted therapy; from 2014 to 2016, that figure rose to 43%; and since 2017, 61% of patients have received targeted therapy (P less than .001).
“Landmark changes in the management of ATC patients as a whole have had a direct impact to the significant increase in overall survival,” said Dr. Maniakas.
He added that the cancer center’s experience could inform future ATC guidelines. Patients with this deadliest of thyroid cancers should all have rapid molecular testing, followed by timely, targeted therapy. Clinical trial eligibility should be considered for all patients. Finally, guideline authors should take note of the ongoing favorable survival rates seen for patients receiving surgery after neoadjuvant therapy.
Dr. Maniakas reported no outside sources of funding and that he had no relevant disclosures.
SOURCE: Maniakas A et al. ATA 2019, Short Call Oral Abstract 9.
CHICAGO –
Median survival for participants in a specialized program who have been able to benefit from targeted therapy and immunotherapy now stands at 16 months, with 43% of patients surviving 2 years or more, said Anastasios Maniakas, MD, at the annual meeting of the American Thyroid Association.
Median survival was 8 months during 2000-2013, before the program, dubbed FAST (Facilitating Anaplastic Thyroid Cancer Specialized Treatment), was initiated at the University of Texas MD Anderson Cancer Center, Houston.
These increased survival rates were driven primarily by better targeting of mutation-specific therapy and by immunotherapy, said Dr. Maniakas, a fellow in head and neck surgery at the center. This targeting, in turn, was facilitated by timely staging and genetic work-up, as well as appropriate clinical trial enrollment.
As word has spread about the program, referrals went up by 44%, said Dr. Maniakas. Members of the FAST team include representatives from oncologic endocrinology, head and neck surgery, radiation oncology, pathology, and basic science.
Historically, anaplastic thyroid cancer (ATC) has had a 12-month overall survival rate of less than 30% for patients who have advanced disease, said Dr. Maniakas, citing a recent analysis showing that, in 1,567 ATC cases, the median survival was just 4 months, and the 6-month survival rate was 35%.
The FAST team’s engagement starts with rapid intake whereby patients see a physician within 3-5 days of initial contact with the center, explained Dr. Maniakas. A prescheduled work-up is completed within another 3-7 days. It includes basic lab work, cell-free DNA testing, BRAF immunohistochemistry, and molecular testing. Additional consults and appropriate medical imaging for staging are also included in the initial work-up.
With these data in hand, physicians meet again with patients in a treatment-planning clinic to assess eligibility for participation in a clinical trial. Patients will otherwise receive standard-of-care therapy that may include surgery or BRAF-directed therapy. However, said Dr. Maniakas, the FAST approach has resulted in a boost of more than 30% in clinical trial participation by ATC patients. Adjunctive therapies are also tailored to patients under the care of the FAST team, which may include stereotactic body-radiation therapy, surgery, and immunotherapy.
The team is tracking a cohort of patients who received surgery with or without radiation therapy, preceded by neoadjuvant BRAF/MEK inhibitor therapy – an approach used since 2017. Of 20 patients who were positive for BRAF-V600E, 16 are still alive at a median 1.21 years of follow-up since diagnosis, said Dr. Maniakas. The median survival time for those who did not receive surgery is 0.8 years, whereas the median survival has not been reached for those who also had surgery.
Molecular testing and initial screening of ATC patients is an essential component of the cancer center’s precision medicine approach, said Dr. Maniakas. “Genetic profiling has become a key player in ATC management and survival.”
In looking at outcomes at the cancer center, Dr. Maniakas and his collaborators divided the patients into three groups. The first included 227 patients seen during 2000-2013, before the program was initiated. The 100 participants in the second group initiated treatment sometime during 2014-2016, after the program was launched but before the targeted therapy and immunotherapy trial was fully implemented. Since 2017, 152 participants in the third group have had the opportunity to participate in the clinical trial, as well as receiving surgery with or without radiation therapy after neoadjuvant immunotherapy.
Since 2017, 97% of ATC patients have had genetic profiling done. Most patients are receiving rapid determination of BRAF-V600E status with immunohistochemistry, with results available in a few days, followed by liquid biopsy (available in about 2 weeks), and then next-generation sequencing. Results for the latter, considered the gold standard, can take up to 3 weeks.
Patients participating in the program were aged a mean 65 years at diagnosis, and just over half were men. The number of patients receiving targeted therapy has continued to rise, said Dr. Maniakas. From 2000 to 2013, just 9% of patients received targeted therapy; from 2014 to 2016, that figure rose to 43%; and since 2017, 61% of patients have received targeted therapy (P less than .001).
“Landmark changes in the management of ATC patients as a whole have had a direct impact to the significant increase in overall survival,” said Dr. Maniakas.
He added that the cancer center’s experience could inform future ATC guidelines. Patients with this deadliest of thyroid cancers should all have rapid molecular testing, followed by timely, targeted therapy. Clinical trial eligibility should be considered for all patients. Finally, guideline authors should take note of the ongoing favorable survival rates seen for patients receiving surgery after neoadjuvant therapy.
Dr. Maniakas reported no outside sources of funding and that he had no relevant disclosures.
SOURCE: Maniakas A et al. ATA 2019, Short Call Oral Abstract 9.
CHICAGO –
Median survival for participants in a specialized program who have been able to benefit from targeted therapy and immunotherapy now stands at 16 months, with 43% of patients surviving 2 years or more, said Anastasios Maniakas, MD, at the annual meeting of the American Thyroid Association.
Median survival was 8 months during 2000-2013, before the program, dubbed FAST (Facilitating Anaplastic Thyroid Cancer Specialized Treatment), was initiated at the University of Texas MD Anderson Cancer Center, Houston.
These increased survival rates were driven primarily by better targeting of mutation-specific therapy and by immunotherapy, said Dr. Maniakas, a fellow in head and neck surgery at the center. This targeting, in turn, was facilitated by timely staging and genetic work-up, as well as appropriate clinical trial enrollment.
As word has spread about the program, referrals went up by 44%, said Dr. Maniakas. Members of the FAST team include representatives from oncologic endocrinology, head and neck surgery, radiation oncology, pathology, and basic science.
Historically, anaplastic thyroid cancer (ATC) has had a 12-month overall survival rate of less than 30% for patients who have advanced disease, said Dr. Maniakas, citing a recent analysis showing that, in 1,567 ATC cases, the median survival was just 4 months, and the 6-month survival rate was 35%.
The FAST team’s engagement starts with rapid intake whereby patients see a physician within 3-5 days of initial contact with the center, explained Dr. Maniakas. A prescheduled work-up is completed within another 3-7 days. It includes basic lab work, cell-free DNA testing, BRAF immunohistochemistry, and molecular testing. Additional consults and appropriate medical imaging for staging are also included in the initial work-up.
With these data in hand, physicians meet again with patients in a treatment-planning clinic to assess eligibility for participation in a clinical trial. Patients will otherwise receive standard-of-care therapy that may include surgery or BRAF-directed therapy. However, said Dr. Maniakas, the FAST approach has resulted in a boost of more than 30% in clinical trial participation by ATC patients. Adjunctive therapies are also tailored to patients under the care of the FAST team, which may include stereotactic body-radiation therapy, surgery, and immunotherapy.
The team is tracking a cohort of patients who received surgery with or without radiation therapy, preceded by neoadjuvant BRAF/MEK inhibitor therapy – an approach used since 2017. Of 20 patients who were positive for BRAF-V600E, 16 are still alive at a median 1.21 years of follow-up since diagnosis, said Dr. Maniakas. The median survival time for those who did not receive surgery is 0.8 years, whereas the median survival has not been reached for those who also had surgery.
Molecular testing and initial screening of ATC patients is an essential component of the cancer center’s precision medicine approach, said Dr. Maniakas. “Genetic profiling has become a key player in ATC management and survival.”
In looking at outcomes at the cancer center, Dr. Maniakas and his collaborators divided the patients into three groups. The first included 227 patients seen during 2000-2013, before the program was initiated. The 100 participants in the second group initiated treatment sometime during 2014-2016, after the program was launched but before the targeted therapy and immunotherapy trial was fully implemented. Since 2017, 152 participants in the third group have had the opportunity to participate in the clinical trial, as well as receiving surgery with or without radiation therapy after neoadjuvant immunotherapy.
Since 2017, 97% of ATC patients have had genetic profiling done. Most patients are receiving rapid determination of BRAF-V600E status with immunohistochemistry, with results available in a few days, followed by liquid biopsy (available in about 2 weeks), and then next-generation sequencing. Results for the latter, considered the gold standard, can take up to 3 weeks.
Patients participating in the program were aged a mean 65 years at diagnosis, and just over half were men. The number of patients receiving targeted therapy has continued to rise, said Dr. Maniakas. From 2000 to 2013, just 9% of patients received targeted therapy; from 2014 to 2016, that figure rose to 43%; and since 2017, 61% of patients have received targeted therapy (P less than .001).
“Landmark changes in the management of ATC patients as a whole have had a direct impact to the significant increase in overall survival,” said Dr. Maniakas.
He added that the cancer center’s experience could inform future ATC guidelines. Patients with this deadliest of thyroid cancers should all have rapid molecular testing, followed by timely, targeted therapy. Clinical trial eligibility should be considered for all patients. Finally, guideline authors should take note of the ongoing favorable survival rates seen for patients receiving surgery after neoadjuvant therapy.
Dr. Maniakas reported no outside sources of funding and that he had no relevant disclosures.
SOURCE: Maniakas A et al. ATA 2019, Short Call Oral Abstract 9.
REPORTING FROM ATA 2019
Frontline ibrutinib saves money over chemoimmunotherapy
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
Ibrutinib monotherapy was associated with lower total health care costs compared with chemoimmunotherapy in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to a retrospective study.
“This study compared time to next treatment, health care resource utilization, and total direct costs among patients with CLL initiating front-line ibrutinib single agent or chemoimmunotherapy,” wrote Bruno Emond, of Analysis Group, Montreal, and colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed data from 1,161 patients with CLL who were started on ibrutinib monotherapy or chemoimmunotherapy from 2014 to 2017. Data were collected from the Optum Clinformatics Extended DataMart De-Identified Databases.
Between the two groups, differences in baseline characteristics were controlled for by way of inverse probability of treatment weighting. Two treatment periods were included in the study: the initial 6 months of treatment and entire duration of frontline therapy.
The team also conducted a subgroup analysis of patients treated with bendamustine and rituximab. This cohort was analyzed independently since the regimen is commonly used in clinical practice.
After analysis, the researchers found that ibrutinib monotherapy was associated with net monthly cost savings of $3,766 (P less than .0001), compared with chemoimmunotherapy and bendamustine/rituximab over the frontline therapy period.
Ibrutinib patients had fewer monthly days with outpatient services (rate ratio, 0.75; 95% confidence interval, 0.60-0.94; P = .0200), compared with those on chemoimmunotherapy; and were less likely to initiate a next line of treatment, compared with chemoimmunotherapy patients (hazard ratio, 0.54; 95% CI, 0.33-0.90; P = .0163).
“Cost savings and reductions in health care resource utilization were even more pronounced when considering only the first 6 months of front-line treatment,” the researchers wrote.
The researchers acknowledged that two key limitations of the study were the potential influence of unobserved confounding factors and the use of claims data, which could include errors and omissions.
“These results suggest that ibrutinib single-agent is associated with lower total costs driven by lower medical costs, despite higher pharmacy costs, compared with chemoimmunotherapy and bendamustine/rituximab,” they concluded.
The authors reported financial affiliations with Janssen Scientific Affairs, which funded the study, and other companies.
SOURCE: Emond B et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 26. doi: 10.1016/j.clml.2019.08.004.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Early lenalidomide may delay progression of smoldering myeloma
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
FROM JOURNAL OF CLINICAL ONCOLOGY
Not all lung cancer patients receive treatment
In the United States, just over 15% of patients with lung cancer receive no treatment, according to the American Lung Association.
“This can happen for multiple reasons, such as the tumor having spread too far, poor health, or refusal of treatment,” the ALA said in its 2019 State of Lung Cancer report.
On the state level, the disparities were considerable. Arizona had the highest rate of nontreatment at 30.4%, followed by the neighboring states of New Mexico (24.2%) and California (24.0%). The lowest rate in the country, 8.0%, came from North Dakota, with Missouri next at 9.4% and Maine third at 9.6%, based on data from the North American Association of Central Cancer Registries’ December 2018 data submission, which covered the years from 2012 to 2016.
Although some cases of lung cancer may be unavoidable, “no one should go untreated because of lack of provider or patient knowledge, stigma associated with lung cancer, fatalism after diagnosis, or cost of treatment. Dismantling these and other barriers is important to reducing the percent of untreated patients,” the ALA said.
In the United States, just over 15% of patients with lung cancer receive no treatment, according to the American Lung Association.

“This can happen for multiple reasons, such as the tumor having spread too far, poor health, or refusal of treatment,” the ALA said in its 2019 State of Lung Cancer report.
On the state level, the disparities were considerable. Arizona had the highest rate of nontreatment at 30.4%, followed by the neighboring states of New Mexico (24.2%) and California (24.0%). The lowest rate in the country, 8.0%, came from North Dakota, with Missouri next at 9.4% and Maine third at 9.6%, based on data from the North American Association of Central Cancer Registries’ December 2018 data submission, which covered the years from 2012 to 2016.
Although some cases of lung cancer may be unavoidable, “no one should go untreated because of lack of provider or patient knowledge, stigma associated with lung cancer, fatalism after diagnosis, or cost of treatment. Dismantling these and other barriers is important to reducing the percent of untreated patients,” the ALA said.
In the United States, just over 15% of patients with lung cancer receive no treatment, according to the American Lung Association.

“This can happen for multiple reasons, such as the tumor having spread too far, poor health, or refusal of treatment,” the ALA said in its 2019 State of Lung Cancer report.
On the state level, the disparities were considerable. Arizona had the highest rate of nontreatment at 30.4%, followed by the neighboring states of New Mexico (24.2%) and California (24.0%). The lowest rate in the country, 8.0%, came from North Dakota, with Missouri next at 9.4% and Maine third at 9.6%, based on data from the North American Association of Central Cancer Registries’ December 2018 data submission, which covered the years from 2012 to 2016.
Although some cases of lung cancer may be unavoidable, “no one should go untreated because of lack of provider or patient knowledge, stigma associated with lung cancer, fatalism after diagnosis, or cost of treatment. Dismantling these and other barriers is important to reducing the percent of untreated patients,” the ALA said.






