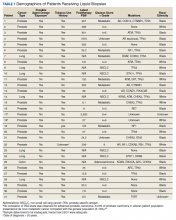
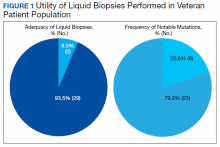
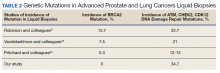
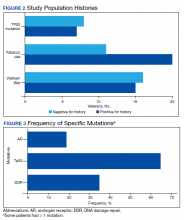
User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


U.S. cancer death rates drop for second year in a row
The study was published online Jan. 12 in CA: A Cancer Journal for Clinicians.
“Mortality rates are a better indicator of progress against cancer than incidence or survival because they are less affected by biases resulting from changes in detection practices,” wrote the authors, led by Rebecca Siegel, MPH, American Cancer Society, Atlanta.
“The overall drop of 31% as of 2018 [since the early 1990s] translates to an estimated 3,188,500 fewer cancer deaths (2,170,700 in men and 1,017,800 in women) than what would have occurred if mortality rates had remained at their peak,” the researchers added.
Lung cancer accounted for 46% of the total decline in cancer mortality in the past 5 years, with a record, single-year drop of 2.4% between 2017 and 2018.
The recent and rapid reductions in lung cancer mortality reflect better treatments for NSCLC, the authors suggested. For example, survival rates at 2 years have increased from 34% for patients diagnosed with NSCLC between 2009 and 2010 to 42% for those diagnosed during 2015 and 2016 – an absolute gain of 5%-6% in survival odds for every stage of diagnosis.
On a more somber note, the authors warned that COVID-19 is predicted to have a negative impact on both the diagnosis and outcomes of patients with cancer in the near future.
“We anticipate that disruptions in access to cancer care in 2020 will lead to downstream increases in advanced stage diagnoses that may impede progress in reducing cancer mortality rates in the years to come,” Ms. Siegel said in a statement.
New cancer cases
The report provides an estimated number of new cancer cases and deaths in 2021 in the United States (nationally and state-by-state) based on the most current population-based data for cancer incidence through 2017 and for mortality through 2018. “An estimated 608,570 Americans will die from cancer in 2021, corresponding to more than 1600 deaths per day,” Ms. Siegel and colleagues reported.
The greatest number of deaths are predicted to be from the most common cancers: Lung, prostate, and colorectal cancer in men and lung, breast, and colorectal cancer in women, they added. However, the mortality rates for all four cancers are continuing to fall.
As of 2018, the death rate from lung cancer had dropped by 54% among males and by 30% among females over the past few decades, the investigators noted.
Mortality from female breast cancer has dropped by 41% since 1989; by 52% for prostate cancer since 1993; and by 53% and 59% for colorectal cancer for men (since 1980) and women (since 1969), respectively.
“However, in recent years, mortality declines have slowed for breast cancer and [colorectal cancer] and have halted for prostate cancer,” the researchers noted.
In contrast, the pace of the annual decline in lung cancer mortality doubled among men from 3.1% between 2009 and 2013 to 5.5% between 2014 and 2018, and from 1.8% to 4.4% among women during the same time intervals.
Increase in incidence at common sites
Despite the steady progress in mortality for most cancers, “rates continue to increase for some common sites,” Ms. Siegel and colleagues reported.
For example, death rates from uterine corpus cancer have accelerated from the late 1990s at twice the pace of the increase in its incidence. Death rates also have increased for cancers of the oral cavity and pharynx – although in this cancer, increases in mortality parallel an increase in its incidence.
“Pancreatic cancer death rates [in turn] continued to increase slowly in men ... but remained stable in women, despite incidence [rates] rising by about 1% per year in both sexes,” the authors observed.
Meanwhile, the incidence of cervical cancer, although declining for decades overall, is increasing for patients who present with more distant-stage disease as well as cervical adenocarcinoma, both of which are often undetected by cytology.
“These findings underscore the need for more targeted efforts to increase both HPV [human papillomavirus] vaccination among all individuals aged [26 and younger] and primary HPV testing or HPV/cytology co-testing every 5 years among women beginning at age 25,” the authors emphasized.
On a more positive note, the long-term increase in mortality from liver cancer has recently slowed among women and has stabilized among men, they added.
Once again, disparities in both cancer occurrence and outcomes varied considerably between racial and ethnic groups. For example, cancer is the leading cause of death in people who are Hispanic, Asian American, and Alaska Native. Survival rates at 5 years for almost all cancers are still higher for White patients than for Black patients, although the disparity in cancer mortality between Black persons and White persons has declined to 13% from a peak of 33% in 1993.
Geographic disparities in cancer mortality rates still prevail; the rates are largest for preventable cancers such as lung and cervical cancer, for which mortality varies by as much as fivefold across states.
And although cancer remains the second most common cause of death among children, death rates from cancer have continuously declined over time among both children and adolescents, largely the result of dramatic declines in death rates from leukemia in both age groups.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was published online Jan. 12 in CA: A Cancer Journal for Clinicians.
“Mortality rates are a better indicator of progress against cancer than incidence or survival because they are less affected by biases resulting from changes in detection practices,” wrote the authors, led by Rebecca Siegel, MPH, American Cancer Society, Atlanta.
“The overall drop of 31% as of 2018 [since the early 1990s] translates to an estimated 3,188,500 fewer cancer deaths (2,170,700 in men and 1,017,800 in women) than what would have occurred if mortality rates had remained at their peak,” the researchers added.
Lung cancer accounted for 46% of the total decline in cancer mortality in the past 5 years, with a record, single-year drop of 2.4% between 2017 and 2018.
The recent and rapid reductions in lung cancer mortality reflect better treatments for NSCLC, the authors suggested. For example, survival rates at 2 years have increased from 34% for patients diagnosed with NSCLC between 2009 and 2010 to 42% for those diagnosed during 2015 and 2016 – an absolute gain of 5%-6% in survival odds for every stage of diagnosis.
On a more somber note, the authors warned that COVID-19 is predicted to have a negative impact on both the diagnosis and outcomes of patients with cancer in the near future.
“We anticipate that disruptions in access to cancer care in 2020 will lead to downstream increases in advanced stage diagnoses that may impede progress in reducing cancer mortality rates in the years to come,” Ms. Siegel said in a statement.
New cancer cases
The report provides an estimated number of new cancer cases and deaths in 2021 in the United States (nationally and state-by-state) based on the most current population-based data for cancer incidence through 2017 and for mortality through 2018. “An estimated 608,570 Americans will die from cancer in 2021, corresponding to more than 1600 deaths per day,” Ms. Siegel and colleagues reported.
The greatest number of deaths are predicted to be from the most common cancers: Lung, prostate, and colorectal cancer in men and lung, breast, and colorectal cancer in women, they added. However, the mortality rates for all four cancers are continuing to fall.
As of 2018, the death rate from lung cancer had dropped by 54% among males and by 30% among females over the past few decades, the investigators noted.
Mortality from female breast cancer has dropped by 41% since 1989; by 52% for prostate cancer since 1993; and by 53% and 59% for colorectal cancer for men (since 1980) and women (since 1969), respectively.
“However, in recent years, mortality declines have slowed for breast cancer and [colorectal cancer] and have halted for prostate cancer,” the researchers noted.
In contrast, the pace of the annual decline in lung cancer mortality doubled among men from 3.1% between 2009 and 2013 to 5.5% between 2014 and 2018, and from 1.8% to 4.4% among women during the same time intervals.
Increase in incidence at common sites
Despite the steady progress in mortality for most cancers, “rates continue to increase for some common sites,” Ms. Siegel and colleagues reported.
For example, death rates from uterine corpus cancer have accelerated from the late 1990s at twice the pace of the increase in its incidence. Death rates also have increased for cancers of the oral cavity and pharynx – although in this cancer, increases in mortality parallel an increase in its incidence.
“Pancreatic cancer death rates [in turn] continued to increase slowly in men ... but remained stable in women, despite incidence [rates] rising by about 1% per year in both sexes,” the authors observed.
Meanwhile, the incidence of cervical cancer, although declining for decades overall, is increasing for patients who present with more distant-stage disease as well as cervical adenocarcinoma, both of which are often undetected by cytology.
“These findings underscore the need for more targeted efforts to increase both HPV [human papillomavirus] vaccination among all individuals aged [26 and younger] and primary HPV testing or HPV/cytology co-testing every 5 years among women beginning at age 25,” the authors emphasized.
On a more positive note, the long-term increase in mortality from liver cancer has recently slowed among women and has stabilized among men, they added.
Once again, disparities in both cancer occurrence and outcomes varied considerably between racial and ethnic groups. For example, cancer is the leading cause of death in people who are Hispanic, Asian American, and Alaska Native. Survival rates at 5 years for almost all cancers are still higher for White patients than for Black patients, although the disparity in cancer mortality between Black persons and White persons has declined to 13% from a peak of 33% in 1993.
Geographic disparities in cancer mortality rates still prevail; the rates are largest for preventable cancers such as lung and cervical cancer, for which mortality varies by as much as fivefold across states.
And although cancer remains the second most common cause of death among children, death rates from cancer have continuously declined over time among both children and adolescents, largely the result of dramatic declines in death rates from leukemia in both age groups.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was published online Jan. 12 in CA: A Cancer Journal for Clinicians.
“Mortality rates are a better indicator of progress against cancer than incidence or survival because they are less affected by biases resulting from changes in detection practices,” wrote the authors, led by Rebecca Siegel, MPH, American Cancer Society, Atlanta.
“The overall drop of 31% as of 2018 [since the early 1990s] translates to an estimated 3,188,500 fewer cancer deaths (2,170,700 in men and 1,017,800 in women) than what would have occurred if mortality rates had remained at their peak,” the researchers added.
Lung cancer accounted for 46% of the total decline in cancer mortality in the past 5 years, with a record, single-year drop of 2.4% between 2017 and 2018.
The recent and rapid reductions in lung cancer mortality reflect better treatments for NSCLC, the authors suggested. For example, survival rates at 2 years have increased from 34% for patients diagnosed with NSCLC between 2009 and 2010 to 42% for those diagnosed during 2015 and 2016 – an absolute gain of 5%-6% in survival odds for every stage of diagnosis.
On a more somber note, the authors warned that COVID-19 is predicted to have a negative impact on both the diagnosis and outcomes of patients with cancer in the near future.
“We anticipate that disruptions in access to cancer care in 2020 will lead to downstream increases in advanced stage diagnoses that may impede progress in reducing cancer mortality rates in the years to come,” Ms. Siegel said in a statement.
New cancer cases
The report provides an estimated number of new cancer cases and deaths in 2021 in the United States (nationally and state-by-state) based on the most current population-based data for cancer incidence through 2017 and for mortality through 2018. “An estimated 608,570 Americans will die from cancer in 2021, corresponding to more than 1600 deaths per day,” Ms. Siegel and colleagues reported.
The greatest number of deaths are predicted to be from the most common cancers: Lung, prostate, and colorectal cancer in men and lung, breast, and colorectal cancer in women, they added. However, the mortality rates for all four cancers are continuing to fall.
As of 2018, the death rate from lung cancer had dropped by 54% among males and by 30% among females over the past few decades, the investigators noted.
Mortality from female breast cancer has dropped by 41% since 1989; by 52% for prostate cancer since 1993; and by 53% and 59% for colorectal cancer for men (since 1980) and women (since 1969), respectively.
“However, in recent years, mortality declines have slowed for breast cancer and [colorectal cancer] and have halted for prostate cancer,” the researchers noted.
In contrast, the pace of the annual decline in lung cancer mortality doubled among men from 3.1% between 2009 and 2013 to 5.5% between 2014 and 2018, and from 1.8% to 4.4% among women during the same time intervals.
Increase in incidence at common sites
Despite the steady progress in mortality for most cancers, “rates continue to increase for some common sites,” Ms. Siegel and colleagues reported.
For example, death rates from uterine corpus cancer have accelerated from the late 1990s at twice the pace of the increase in its incidence. Death rates also have increased for cancers of the oral cavity and pharynx – although in this cancer, increases in mortality parallel an increase in its incidence.
“Pancreatic cancer death rates [in turn] continued to increase slowly in men ... but remained stable in women, despite incidence [rates] rising by about 1% per year in both sexes,” the authors observed.
Meanwhile, the incidence of cervical cancer, although declining for decades overall, is increasing for patients who present with more distant-stage disease as well as cervical adenocarcinoma, both of which are often undetected by cytology.
“These findings underscore the need for more targeted efforts to increase both HPV [human papillomavirus] vaccination among all individuals aged [26 and younger] and primary HPV testing or HPV/cytology co-testing every 5 years among women beginning at age 25,” the authors emphasized.
On a more positive note, the long-term increase in mortality from liver cancer has recently slowed among women and has stabilized among men, they added.
Once again, disparities in both cancer occurrence and outcomes varied considerably between racial and ethnic groups. For example, cancer is the leading cause of death in people who are Hispanic, Asian American, and Alaska Native. Survival rates at 5 years for almost all cancers are still higher for White patients than for Black patients, although the disparity in cancer mortality between Black persons and White persons has declined to 13% from a peak of 33% in 1993.
Geographic disparities in cancer mortality rates still prevail; the rates are largest for preventable cancers such as lung and cervical cancer, for which mortality varies by as much as fivefold across states.
And although cancer remains the second most common cause of death among children, death rates from cancer have continuously declined over time among both children and adolescents, largely the result of dramatic declines in death rates from leukemia in both age groups.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adaptive biomarker approach may spare some breast cancer patients chemo
The findings were reported at the 2020 San Antonio Breast Cancer Symposium.
“In early luminal breast cancer, optimal patient selection for omission of adjuvant chemotherapy, particularly in patients with one to three involved lymph nodes, is still unclear,” noted principal investigator Nadia Harbeck, MD, PhD, of the University of Munich.
Successive trials have used nodal status, genomic risk scores, and response to preoperative therapy to home in on subsets of women for whom this practice is safe.
The ADAPT HR+/HER2– trial is a phase 3 trial that enrolled 5,625 patients with luminal (hormone receptor–positive, HER2-negative) early breast cancer who were candidates for adjuvant chemotherapy based on conventional criteria.
The trial combined a static biomarker – Oncotype Dx recurrence score (RS) in the baseline core biopsy – and a dynamic biomarker – Ki-67 response to a 3-week course of preoperative endocrine therapy – to personalize adjuvant therapy.
At SABCS 2020, Dr. Harbeck reported results for 2,290 patients having zero to three involved lymph nodes: 868 patients with RS 0-11 and 1,422 patients with RS 12-25 who had a response to brief preoperative endocrine therapy (a Ki-67 fraction ≤10% at surgery). All were treated with endocrine therapy alone as adjuvant therapy.
Similar outcomes
The median follow-up was 60 months. The 5-year rate of invasive disease–free survival was 93.9% for the group with RS 0-11 and 92.6% for the group with RS 12-25 and a response to the preoperative endocrine therapy.
The study met its primary endpoint, as the lower limit of the 95% confidence interval for the difference between groups of –3.3% fell just within the predefined margin of –3.3% or less for noninferiority (P = .05).
The groups also had similarly “excellent” distant disease–free survival (96.3% for RS 0-11 and 95.6% for RS 12-25; P = .247) and overall survival (98.0% for RS 0-11 and 97.3% for RS 12-25; P = .160), Dr. Harbeck reported.
The similar distant disease–free survival was consistent regardless of whether women were younger or older than 50 years and regardless of whether women had involved nodes or not.
In multivariate analysis, women had greater risk of distant disease–free survival events if they had three positive lymph nodes versus zero to two (hazard ratio, 3.40) or a pathologic T stage of 2-4 versus 0-1 (HR, 2.24), whereas risk fell with increasing baseline progesterone receptor expression (HR, 0.92).
“Neither patient age nor study arm were prognostic factors for patient outcome,” Dr. Harbeck noted.
In stratified analysis, the negative impact of having three positive nodes was seen only in the group with RS 12-25 and response to preoperative endocrine therapy, suggesting this subgroup may not be good candidates for omission of chemotherapy, she said.
Applying results to practice
“In luminal early breast cancer, the following patients – irrespective of their age – can safely be treated by endocrine therapy alone: patients with zero to three involved lymph nodes and recurrence score 0-11, and those with limited nodal burden (zero to two lymph nodes), recurrence score 12-25, and endocrine response after short preoperative endocrine therapy,” Dr. Harbeck summarized.
“Oncotype Dx testing can spare chemotherapy for the majority of patients with up to three involved lymph nodes. Dynamic Ki-67 response testing is feasible in clinical routine and complements baseline risk assessment to define patient selection for therapy deescalation or escalation,” she added.
The investigators have used the trial’s data to develop an algorithm for predicting the probability of response to short-course preoperative endocrine therapy that is available free of charge online (www.enrep.info).
“This may support everyday clinical decision-making in luminal early breast cancer; for example, whether to start a short period of preoperative endocrine therapy at all, and whether to rely on adjuvant endocrine therapy alone, but also in times like these, whether it’s safe to delay surgery by putting patients on prolonged preoperative endocrine therapy if surgical resources are scarce,” Dr. Harbeck commented.
Her clinic is now recruiting patients for the ADAPT Cycle trial, which is testing an endocrine-based approach with a CDK4/6 inhibitor versus chemotherapy in patients who are not candidates for adjuvant endocrine therapy alone. Therefore, all eligible patients receive the short course of endocrine therapy up front as the standard.
“But if you don’t have a trial, what are you going to do on Monday morning? Please let your patient know whether her tumor is endocrine responsive by doing this 3-week preoperative endocrine therapy,” Dr. Harbeck recommended. “It’s easy to do, you can schedule your surgeries better, and in patients with up to three lymph nodes, it helps with your decision-making, not just in the postmenopausal patients but also in the premenopausal patients, regarding whether they can forgo chemotherapy.”
Findings in context
More than 75% of ADAPT patients with RS 12-25 had a response to short-course endocrine therapy, noted invited discussant Lajos Pusztai, MD, DPhil, of the Yale Cancer Center in New Haven, Conn.
“This implies that the endocrine challenge is not informative for most patients,” he said, adding that a related question is whether the 25% of patients who did not have a response and were therefore given chemotherapy benefited from that therapy.
Dr. Pusztai cautioned that, among patients in the group with RS 12-25 who had a response to preoperative endocrine therapy, certain subgroups were fairly or very small: those aged 50 years or younger (330 patients) and those with two or three positive nodes (75 and 22 patients, respectively).
And collective findings of the similar but much larger TAILORx trial and RxPONDER trial (also reported at SABCS 2020) do suggest a benefit of chemotherapy in younger women, regardless of the number of positive nodes.
“Selection of [estrogen receptor]–positive patients with zero to three lymph nodes for adjuvant chemotherapy currently should be based on age and baseline recurrence score or a similar validated molecular assay,” Dr. Pusztai recommended. “TAILORx results guide us in regard to the use of the recurrence score in node-negative patients with a recurrence score of less than 26, and the recently presented RxPONDER results provide evidence for the use of recurrence score in patients with one to three positive nodes with a recurrence score in the range of 0-26. Both of these trials showed benefit in younger women from adjuvant chemotherapy.”
The ADAPT trial was sponsored by Roche, Genomic Health/Exact Sciences, Celgene, Bayer, Teva, and Amgen. Dr. Harbeck disclosed relationships with Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Lilly, Merck, Novartis, Odonate Therapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Samsung, Sandoz, and Seattle Genetics. Dr. Pusztai disclosed relationships with AstraZeneca, Athenex, Almac, Bristol-Myers Squibb, Biotheranostics, Clovis, Daiichi, Eisai, Genentech, H2Bio, H3 Biomedicine, Immunomedics, Merck, Novartis, Pfizer, Pieris, Radius Health, Syndax, and Seattle Genetics,.
The findings were reported at the 2020 San Antonio Breast Cancer Symposium.
“In early luminal breast cancer, optimal patient selection for omission of adjuvant chemotherapy, particularly in patients with one to three involved lymph nodes, is still unclear,” noted principal investigator Nadia Harbeck, MD, PhD, of the University of Munich.
Successive trials have used nodal status, genomic risk scores, and response to preoperative therapy to home in on subsets of women for whom this practice is safe.
The ADAPT HR+/HER2– trial is a phase 3 trial that enrolled 5,625 patients with luminal (hormone receptor–positive, HER2-negative) early breast cancer who were candidates for adjuvant chemotherapy based on conventional criteria.
The trial combined a static biomarker – Oncotype Dx recurrence score (RS) in the baseline core biopsy – and a dynamic biomarker – Ki-67 response to a 3-week course of preoperative endocrine therapy – to personalize adjuvant therapy.
At SABCS 2020, Dr. Harbeck reported results for 2,290 patients having zero to three involved lymph nodes: 868 patients with RS 0-11 and 1,422 patients with RS 12-25 who had a response to brief preoperative endocrine therapy (a Ki-67 fraction ≤10% at surgery). All were treated with endocrine therapy alone as adjuvant therapy.
Similar outcomes
The median follow-up was 60 months. The 5-year rate of invasive disease–free survival was 93.9% for the group with RS 0-11 and 92.6% for the group with RS 12-25 and a response to the preoperative endocrine therapy.
The study met its primary endpoint, as the lower limit of the 95% confidence interval for the difference between groups of –3.3% fell just within the predefined margin of –3.3% or less for noninferiority (P = .05).
The groups also had similarly “excellent” distant disease–free survival (96.3% for RS 0-11 and 95.6% for RS 12-25; P = .247) and overall survival (98.0% for RS 0-11 and 97.3% for RS 12-25; P = .160), Dr. Harbeck reported.
The similar distant disease–free survival was consistent regardless of whether women were younger or older than 50 years and regardless of whether women had involved nodes or not.
In multivariate analysis, women had greater risk of distant disease–free survival events if they had three positive lymph nodes versus zero to two (hazard ratio, 3.40) or a pathologic T stage of 2-4 versus 0-1 (HR, 2.24), whereas risk fell with increasing baseline progesterone receptor expression (HR, 0.92).
“Neither patient age nor study arm were prognostic factors for patient outcome,” Dr. Harbeck noted.
In stratified analysis, the negative impact of having three positive nodes was seen only in the group with RS 12-25 and response to preoperative endocrine therapy, suggesting this subgroup may not be good candidates for omission of chemotherapy, she said.
Applying results to practice
“In luminal early breast cancer, the following patients – irrespective of their age – can safely be treated by endocrine therapy alone: patients with zero to three involved lymph nodes and recurrence score 0-11, and those with limited nodal burden (zero to two lymph nodes), recurrence score 12-25, and endocrine response after short preoperative endocrine therapy,” Dr. Harbeck summarized.
“Oncotype Dx testing can spare chemotherapy for the majority of patients with up to three involved lymph nodes. Dynamic Ki-67 response testing is feasible in clinical routine and complements baseline risk assessment to define patient selection for therapy deescalation or escalation,” she added.
The investigators have used the trial’s data to develop an algorithm for predicting the probability of response to short-course preoperative endocrine therapy that is available free of charge online (www.enrep.info).
“This may support everyday clinical decision-making in luminal early breast cancer; for example, whether to start a short period of preoperative endocrine therapy at all, and whether to rely on adjuvant endocrine therapy alone, but also in times like these, whether it’s safe to delay surgery by putting patients on prolonged preoperative endocrine therapy if surgical resources are scarce,” Dr. Harbeck commented.
Her clinic is now recruiting patients for the ADAPT Cycle trial, which is testing an endocrine-based approach with a CDK4/6 inhibitor versus chemotherapy in patients who are not candidates for adjuvant endocrine therapy alone. Therefore, all eligible patients receive the short course of endocrine therapy up front as the standard.
“But if you don’t have a trial, what are you going to do on Monday morning? Please let your patient know whether her tumor is endocrine responsive by doing this 3-week preoperative endocrine therapy,” Dr. Harbeck recommended. “It’s easy to do, you can schedule your surgeries better, and in patients with up to three lymph nodes, it helps with your decision-making, not just in the postmenopausal patients but also in the premenopausal patients, regarding whether they can forgo chemotherapy.”
Findings in context
More than 75% of ADAPT patients with RS 12-25 had a response to short-course endocrine therapy, noted invited discussant Lajos Pusztai, MD, DPhil, of the Yale Cancer Center in New Haven, Conn.
“This implies that the endocrine challenge is not informative for most patients,” he said, adding that a related question is whether the 25% of patients who did not have a response and were therefore given chemotherapy benefited from that therapy.
Dr. Pusztai cautioned that, among patients in the group with RS 12-25 who had a response to preoperative endocrine therapy, certain subgroups were fairly or very small: those aged 50 years or younger (330 patients) and those with two or three positive nodes (75 and 22 patients, respectively).
And collective findings of the similar but much larger TAILORx trial and RxPONDER trial (also reported at SABCS 2020) do suggest a benefit of chemotherapy in younger women, regardless of the number of positive nodes.
“Selection of [estrogen receptor]–positive patients with zero to three lymph nodes for adjuvant chemotherapy currently should be based on age and baseline recurrence score or a similar validated molecular assay,” Dr. Pusztai recommended. “TAILORx results guide us in regard to the use of the recurrence score in node-negative patients with a recurrence score of less than 26, and the recently presented RxPONDER results provide evidence for the use of recurrence score in patients with one to three positive nodes with a recurrence score in the range of 0-26. Both of these trials showed benefit in younger women from adjuvant chemotherapy.”
The ADAPT trial was sponsored by Roche, Genomic Health/Exact Sciences, Celgene, Bayer, Teva, and Amgen. Dr. Harbeck disclosed relationships with Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Lilly, Merck, Novartis, Odonate Therapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Samsung, Sandoz, and Seattle Genetics. Dr. Pusztai disclosed relationships with AstraZeneca, Athenex, Almac, Bristol-Myers Squibb, Biotheranostics, Clovis, Daiichi, Eisai, Genentech, H2Bio, H3 Biomedicine, Immunomedics, Merck, Novartis, Pfizer, Pieris, Radius Health, Syndax, and Seattle Genetics,.
The findings were reported at the 2020 San Antonio Breast Cancer Symposium.
“In early luminal breast cancer, optimal patient selection for omission of adjuvant chemotherapy, particularly in patients with one to three involved lymph nodes, is still unclear,” noted principal investigator Nadia Harbeck, MD, PhD, of the University of Munich.
Successive trials have used nodal status, genomic risk scores, and response to preoperative therapy to home in on subsets of women for whom this practice is safe.
The ADAPT HR+/HER2– trial is a phase 3 trial that enrolled 5,625 patients with luminal (hormone receptor–positive, HER2-negative) early breast cancer who were candidates for adjuvant chemotherapy based on conventional criteria.
The trial combined a static biomarker – Oncotype Dx recurrence score (RS) in the baseline core biopsy – and a dynamic biomarker – Ki-67 response to a 3-week course of preoperative endocrine therapy – to personalize adjuvant therapy.
At SABCS 2020, Dr. Harbeck reported results for 2,290 patients having zero to three involved lymph nodes: 868 patients with RS 0-11 and 1,422 patients with RS 12-25 who had a response to brief preoperative endocrine therapy (a Ki-67 fraction ≤10% at surgery). All were treated with endocrine therapy alone as adjuvant therapy.
Similar outcomes
The median follow-up was 60 months. The 5-year rate of invasive disease–free survival was 93.9% for the group with RS 0-11 and 92.6% for the group with RS 12-25 and a response to the preoperative endocrine therapy.
The study met its primary endpoint, as the lower limit of the 95% confidence interval for the difference between groups of –3.3% fell just within the predefined margin of –3.3% or less for noninferiority (P = .05).
The groups also had similarly “excellent” distant disease–free survival (96.3% for RS 0-11 and 95.6% for RS 12-25; P = .247) and overall survival (98.0% for RS 0-11 and 97.3% for RS 12-25; P = .160), Dr. Harbeck reported.
The similar distant disease–free survival was consistent regardless of whether women were younger or older than 50 years and regardless of whether women had involved nodes or not.
In multivariate analysis, women had greater risk of distant disease–free survival events if they had three positive lymph nodes versus zero to two (hazard ratio, 3.40) or a pathologic T stage of 2-4 versus 0-1 (HR, 2.24), whereas risk fell with increasing baseline progesterone receptor expression (HR, 0.92).
“Neither patient age nor study arm were prognostic factors for patient outcome,” Dr. Harbeck noted.
In stratified analysis, the negative impact of having three positive nodes was seen only in the group with RS 12-25 and response to preoperative endocrine therapy, suggesting this subgroup may not be good candidates for omission of chemotherapy, she said.
Applying results to practice
“In luminal early breast cancer, the following patients – irrespective of their age – can safely be treated by endocrine therapy alone: patients with zero to three involved lymph nodes and recurrence score 0-11, and those with limited nodal burden (zero to two lymph nodes), recurrence score 12-25, and endocrine response after short preoperative endocrine therapy,” Dr. Harbeck summarized.
“Oncotype Dx testing can spare chemotherapy for the majority of patients with up to three involved lymph nodes. Dynamic Ki-67 response testing is feasible in clinical routine and complements baseline risk assessment to define patient selection for therapy deescalation or escalation,” she added.
The investigators have used the trial’s data to develop an algorithm for predicting the probability of response to short-course preoperative endocrine therapy that is available free of charge online (www.enrep.info).
“This may support everyday clinical decision-making in luminal early breast cancer; for example, whether to start a short period of preoperative endocrine therapy at all, and whether to rely on adjuvant endocrine therapy alone, but also in times like these, whether it’s safe to delay surgery by putting patients on prolonged preoperative endocrine therapy if surgical resources are scarce,” Dr. Harbeck commented.
Her clinic is now recruiting patients for the ADAPT Cycle trial, which is testing an endocrine-based approach with a CDK4/6 inhibitor versus chemotherapy in patients who are not candidates for adjuvant endocrine therapy alone. Therefore, all eligible patients receive the short course of endocrine therapy up front as the standard.
“But if you don’t have a trial, what are you going to do on Monday morning? Please let your patient know whether her tumor is endocrine responsive by doing this 3-week preoperative endocrine therapy,” Dr. Harbeck recommended. “It’s easy to do, you can schedule your surgeries better, and in patients with up to three lymph nodes, it helps with your decision-making, not just in the postmenopausal patients but also in the premenopausal patients, regarding whether they can forgo chemotherapy.”
Findings in context
More than 75% of ADAPT patients with RS 12-25 had a response to short-course endocrine therapy, noted invited discussant Lajos Pusztai, MD, DPhil, of the Yale Cancer Center in New Haven, Conn.
“This implies that the endocrine challenge is not informative for most patients,” he said, adding that a related question is whether the 25% of patients who did not have a response and were therefore given chemotherapy benefited from that therapy.
Dr. Pusztai cautioned that, among patients in the group with RS 12-25 who had a response to preoperative endocrine therapy, certain subgroups were fairly or very small: those aged 50 years or younger (330 patients) and those with two or three positive nodes (75 and 22 patients, respectively).
And collective findings of the similar but much larger TAILORx trial and RxPONDER trial (also reported at SABCS 2020) do suggest a benefit of chemotherapy in younger women, regardless of the number of positive nodes.
“Selection of [estrogen receptor]–positive patients with zero to three lymph nodes for adjuvant chemotherapy currently should be based on age and baseline recurrence score or a similar validated molecular assay,” Dr. Pusztai recommended. “TAILORx results guide us in regard to the use of the recurrence score in node-negative patients with a recurrence score of less than 26, and the recently presented RxPONDER results provide evidence for the use of recurrence score in patients with one to three positive nodes with a recurrence score in the range of 0-26. Both of these trials showed benefit in younger women from adjuvant chemotherapy.”
The ADAPT trial was sponsored by Roche, Genomic Health/Exact Sciences, Celgene, Bayer, Teva, and Amgen. Dr. Harbeck disclosed relationships with Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Lilly, Merck, Novartis, Odonate Therapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Samsung, Sandoz, and Seattle Genetics. Dr. Pusztai disclosed relationships with AstraZeneca, Athenex, Almac, Bristol-Myers Squibb, Biotheranostics, Clovis, Daiichi, Eisai, Genentech, H2Bio, H3 Biomedicine, Immunomedics, Merck, Novartis, Pfizer, Pieris, Radius Health, Syndax, and Seattle Genetics,.
FROM SABCS 2020
Concern over response to COVID-19 in patients with blood cancers
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In COVID-19 patients, risk of bleeding rivals risk of thromboembolism
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE GOING BACK TO THE HEART OF CARDIOLOGY MEETING
A 4-point thrombocytopenia score was found able to rule out suspected HIT
The real strength of the 4T score for heparin-induced thrombocytopenia (HIT) is its negative predictive value, according to hematologist Adam Cuker, MD, of the department of medicine at the University of Pennsylvania, Philadelphia.
The score assigns patients points based on degree of thrombocytopenia, timing of platelet count fall in relation to heparin exposure, presence of thrombosis and other sequelae, and the likelihood of other causes of thrombocytopenia.
A low score – 3 points or less – has a negative predictive value of 99.8%, “so HIT is basically ruled out; you do not need to order lab testing for HIT or manage the patient empirically for HIT,” and should look for other causes of thrombocytopenia, said Dr. Cuker, lead author of the American Society of Hematology’s most recent HIT guidelines.
Intermediate scores of 4 or 5 points, and high scores of 6-8 points, are a different story. The positive predictive value of an intermediate score is only 14%, and of a high score, 64%, so although they don’t confirm the diagnosis, “you have to take the possibility of HIT seriously.” Discontinue heparin, start a nonheparin anticoagulant, and order a HIT immunoassay. If it’s positive, order a functional assay to confirm the diagnosis, he said.
Suspicion of HIT “is perhaps the most common consult that we get on the hematology service. These are tough consults because it is a high-stakes decision.” There is about a 6% risk of thromboembolism, amputation, and death for every day treatment is delayed. “On the other hand, the nonheparin anticoagulants are expensive, and they carry about a 1% daily risk of major bleeding,” Dr. Cuker explained during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
ELISA immunoassay detects antiplatelet factor 4 heparin antibodies but doesn’t tell whether or not they are able to activate platelets and cause HIT. Functional tests such as the serotonin-release assay detect only those antibodies able to do so, but the assays are difficult to perform, and often require samples to be sent out to a reference lab.
ASH did not specify a particular nonheparin anticoagulant in its 2018 guidelines because “the best choice for your patient” depends on which drugs you have available, your familiarity with them, and patient factors, Dr. Cuker said at the conference sponsored by MedscapeLive.
It makes sense, for instance, to use a short-acting agent such as argatroban or bivalirudin in patients who are critically ill, at high risk of bleeding, or likely to need an urgent unplanned procedure. Fondaparinux or direct oral anticoagulants (DOACs) make sense if patients are clinically stable with good organ function and no more than average bleeding risk, because they are easier to administer and facilitate transition to the outpatient setting.
DOACs are newcomers to ASH’s guidelines. Just 81 patients had been reported in the literature when they were being drafted, but only 2 patients had recurrence or progression of thromboembolic events, and there were no major bleeds. The results compared favorably with other options.
The studies were subject to selection and reporting biases, “but, nonetheless, the panel felt the results were positive enough that DOACs ought to be listed as an option,” Dr. Cuker said.
The guidelines note that parenteral options may be the best choice for life- or limb-threatening thrombosis “because few such patients have been treated with a DOAC.” Anticoagulation must continue until platelet counts recover.
Dr. Cuker is a consultant for Synergy and has institutional research support from Alexion, Bayer, Sanofi, and other companies. MedscapeLive and this news organization are owned by the same parent company.
The real strength of the 4T score for heparin-induced thrombocytopenia (HIT) is its negative predictive value, according to hematologist Adam Cuker, MD, of the department of medicine at the University of Pennsylvania, Philadelphia.
The score assigns patients points based on degree of thrombocytopenia, timing of platelet count fall in relation to heparin exposure, presence of thrombosis and other sequelae, and the likelihood of other causes of thrombocytopenia.
A low score – 3 points or less – has a negative predictive value of 99.8%, “so HIT is basically ruled out; you do not need to order lab testing for HIT or manage the patient empirically for HIT,” and should look for other causes of thrombocytopenia, said Dr. Cuker, lead author of the American Society of Hematology’s most recent HIT guidelines.
Intermediate scores of 4 or 5 points, and high scores of 6-8 points, are a different story. The positive predictive value of an intermediate score is only 14%, and of a high score, 64%, so although they don’t confirm the diagnosis, “you have to take the possibility of HIT seriously.” Discontinue heparin, start a nonheparin anticoagulant, and order a HIT immunoassay. If it’s positive, order a functional assay to confirm the diagnosis, he said.
Suspicion of HIT “is perhaps the most common consult that we get on the hematology service. These are tough consults because it is a high-stakes decision.” There is about a 6% risk of thromboembolism, amputation, and death for every day treatment is delayed. “On the other hand, the nonheparin anticoagulants are expensive, and they carry about a 1% daily risk of major bleeding,” Dr. Cuker explained during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
ELISA immunoassay detects antiplatelet factor 4 heparin antibodies but doesn’t tell whether or not they are able to activate platelets and cause HIT. Functional tests such as the serotonin-release assay detect only those antibodies able to do so, but the assays are difficult to perform, and often require samples to be sent out to a reference lab.
ASH did not specify a particular nonheparin anticoagulant in its 2018 guidelines because “the best choice for your patient” depends on which drugs you have available, your familiarity with them, and patient factors, Dr. Cuker said at the conference sponsored by MedscapeLive.
It makes sense, for instance, to use a short-acting agent such as argatroban or bivalirudin in patients who are critically ill, at high risk of bleeding, or likely to need an urgent unplanned procedure. Fondaparinux or direct oral anticoagulants (DOACs) make sense if patients are clinically stable with good organ function and no more than average bleeding risk, because they are easier to administer and facilitate transition to the outpatient setting.
DOACs are newcomers to ASH’s guidelines. Just 81 patients had been reported in the literature when they were being drafted, but only 2 patients had recurrence or progression of thromboembolic events, and there were no major bleeds. The results compared favorably with other options.
The studies were subject to selection and reporting biases, “but, nonetheless, the panel felt the results were positive enough that DOACs ought to be listed as an option,” Dr. Cuker said.
The guidelines note that parenteral options may be the best choice for life- or limb-threatening thrombosis “because few such patients have been treated with a DOAC.” Anticoagulation must continue until platelet counts recover.
Dr. Cuker is a consultant for Synergy and has institutional research support from Alexion, Bayer, Sanofi, and other companies. MedscapeLive and this news organization are owned by the same parent company.
The real strength of the 4T score for heparin-induced thrombocytopenia (HIT) is its negative predictive value, according to hematologist Adam Cuker, MD, of the department of medicine at the University of Pennsylvania, Philadelphia.
The score assigns patients points based on degree of thrombocytopenia, timing of platelet count fall in relation to heparin exposure, presence of thrombosis and other sequelae, and the likelihood of other causes of thrombocytopenia.
A low score – 3 points or less – has a negative predictive value of 99.8%, “so HIT is basically ruled out; you do not need to order lab testing for HIT or manage the patient empirically for HIT,” and should look for other causes of thrombocytopenia, said Dr. Cuker, lead author of the American Society of Hematology’s most recent HIT guidelines.
Intermediate scores of 4 or 5 points, and high scores of 6-8 points, are a different story. The positive predictive value of an intermediate score is only 14%, and of a high score, 64%, so although they don’t confirm the diagnosis, “you have to take the possibility of HIT seriously.” Discontinue heparin, start a nonheparin anticoagulant, and order a HIT immunoassay. If it’s positive, order a functional assay to confirm the diagnosis, he said.
Suspicion of HIT “is perhaps the most common consult that we get on the hematology service. These are tough consults because it is a high-stakes decision.” There is about a 6% risk of thromboembolism, amputation, and death for every day treatment is delayed. “On the other hand, the nonheparin anticoagulants are expensive, and they carry about a 1% daily risk of major bleeding,” Dr. Cuker explained during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
ELISA immunoassay detects antiplatelet factor 4 heparin antibodies but doesn’t tell whether or not they are able to activate platelets and cause HIT. Functional tests such as the serotonin-release assay detect only those antibodies able to do so, but the assays are difficult to perform, and often require samples to be sent out to a reference lab.
ASH did not specify a particular nonheparin anticoagulant in its 2018 guidelines because “the best choice for your patient” depends on which drugs you have available, your familiarity with them, and patient factors, Dr. Cuker said at the conference sponsored by MedscapeLive.
It makes sense, for instance, to use a short-acting agent such as argatroban or bivalirudin in patients who are critically ill, at high risk of bleeding, or likely to need an urgent unplanned procedure. Fondaparinux or direct oral anticoagulants (DOACs) make sense if patients are clinically stable with good organ function and no more than average bleeding risk, because they are easier to administer and facilitate transition to the outpatient setting.
DOACs are newcomers to ASH’s guidelines. Just 81 patients had been reported in the literature when they were being drafted, but only 2 patients had recurrence or progression of thromboembolic events, and there were no major bleeds. The results compared favorably with other options.
The studies were subject to selection and reporting biases, “but, nonetheless, the panel felt the results were positive enough that DOACs ought to be listed as an option,” Dr. Cuker said.
The guidelines note that parenteral options may be the best choice for life- or limb-threatening thrombosis “because few such patients have been treated with a DOAC.” Anticoagulation must continue until platelet counts recover.
Dr. Cuker is a consultant for Synergy and has institutional research support from Alexion, Bayer, Sanofi, and other companies. MedscapeLive and this news organization are owned by the same parent company.
FROM 2020 UNNH
Risk of HPV-related oropharyngeal cancer linked to number of oral sex partners
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One-week radiotherapy course should be standard for early invasive breast cancer, experts say
The trial was designed to compare the standard regimen (40 Gy in 15 fractions over 3 weeks) with a higher-dose hypofractionated regimen (27 Gy in 5 fractions over 5 days) and a lower-dose hypofractionated regimen (26 Gy in 5 fractions over 5 days) in women who had undergone surgery for early invasive breast cancer.
The 5-year rate of ipsilateral breast tumor relapse was similar with all regimens – 2.1% with the 40-Gy regimen, 1.7% with the 27-Gy regimen, and 1.4% with the 26-Gy regimen. The 26-Gy regimen also had similar safety as the 40-Gy regimen.
These results were presented at the European Society for Radiology and Oncology 2020 Online Congress by Joanne S. Haviland, MSc, of the Institute of Cancer Research in London. Results were also published in The Lancet.
Ms. Haviland said that hypofractionated regimens are attractive because of their shorter overall treatment times, which translate to greater convenience and lower treatment costs.
The historic 5-week regimen (50 Gy in 25 fractions) has been replaced by a 3-week regimen (40 Gy in 15 fractions) in the United Kingdom and elsewhere, and ongoing efforts are exploring whether further hypofractionation can be achieved without compromising efficacy and safety.
“The FAST-Forward trial was the next step on from testing hypofractionated schedules evaluated in earlier trials, including the START trials in the early 2000s and the FAST trial, which published its 10-year results earlier this year,” Ms. Haviland explained.
FAST-Forward enrolled 4,096 women who had undergone breast-conserving surgery or mastectomy for early invasive breast cancer. The patients were randomized into the aforementioned groups for adjuvant whole-breast or chest-wall radiotherapy: 40 Gy in 15 fractions over 3 weeks, 27 Gy in 5 fractions over 5 days, or 26 Gy in 5 fractions over 5 days. Boosts were permitted for all regimens.
Relapse, safety, and patient reports
The median follow-up was 6 years. The 5-year rate of ipsilateral breast tumor relapse was 2.1% with the 40-Gy standard regimen, 1.7% with the 27-Gy hypofractionated regimen, and 1.4% with the 26-Gy hypofractionated regimen.
The upper bound of the 95% confidence interval for the difference comparing the hypofractionated regimens against the standard fell well within the 1.6% excess predefined for noninferiority for both the 27-Gy regimen and the 26-Gy regimen (0.9% and 0.3%, respectively).
The hazard ratio for ipsilateral breast tumor relapse, compared with the standard regimen, was 0.86 for the 27-Gy hypofractionated regimen and 0.67 for the 26-Gy hypofractionated regimen.
In terms of safety, the 5-year rate of late adverse effects of the breast or chest wall – distortion, shrinkage, induration, telangiectasia, or edema – rated as “moderate” or “marked” by clinicians was 10% with the standard regimen, 15% with the 27-Gy regimen (relative risk, 1.55 ; P < .001), and 12% with the 26-Gy regimen (RR, 1.19; P = .17).
Over the entire follow-up, women had significantly higher odds of all moderate or marked individual late adverse effects (except discomfort) with the 27-Gy regimen versus the standard regimen, whereas their odds were significantly higher only for induration and edema with the 26-Gy regimen.
However, absolute rates and risk differences between groups were small, Ms. Haviland pointed out. For example, the most common moderate or marked late adverse effect with the standard regimen was breast shrinkage, seen in 5% of patients, followed by discomfort, seen in 4%.
Patient-assessed change in breast appearance and shrinkage did not differ significantly across groups. But women in the 27-Gy group were more likely than peers in the standard regimen group to report a moderate or marked increase in breast hardness/firmness (21% vs. 14%; P = .008), and women in both the 27-Gy and 26-Gy groups were more likely to report moderate or marked breast swelling (5%; P = .007 and 4%; P = .02, respectively, vs. 2%).
A new standard
“We have shown noninferiority in terms of local tumor control for both 5-fraction schedules, compared with the control group of 40 Gy in 15 fractions,” Ms. Haviland summarized. “Late adverse effects in normal tissues were similar after 26 Gy in 5 fractions to 40 Gy in 15 fractions, and although rates were higher for the 27-Gy schedule, we noted that these are consistent with the historic standard of 50 Gy in 25 fractions.”
“There are obvious benefits to patients and health care systems of shorter radiotherapy treatments, particularly at the current time, and in fact, the COVID pandemic has accelerated uptake of the 26-Gy schedule around the world,” she added. “At a recent consensus meeting organized by the Royal College of Radiologists, the U.K. adopted the 26-Gy schedule as a new standard, also integrating this with partial breast irradiation, in close collaboration with the U.K. IMPORT Low trial.”
“This is very important work. I think this is one of the most important trials in the past few years. It has really changed practice,” commented session co-chair Ben Slotman, MD, PhD, of Vrije Universiteit Medical Center, Amsterdam, and AMC Amsterdam, who was not involved the trial.
Dr. Slotman wondered how extensive uptake of the new hypofractionated regimen has been. “I know it’s being used in the U.K. and the Netherlands, but do you have any idea about the rest of Europe? What do we need to make it the new standard?”
“I think there has been uptake in other countries in Europe and elsewhere around the world as well,” Ms. Haviland replied. But feedback suggests adoption has been tempered because of reservations related to the regimen’s safety in certain patient subgroups.
“We haven’t found any cause for concern in the subgroups, and also backed up by meta-analysis in the many patients randomized in the START trials,” she noted. “So I think there is very convincing evidence that it is safe as a new standard.”
FAST-Forward was sponsored by the Institute of Cancer Research and funded by the National Institute for Health Research Health Technology Assessment Programme. Ms. Haviland disclosed no conflicts of interest. Dr. Slotman has relationships with ViewRay and Varian Medical Systems.
SOURCE: Haviland J et al. ESTRO 2020, Abstract OC-0610.
The trial was designed to compare the standard regimen (40 Gy in 15 fractions over 3 weeks) with a higher-dose hypofractionated regimen (27 Gy in 5 fractions over 5 days) and a lower-dose hypofractionated regimen (26 Gy in 5 fractions over 5 days) in women who had undergone surgery for early invasive breast cancer.
The 5-year rate of ipsilateral breast tumor relapse was similar with all regimens – 2.1% with the 40-Gy regimen, 1.7% with the 27-Gy regimen, and 1.4% with the 26-Gy regimen. The 26-Gy regimen also had similar safety as the 40-Gy regimen.
These results were presented at the European Society for Radiology and Oncology 2020 Online Congress by Joanne S. Haviland, MSc, of the Institute of Cancer Research in London. Results were also published in The Lancet.
Ms. Haviland said that hypofractionated regimens are attractive because of their shorter overall treatment times, which translate to greater convenience and lower treatment costs.
The historic 5-week regimen (50 Gy in 25 fractions) has been replaced by a 3-week regimen (40 Gy in 15 fractions) in the United Kingdom and elsewhere, and ongoing efforts are exploring whether further hypofractionation can be achieved without compromising efficacy and safety.
“The FAST-Forward trial was the next step on from testing hypofractionated schedules evaluated in earlier trials, including the START trials in the early 2000s and the FAST trial, which published its 10-year results earlier this year,” Ms. Haviland explained.
FAST-Forward enrolled 4,096 women who had undergone breast-conserving surgery or mastectomy for early invasive breast cancer. The patients were randomized into the aforementioned groups for adjuvant whole-breast or chest-wall radiotherapy: 40 Gy in 15 fractions over 3 weeks, 27 Gy in 5 fractions over 5 days, or 26 Gy in 5 fractions over 5 days. Boosts were permitted for all regimens.
Relapse, safety, and patient reports
The median follow-up was 6 years. The 5-year rate of ipsilateral breast tumor relapse was 2.1% with the 40-Gy standard regimen, 1.7% with the 27-Gy hypofractionated regimen, and 1.4% with the 26-Gy hypofractionated regimen.
The upper bound of the 95% confidence interval for the difference comparing the hypofractionated regimens against the standard fell well within the 1.6% excess predefined for noninferiority for both the 27-Gy regimen and the 26-Gy regimen (0.9% and 0.3%, respectively).
The hazard ratio for ipsilateral breast tumor relapse, compared with the standard regimen, was 0.86 for the 27-Gy hypofractionated regimen and 0.67 for the 26-Gy hypofractionated regimen.
In terms of safety, the 5-year rate of late adverse effects of the breast or chest wall – distortion, shrinkage, induration, telangiectasia, or edema – rated as “moderate” or “marked” by clinicians was 10% with the standard regimen, 15% with the 27-Gy regimen (relative risk, 1.55 ; P < .001), and 12% with the 26-Gy regimen (RR, 1.19; P = .17).
Over the entire follow-up, women had significantly higher odds of all moderate or marked individual late adverse effects (except discomfort) with the 27-Gy regimen versus the standard regimen, whereas their odds were significantly higher only for induration and edema with the 26-Gy regimen.
However, absolute rates and risk differences between groups were small, Ms. Haviland pointed out. For example, the most common moderate or marked late adverse effect with the standard regimen was breast shrinkage, seen in 5% of patients, followed by discomfort, seen in 4%.
Patient-assessed change in breast appearance and shrinkage did not differ significantly across groups. But women in the 27-Gy group were more likely than peers in the standard regimen group to report a moderate or marked increase in breast hardness/firmness (21% vs. 14%; P = .008), and women in both the 27-Gy and 26-Gy groups were more likely to report moderate or marked breast swelling (5%; P = .007 and 4%; P = .02, respectively, vs. 2%).
A new standard
“We have shown noninferiority in terms of local tumor control for both 5-fraction schedules, compared with the control group of 40 Gy in 15 fractions,” Ms. Haviland summarized. “Late adverse effects in normal tissues were similar after 26 Gy in 5 fractions to 40 Gy in 15 fractions, and although rates were higher for the 27-Gy schedule, we noted that these are consistent with the historic standard of 50 Gy in 25 fractions.”
“There are obvious benefits to patients and health care systems of shorter radiotherapy treatments, particularly at the current time, and in fact, the COVID pandemic has accelerated uptake of the 26-Gy schedule around the world,” she added. “At a recent consensus meeting organized by the Royal College of Radiologists, the U.K. adopted the 26-Gy schedule as a new standard, also integrating this with partial breast irradiation, in close collaboration with the U.K. IMPORT Low trial.”
“This is very important work. I think this is one of the most important trials in the past few years. It has really changed practice,” commented session co-chair Ben Slotman, MD, PhD, of Vrije Universiteit Medical Center, Amsterdam, and AMC Amsterdam, who was not involved the trial.
Dr. Slotman wondered how extensive uptake of the new hypofractionated regimen has been. “I know it’s being used in the U.K. and the Netherlands, but do you have any idea about the rest of Europe? What do we need to make it the new standard?”
“I think there has been uptake in other countries in Europe and elsewhere around the world as well,” Ms. Haviland replied. But feedback suggests adoption has been tempered because of reservations related to the regimen’s safety in certain patient subgroups.
“We haven’t found any cause for concern in the subgroups, and also backed up by meta-analysis in the many patients randomized in the START trials,” she noted. “So I think there is very convincing evidence that it is safe as a new standard.”
FAST-Forward was sponsored by the Institute of Cancer Research and funded by the National Institute for Health Research Health Technology Assessment Programme. Ms. Haviland disclosed no conflicts of interest. Dr. Slotman has relationships with ViewRay and Varian Medical Systems.
SOURCE: Haviland J et al. ESTRO 2020, Abstract OC-0610.
The trial was designed to compare the standard regimen (40 Gy in 15 fractions over 3 weeks) with a higher-dose hypofractionated regimen (27 Gy in 5 fractions over 5 days) and a lower-dose hypofractionated regimen (26 Gy in 5 fractions over 5 days) in women who had undergone surgery for early invasive breast cancer.
The 5-year rate of ipsilateral breast tumor relapse was similar with all regimens – 2.1% with the 40-Gy regimen, 1.7% with the 27-Gy regimen, and 1.4% with the 26-Gy regimen. The 26-Gy regimen also had similar safety as the 40-Gy regimen.
These results were presented at the European Society for Radiology and Oncology 2020 Online Congress by Joanne S. Haviland, MSc, of the Institute of Cancer Research in London. Results were also published in The Lancet.
Ms. Haviland said that hypofractionated regimens are attractive because of their shorter overall treatment times, which translate to greater convenience and lower treatment costs.
The historic 5-week regimen (50 Gy in 25 fractions) has been replaced by a 3-week regimen (40 Gy in 15 fractions) in the United Kingdom and elsewhere, and ongoing efforts are exploring whether further hypofractionation can be achieved without compromising efficacy and safety.
“The FAST-Forward trial was the next step on from testing hypofractionated schedules evaluated in earlier trials, including the START trials in the early 2000s and the FAST trial, which published its 10-year results earlier this year,” Ms. Haviland explained.
FAST-Forward enrolled 4,096 women who had undergone breast-conserving surgery or mastectomy for early invasive breast cancer. The patients were randomized into the aforementioned groups for adjuvant whole-breast or chest-wall radiotherapy: 40 Gy in 15 fractions over 3 weeks, 27 Gy in 5 fractions over 5 days, or 26 Gy in 5 fractions over 5 days. Boosts were permitted for all regimens.
Relapse, safety, and patient reports
The median follow-up was 6 years. The 5-year rate of ipsilateral breast tumor relapse was 2.1% with the 40-Gy standard regimen, 1.7% with the 27-Gy hypofractionated regimen, and 1.4% with the 26-Gy hypofractionated regimen.
The upper bound of the 95% confidence interval for the difference comparing the hypofractionated regimens against the standard fell well within the 1.6% excess predefined for noninferiority for both the 27-Gy regimen and the 26-Gy regimen (0.9% and 0.3%, respectively).
The hazard ratio for ipsilateral breast tumor relapse, compared with the standard regimen, was 0.86 for the 27-Gy hypofractionated regimen and 0.67 for the 26-Gy hypofractionated regimen.
In terms of safety, the 5-year rate of late adverse effects of the breast or chest wall – distortion, shrinkage, induration, telangiectasia, or edema – rated as “moderate” or “marked” by clinicians was 10% with the standard regimen, 15% with the 27-Gy regimen (relative risk, 1.55 ; P < .001), and 12% with the 26-Gy regimen (RR, 1.19; P = .17).
Over the entire follow-up, women had significantly higher odds of all moderate or marked individual late adverse effects (except discomfort) with the 27-Gy regimen versus the standard regimen, whereas their odds were significantly higher only for induration and edema with the 26-Gy regimen.
However, absolute rates and risk differences between groups were small, Ms. Haviland pointed out. For example, the most common moderate or marked late adverse effect with the standard regimen was breast shrinkage, seen in 5% of patients, followed by discomfort, seen in 4%.
Patient-assessed change in breast appearance and shrinkage did not differ significantly across groups. But women in the 27-Gy group were more likely than peers in the standard regimen group to report a moderate or marked increase in breast hardness/firmness (21% vs. 14%; P = .008), and women in both the 27-Gy and 26-Gy groups were more likely to report moderate or marked breast swelling (5%; P = .007 and 4%; P = .02, respectively, vs. 2%).
A new standard
“We have shown noninferiority in terms of local tumor control for both 5-fraction schedules, compared with the control group of 40 Gy in 15 fractions,” Ms. Haviland summarized. “Late adverse effects in normal tissues were similar after 26 Gy in 5 fractions to 40 Gy in 15 fractions, and although rates were higher for the 27-Gy schedule, we noted that these are consistent with the historic standard of 50 Gy in 25 fractions.”
“There are obvious benefits to patients and health care systems of shorter radiotherapy treatments, particularly at the current time, and in fact, the COVID pandemic has accelerated uptake of the 26-Gy schedule around the world,” she added. “At a recent consensus meeting organized by the Royal College of Radiologists, the U.K. adopted the 26-Gy schedule as a new standard, also integrating this with partial breast irradiation, in close collaboration with the U.K. IMPORT Low trial.”
“This is very important work. I think this is one of the most important trials in the past few years. It has really changed practice,” commented session co-chair Ben Slotman, MD, PhD, of Vrije Universiteit Medical Center, Amsterdam, and AMC Amsterdam, who was not involved the trial.
Dr. Slotman wondered how extensive uptake of the new hypofractionated regimen has been. “I know it’s being used in the U.K. and the Netherlands, but do you have any idea about the rest of Europe? What do we need to make it the new standard?”
“I think there has been uptake in other countries in Europe and elsewhere around the world as well,” Ms. Haviland replied. But feedback suggests adoption has been tempered because of reservations related to the regimen’s safety in certain patient subgroups.
“We haven’t found any cause for concern in the subgroups, and also backed up by meta-analysis in the many patients randomized in the START trials,” she noted. “So I think there is very convincing evidence that it is safe as a new standard.”
FAST-Forward was sponsored by the Institute of Cancer Research and funded by the National Institute for Health Research Health Technology Assessment Programme. Ms. Haviland disclosed no conflicts of interest. Dr. Slotman has relationships with ViewRay and Varian Medical Systems.
SOURCE: Haviland J et al. ESTRO 2020, Abstract OC-0610.
FROM ESTRO 2020
Differences in right vs. left colon in Black vs. White individuals
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liquid Biopsies in a Veteran Patient Population With Advanced Prostate and Lung Non-Small Cell Carcinomas: A New Paradigm and Unique Challenge in Personalized Medicine
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
Sequential Targeted Treatment for a Geriatric Patient with Acute Myeloid Leukemia with Concurrent FLT3-TKD and IDH1 Mutations
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.