User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
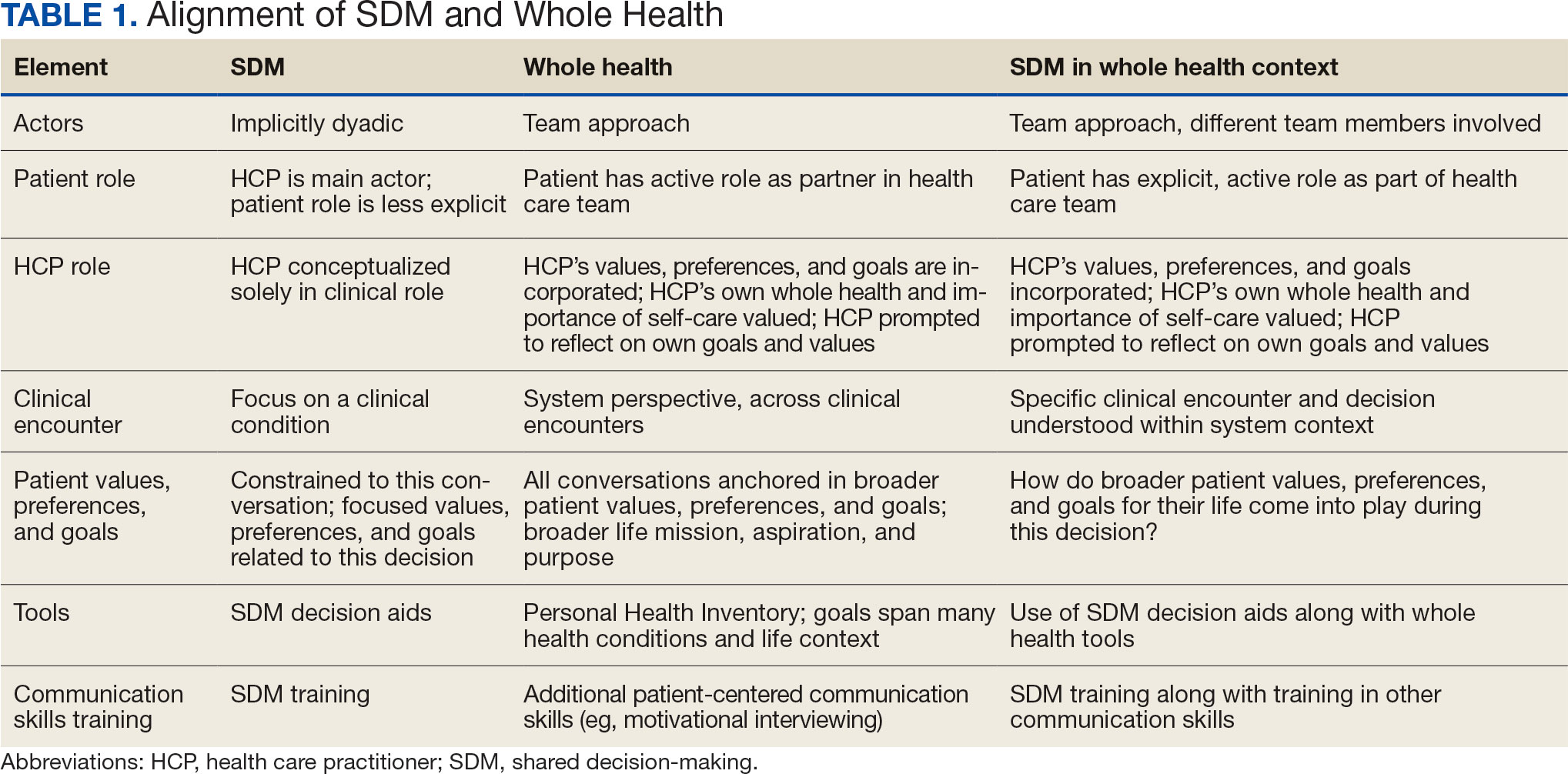
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.

STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Lifestyle Changes Save Lives in Colon Cancer?
Can Lifestyle Changes Save Lives in Colon Cancer?
Can exercise “therapy” and diet improve survival in patients with colon cancer? It appears so, according to two pivotal studies presented at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
In the CHALLENGE trial, a structured exercise program after surgery and adjuvant chemotherapy cut the risk for colon cancer recurrence in patients with stage III and high-risk stage II disease by more than one quarter and the risk for death by more than one third.
“The magnitude of benefit with exercise is substantial. In fact, it is comparable, and in some cases exceeds the magnitude of benefit of many of our very good standard medical therapies in oncology,” study presenter Christopher Booth, MD, with Queen’s University, Kingston, Ontario, Canada, told attendees.
Results of the study were published online in The New England Journal of Medicine to coincide with the presentation at the meeting.
The findings are “nothing short of a major milestone,” said study discussant Peter Campbell, PhD, with Montefiore Einstein Comprehensive Cancer Center, Bronx, New York.
The other study showed that eating a less inflammatory diet may reduce the risk for death in patients with colon cancer, with the greatest benefits seen in those who embraced anti-inflammatory foods and exercised regularly.
“Putting these two abstracts into perspective, we as physicians need to be essentially prescribing healthy diet and exercise. The combination of the two are synergistic,” Julie Gralow, MD, ASCO chief medical officer and executive vice president, told attendees.
Despite the benefits of these lifestyle changes, exercise and diet are meant to supplement, not replace, established colon cancer treatments.
It would be a false binary to frame this as lifestyle vs cancer treatment, explained Mark Lewis, MD, director of Gastrointestinal Oncology at Intermountain Healthcare in Salt Lake City, Utah. With exercise, for instance, “the key is giving enough chemo to protect against recurrence and eliminate micrometastases but not so much that we cause neuropathy and reduce function and ability to follow the CHALLENGE structured program,” Lewis said.
Exercise and Survival
Colon cancer remains the second-leading cause of cancer death worldwide. Even with surgery and chemotherapy, roughly 30% of patients with stage III and high-risk stage II colon cancer will experience disease recurrence.
“As oncologists, one of the most common questions we get asked by patients is — what else can I do to improve my outcome?” Booth said.
Observational studies published nearly two decades ago hinted that physically active cancer survivors fare better, but no randomized trial has definitively tested whether exercise could alter disease course. That knowledge gap prompted the Canadian Cancer Trials Group to launch the CHALLENGE trial.
Between 2009 and 2023, the phase 3 study enrolled 889 adults (median age, 61 years; 51% women) who had completed surgery and adjuvant chemotherapy for stage III (90%) or high-risk stage II (10%) colon cancer. Most patients were from Canada and Australia and were enrolled 2-6 months after completing chemotherapy.
Half of study participants were randomly allocated to a structured exercise program (n = 445) and half to receive standard health education materials promoting physical activity and healthy eating (control individuals, n = 444).
As part of the structured exercise intervention, patients met with a physical activity consultant twice a month for the first 6 months. These sessions included exercise coaching and supervised exercise. Patients could choose their preferred aerobic exercise and most picked brisk walking.
The consultants gave each patient an “exercise prescription” to hit a specific amount of exercise. The target was an additional 10 metabolic equivalent (MET)–hours of aerobic activity per week — about three to four brisk walks each lasting 45-60 minutes. After 6 months, patients met with their consultants once a month, with additional sessions available for extra support if needed.
Structured exercise led to “substantial and sustained” increases in the amount of exercise participants did, as well as physiologic measures of their fitness, with “highly relevant” improvements in VO2 max, 6-minute walk test, and patient-reported physical function, underscoring that participants were not only exercising more but also getting fitter, Booth said.
Exercise was associated with a clinically meaningful and statistically significant 28% reduction in the risk for recurrent or new cancer (hazard ratio [HR], 0.72; P = .017), with a 5-year disease free survival rate of 80% in the exercise group and 74% in the control group.
In other words, “for every 16 patients that went on the exercise program, exercise prevented 1 person from recurrent or new cancer” at 5 years, Booth reported.
Overall survival results were “even more impressive,” he said.
At 8 years, 90% of patients in the exercise program were alive vs 83% of those in the control group, which translated to a 37% lower risk for death (HR, 0.63; P = .022).
“For every 14 patients who went on the exercise program, exercise prevented 1 person from dying” at the 8-year mark, Booth noted.
“Notably, this difference in survival was not driven by difference in cardiovascular deaths but by a reduction in the risk of death from colon cancer,” he said.
Besides a slight uptick in musculoskeletal aches, no major safety signals emerged in the exercise group.
It’s important to note that the survival benefit associated with exercise came after patients had received surgery followed by chemotherapy — in other words, exercise did not replace established cancer treatments. It’s also unclear whether initiating an exercise intervention earlier in the treatment trajectory — before surgery or during chemotherapy, instead of after chemotherapy — could further improve cancer outcomes, the authors noted.
Still, “exercise as an intervention is a no brainer and should be implemented broadly,” said ASCO expert Pamela Kunz, MD, with Yale School of Medicine, New Haven, Connecticut.
Marco Gerlinger, MD, with Barts Cancer Institute, London, England, agreed.
“Oncologists can now make a very clear evidence-based recommendation for patients who just completed their chemotherapy for bowel cancer and are fit enough for such an exercise program,” Gerlinger said in a statement from the nonprofit UK Science Media Centre.
Booth noted that knowledge alone will not be sufficient to allow most patients to change their lifestyle and realize the health benefits.
“The policy implementation piece of this is really key, and we need health systems, hospitals, and payers to invest in these behavior support programs so that patients have access to a physical activity consultant and can realize the health benefits,” he said.
“This intervention is empowering and achievable for patients and with much, much lower cost than many of our therapies. It is also sustainable for health systems,” he concluded.
Diet and Survival
Diet can also affect outcomes in patients with colon cancer.
In the same session describing the CHALLENGE results, Sara Char, MD, with Dana-Farber Cancer Institute in Boston, reported findings showing that consuming a diet high in proinflammatory foods was associated with worse overall survival in patients with stage III colon cancer. A proinflammatory diet includes red and processed meats, sugary drinks, and refined grains, while an anti-inflammatory diet focuses on fruits, vegetables, whole grains, fish, and olive oil.
Chronic systemic inflammation has been implicated in both colon cancer development and in its progression, and elevated levels of inflammatory markers in the blood have previously been associated with worse survival outcomes in patients with stage III colon cancer.
Char and colleagues analyzed dietary patterns of a subset of 1625 patients (mean age, 61 years) with resected stage III colon cancer enrolled in the phase 3 CALGB/SWOG 80702 (Alliance) clinical trial, which compared 3 months of adjuvant chemotherapy with 6 months of adjuvant chemotherapy, with or without the anti-inflammatory medication celecoxib.
As part of the trial, participants reported their diet and exercise habits at various timepoints. Their diets were scored using the validated empirical dietary inflammatory pattern (EDIP) tool, which is a weighted sum of 18 food groups — nine proinflammatory and nine anti-inflammatory. A high EDIP score marks a proinflammatory diet, and a low EDIP score indicates a less inflammatory diet.
During median follow-up of nearly 4 years, researchers noted a trend toward worse disease-free survival in patients with high proinflammatory diets (HR, 1.46), but this association was not significant in the multivariable adjusted model (HR, 1.36; P = .22), Char reported.
However, higher intake of proinflammatory foods was associated with significantly worse overall survival.
Patients who consumed the most proinflammatory foods (top 20%) had an 87% higher risk for death compared with those who consumed the least (bottom 20%; HR, 1.87). The median overall survival in the highest quintile was 7.7 years and was not reached in the lowest quintile.
Combine Exercise and Diet for Best Results
To examine the joint effect of physical activity and diet on overall survival, patients were divided into higher and lower levels of physical activity using a cut-off of 9 MET hours per week, which roughly correlates to 30 minutes of vigorous walking five days a week with a little bit of light yoga, Char explained.
In this analysis, patients with less proinflammatory diets and higher physical activity levels had the best overall survival outcomes, with a 63% lower risk for death compared with peers who consumed more pro-inflammatory diets and exercised less (HR, 0.37; P < .0001).
Daily celecoxib use and low-dose aspirin use (< 100 mg/d) did not affect the association between inflammatory diet and survival.
Char cautioned, that while the EDIP tool is useful to measure the inflammatory potential of a diet, “this is not a dietary recommendation, and we need further studies to be able to tailor our findings into dietary recommendations that can be provided to patients at the bedside.”
Gralow said this “early but promising observational study suggests a powerful synergy: Patients with stage III colon cancer who embraced anti-inflammatory foods and exercised regularly showed the best overall survival compared to those with inflammatory diets and limited exercise.”
The CHALLENGE trial was funded by the Canadian Cancer Society, the National Health and Medical Research Council, Cancer Research UK, and the University of Sydney Cancer Research Fund. Booth had no disclosures. The diet study was funded by the National Institutes of Health, Pfizer, and the Project P Fund. Char disclosed an advisory/consultant role with Goodpath. Kunz, Gralow and Campbell had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Can exercise “therapy” and diet improve survival in patients with colon cancer? It appears so, according to two pivotal studies presented at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
In the CHALLENGE trial, a structured exercise program after surgery and adjuvant chemotherapy cut the risk for colon cancer recurrence in patients with stage III and high-risk stage II disease by more than one quarter and the risk for death by more than one third.
“The magnitude of benefit with exercise is substantial. In fact, it is comparable, and in some cases exceeds the magnitude of benefit of many of our very good standard medical therapies in oncology,” study presenter Christopher Booth, MD, with Queen’s University, Kingston, Ontario, Canada, told attendees.
Results of the study were published online in The New England Journal of Medicine to coincide with the presentation at the meeting.
The findings are “nothing short of a major milestone,” said study discussant Peter Campbell, PhD, with Montefiore Einstein Comprehensive Cancer Center, Bronx, New York.
The other study showed that eating a less inflammatory diet may reduce the risk for death in patients with colon cancer, with the greatest benefits seen in those who embraced anti-inflammatory foods and exercised regularly.
“Putting these two abstracts into perspective, we as physicians need to be essentially prescribing healthy diet and exercise. The combination of the two are synergistic,” Julie Gralow, MD, ASCO chief medical officer and executive vice president, told attendees.
Despite the benefits of these lifestyle changes, exercise and diet are meant to supplement, not replace, established colon cancer treatments.
It would be a false binary to frame this as lifestyle vs cancer treatment, explained Mark Lewis, MD, director of Gastrointestinal Oncology at Intermountain Healthcare in Salt Lake City, Utah. With exercise, for instance, “the key is giving enough chemo to protect against recurrence and eliminate micrometastases but not so much that we cause neuropathy and reduce function and ability to follow the CHALLENGE structured program,” Lewis said.
Exercise and Survival
Colon cancer remains the second-leading cause of cancer death worldwide. Even with surgery and chemotherapy, roughly 30% of patients with stage III and high-risk stage II colon cancer will experience disease recurrence.
“As oncologists, one of the most common questions we get asked by patients is — what else can I do to improve my outcome?” Booth said.
Observational studies published nearly two decades ago hinted that physically active cancer survivors fare better, but no randomized trial has definitively tested whether exercise could alter disease course. That knowledge gap prompted the Canadian Cancer Trials Group to launch the CHALLENGE trial.
Between 2009 and 2023, the phase 3 study enrolled 889 adults (median age, 61 years; 51% women) who had completed surgery and adjuvant chemotherapy for stage III (90%) or high-risk stage II (10%) colon cancer. Most patients were from Canada and Australia and were enrolled 2-6 months after completing chemotherapy.
Half of study participants were randomly allocated to a structured exercise program (n = 445) and half to receive standard health education materials promoting physical activity and healthy eating (control individuals, n = 444).
As part of the structured exercise intervention, patients met with a physical activity consultant twice a month for the first 6 months. These sessions included exercise coaching and supervised exercise. Patients could choose their preferred aerobic exercise and most picked brisk walking.
The consultants gave each patient an “exercise prescription” to hit a specific amount of exercise. The target was an additional 10 metabolic equivalent (MET)–hours of aerobic activity per week — about three to four brisk walks each lasting 45-60 minutes. After 6 months, patients met with their consultants once a month, with additional sessions available for extra support if needed.
Structured exercise led to “substantial and sustained” increases in the amount of exercise participants did, as well as physiologic measures of their fitness, with “highly relevant” improvements in VO2 max, 6-minute walk test, and patient-reported physical function, underscoring that participants were not only exercising more but also getting fitter, Booth said.
Exercise was associated with a clinically meaningful and statistically significant 28% reduction in the risk for recurrent or new cancer (hazard ratio [HR], 0.72; P = .017), with a 5-year disease free survival rate of 80% in the exercise group and 74% in the control group.
In other words, “for every 16 patients that went on the exercise program, exercise prevented 1 person from recurrent or new cancer” at 5 years, Booth reported.
Overall survival results were “even more impressive,” he said.
At 8 years, 90% of patients in the exercise program were alive vs 83% of those in the control group, which translated to a 37% lower risk for death (HR, 0.63; P = .022).
“For every 14 patients who went on the exercise program, exercise prevented 1 person from dying” at the 8-year mark, Booth noted.
“Notably, this difference in survival was not driven by difference in cardiovascular deaths but by a reduction in the risk of death from colon cancer,” he said.
Besides a slight uptick in musculoskeletal aches, no major safety signals emerged in the exercise group.
It’s important to note that the survival benefit associated with exercise came after patients had received surgery followed by chemotherapy — in other words, exercise did not replace established cancer treatments. It’s also unclear whether initiating an exercise intervention earlier in the treatment trajectory — before surgery or during chemotherapy, instead of after chemotherapy — could further improve cancer outcomes, the authors noted.
Still, “exercise as an intervention is a no brainer and should be implemented broadly,” said ASCO expert Pamela Kunz, MD, with Yale School of Medicine, New Haven, Connecticut.
Marco Gerlinger, MD, with Barts Cancer Institute, London, England, agreed.
“Oncologists can now make a very clear evidence-based recommendation for patients who just completed their chemotherapy for bowel cancer and are fit enough for such an exercise program,” Gerlinger said in a statement from the nonprofit UK Science Media Centre.
Booth noted that knowledge alone will not be sufficient to allow most patients to change their lifestyle and realize the health benefits.
“The policy implementation piece of this is really key, and we need health systems, hospitals, and payers to invest in these behavior support programs so that patients have access to a physical activity consultant and can realize the health benefits,” he said.
“This intervention is empowering and achievable for patients and with much, much lower cost than many of our therapies. It is also sustainable for health systems,” he concluded.
Diet and Survival
Diet can also affect outcomes in patients with colon cancer.
In the same session describing the CHALLENGE results, Sara Char, MD, with Dana-Farber Cancer Institute in Boston, reported findings showing that consuming a diet high in proinflammatory foods was associated with worse overall survival in patients with stage III colon cancer. A proinflammatory diet includes red and processed meats, sugary drinks, and refined grains, while an anti-inflammatory diet focuses on fruits, vegetables, whole grains, fish, and olive oil.
Chronic systemic inflammation has been implicated in both colon cancer development and in its progression, and elevated levels of inflammatory markers in the blood have previously been associated with worse survival outcomes in patients with stage III colon cancer.
Char and colleagues analyzed dietary patterns of a subset of 1625 patients (mean age, 61 years) with resected stage III colon cancer enrolled in the phase 3 CALGB/SWOG 80702 (Alliance) clinical trial, which compared 3 months of adjuvant chemotherapy with 6 months of adjuvant chemotherapy, with or without the anti-inflammatory medication celecoxib.
As part of the trial, participants reported their diet and exercise habits at various timepoints. Their diets were scored using the validated empirical dietary inflammatory pattern (EDIP) tool, which is a weighted sum of 18 food groups — nine proinflammatory and nine anti-inflammatory. A high EDIP score marks a proinflammatory diet, and a low EDIP score indicates a less inflammatory diet.
During median follow-up of nearly 4 years, researchers noted a trend toward worse disease-free survival in patients with high proinflammatory diets (HR, 1.46), but this association was not significant in the multivariable adjusted model (HR, 1.36; P = .22), Char reported.
However, higher intake of proinflammatory foods was associated with significantly worse overall survival.
Patients who consumed the most proinflammatory foods (top 20%) had an 87% higher risk for death compared with those who consumed the least (bottom 20%; HR, 1.87). The median overall survival in the highest quintile was 7.7 years and was not reached in the lowest quintile.
Combine Exercise and Diet for Best Results
To examine the joint effect of physical activity and diet on overall survival, patients were divided into higher and lower levels of physical activity using a cut-off of 9 MET hours per week, which roughly correlates to 30 minutes of vigorous walking five days a week with a little bit of light yoga, Char explained.
In this analysis, patients with less proinflammatory diets and higher physical activity levels had the best overall survival outcomes, with a 63% lower risk for death compared with peers who consumed more pro-inflammatory diets and exercised less (HR, 0.37; P < .0001).
Daily celecoxib use and low-dose aspirin use (< 100 mg/d) did not affect the association between inflammatory diet and survival.
Char cautioned, that while the EDIP tool is useful to measure the inflammatory potential of a diet, “this is not a dietary recommendation, and we need further studies to be able to tailor our findings into dietary recommendations that can be provided to patients at the bedside.”
Gralow said this “early but promising observational study suggests a powerful synergy: Patients with stage III colon cancer who embraced anti-inflammatory foods and exercised regularly showed the best overall survival compared to those with inflammatory diets and limited exercise.”
The CHALLENGE trial was funded by the Canadian Cancer Society, the National Health and Medical Research Council, Cancer Research UK, and the University of Sydney Cancer Research Fund. Booth had no disclosures. The diet study was funded by the National Institutes of Health, Pfizer, and the Project P Fund. Char disclosed an advisory/consultant role with Goodpath. Kunz, Gralow and Campbell had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Can exercise “therapy” and diet improve survival in patients with colon cancer? It appears so, according to two pivotal studies presented at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
In the CHALLENGE trial, a structured exercise program after surgery and adjuvant chemotherapy cut the risk for colon cancer recurrence in patients with stage III and high-risk stage II disease by more than one quarter and the risk for death by more than one third.
“The magnitude of benefit with exercise is substantial. In fact, it is comparable, and in some cases exceeds the magnitude of benefit of many of our very good standard medical therapies in oncology,” study presenter Christopher Booth, MD, with Queen’s University, Kingston, Ontario, Canada, told attendees.
Results of the study were published online in The New England Journal of Medicine to coincide with the presentation at the meeting.
The findings are “nothing short of a major milestone,” said study discussant Peter Campbell, PhD, with Montefiore Einstein Comprehensive Cancer Center, Bronx, New York.
The other study showed that eating a less inflammatory diet may reduce the risk for death in patients with colon cancer, with the greatest benefits seen in those who embraced anti-inflammatory foods and exercised regularly.
“Putting these two abstracts into perspective, we as physicians need to be essentially prescribing healthy diet and exercise. The combination of the two are synergistic,” Julie Gralow, MD, ASCO chief medical officer and executive vice president, told attendees.
Despite the benefits of these lifestyle changes, exercise and diet are meant to supplement, not replace, established colon cancer treatments.
It would be a false binary to frame this as lifestyle vs cancer treatment, explained Mark Lewis, MD, director of Gastrointestinal Oncology at Intermountain Healthcare in Salt Lake City, Utah. With exercise, for instance, “the key is giving enough chemo to protect against recurrence and eliminate micrometastases but not so much that we cause neuropathy and reduce function and ability to follow the CHALLENGE structured program,” Lewis said.
Exercise and Survival
Colon cancer remains the second-leading cause of cancer death worldwide. Even with surgery and chemotherapy, roughly 30% of patients with stage III and high-risk stage II colon cancer will experience disease recurrence.
“As oncologists, one of the most common questions we get asked by patients is — what else can I do to improve my outcome?” Booth said.
Observational studies published nearly two decades ago hinted that physically active cancer survivors fare better, but no randomized trial has definitively tested whether exercise could alter disease course. That knowledge gap prompted the Canadian Cancer Trials Group to launch the CHALLENGE trial.
Between 2009 and 2023, the phase 3 study enrolled 889 adults (median age, 61 years; 51% women) who had completed surgery and adjuvant chemotherapy for stage III (90%) or high-risk stage II (10%) colon cancer. Most patients were from Canada and Australia and were enrolled 2-6 months after completing chemotherapy.
Half of study participants were randomly allocated to a structured exercise program (n = 445) and half to receive standard health education materials promoting physical activity and healthy eating (control individuals, n = 444).
As part of the structured exercise intervention, patients met with a physical activity consultant twice a month for the first 6 months. These sessions included exercise coaching and supervised exercise. Patients could choose their preferred aerobic exercise and most picked brisk walking.
The consultants gave each patient an “exercise prescription” to hit a specific amount of exercise. The target was an additional 10 metabolic equivalent (MET)–hours of aerobic activity per week — about three to four brisk walks each lasting 45-60 minutes. After 6 months, patients met with their consultants once a month, with additional sessions available for extra support if needed.
Structured exercise led to “substantial and sustained” increases in the amount of exercise participants did, as well as physiologic measures of their fitness, with “highly relevant” improvements in VO2 max, 6-minute walk test, and patient-reported physical function, underscoring that participants were not only exercising more but also getting fitter, Booth said.
Exercise was associated with a clinically meaningful and statistically significant 28% reduction in the risk for recurrent or new cancer (hazard ratio [HR], 0.72; P = .017), with a 5-year disease free survival rate of 80% in the exercise group and 74% in the control group.
In other words, “for every 16 patients that went on the exercise program, exercise prevented 1 person from recurrent or new cancer” at 5 years, Booth reported.
Overall survival results were “even more impressive,” he said.
At 8 years, 90% of patients in the exercise program were alive vs 83% of those in the control group, which translated to a 37% lower risk for death (HR, 0.63; P = .022).
“For every 14 patients who went on the exercise program, exercise prevented 1 person from dying” at the 8-year mark, Booth noted.
“Notably, this difference in survival was not driven by difference in cardiovascular deaths but by a reduction in the risk of death from colon cancer,” he said.
Besides a slight uptick in musculoskeletal aches, no major safety signals emerged in the exercise group.
It’s important to note that the survival benefit associated with exercise came after patients had received surgery followed by chemotherapy — in other words, exercise did not replace established cancer treatments. It’s also unclear whether initiating an exercise intervention earlier in the treatment trajectory — before surgery or during chemotherapy, instead of after chemotherapy — could further improve cancer outcomes, the authors noted.
Still, “exercise as an intervention is a no brainer and should be implemented broadly,” said ASCO expert Pamela Kunz, MD, with Yale School of Medicine, New Haven, Connecticut.
Marco Gerlinger, MD, with Barts Cancer Institute, London, England, agreed.
“Oncologists can now make a very clear evidence-based recommendation for patients who just completed their chemotherapy for bowel cancer and are fit enough for such an exercise program,” Gerlinger said in a statement from the nonprofit UK Science Media Centre.
Booth noted that knowledge alone will not be sufficient to allow most patients to change their lifestyle and realize the health benefits.
“The policy implementation piece of this is really key, and we need health systems, hospitals, and payers to invest in these behavior support programs so that patients have access to a physical activity consultant and can realize the health benefits,” he said.
“This intervention is empowering and achievable for patients and with much, much lower cost than many of our therapies. It is also sustainable for health systems,” he concluded.
Diet and Survival
Diet can also affect outcomes in patients with colon cancer.
In the same session describing the CHALLENGE results, Sara Char, MD, with Dana-Farber Cancer Institute in Boston, reported findings showing that consuming a diet high in proinflammatory foods was associated with worse overall survival in patients with stage III colon cancer. A proinflammatory diet includes red and processed meats, sugary drinks, and refined grains, while an anti-inflammatory diet focuses on fruits, vegetables, whole grains, fish, and olive oil.
Chronic systemic inflammation has been implicated in both colon cancer development and in its progression, and elevated levels of inflammatory markers in the blood have previously been associated with worse survival outcomes in patients with stage III colon cancer.
Char and colleagues analyzed dietary patterns of a subset of 1625 patients (mean age, 61 years) with resected stage III colon cancer enrolled in the phase 3 CALGB/SWOG 80702 (Alliance) clinical trial, which compared 3 months of adjuvant chemotherapy with 6 months of adjuvant chemotherapy, with or without the anti-inflammatory medication celecoxib.
As part of the trial, participants reported their diet and exercise habits at various timepoints. Their diets were scored using the validated empirical dietary inflammatory pattern (EDIP) tool, which is a weighted sum of 18 food groups — nine proinflammatory and nine anti-inflammatory. A high EDIP score marks a proinflammatory diet, and a low EDIP score indicates a less inflammatory diet.
During median follow-up of nearly 4 years, researchers noted a trend toward worse disease-free survival in patients with high proinflammatory diets (HR, 1.46), but this association was not significant in the multivariable adjusted model (HR, 1.36; P = .22), Char reported.
However, higher intake of proinflammatory foods was associated with significantly worse overall survival.
Patients who consumed the most proinflammatory foods (top 20%) had an 87% higher risk for death compared with those who consumed the least (bottom 20%; HR, 1.87). The median overall survival in the highest quintile was 7.7 years and was not reached in the lowest quintile.
Combine Exercise and Diet for Best Results
To examine the joint effect of physical activity and diet on overall survival, patients were divided into higher and lower levels of physical activity using a cut-off of 9 MET hours per week, which roughly correlates to 30 minutes of vigorous walking five days a week with a little bit of light yoga, Char explained.
In this analysis, patients with less proinflammatory diets and higher physical activity levels had the best overall survival outcomes, with a 63% lower risk for death compared with peers who consumed more pro-inflammatory diets and exercised less (HR, 0.37; P < .0001).
Daily celecoxib use and low-dose aspirin use (< 100 mg/d) did not affect the association between inflammatory diet and survival.
Char cautioned, that while the EDIP tool is useful to measure the inflammatory potential of a diet, “this is not a dietary recommendation, and we need further studies to be able to tailor our findings into dietary recommendations that can be provided to patients at the bedside.”
Gralow said this “early but promising observational study suggests a powerful synergy: Patients with stage III colon cancer who embraced anti-inflammatory foods and exercised regularly showed the best overall survival compared to those with inflammatory diets and limited exercise.”
The CHALLENGE trial was funded by the Canadian Cancer Society, the National Health and Medical Research Council, Cancer Research UK, and the University of Sydney Cancer Research Fund. Booth had no disclosures. The diet study was funded by the National Institutes of Health, Pfizer, and the Project P Fund. Char disclosed an advisory/consultant role with Goodpath. Kunz, Gralow and Campbell had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Can Lifestyle Changes Save Lives in Colon Cancer?
Can Lifestyle Changes Save Lives in Colon Cancer?
Genomic Testing Reveals Distinct Mutation Patterns in Black and White Veterans With Metastatic Prostate Cancer
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Next-generation sequencing (NGS) analysis of 5015 veterans with metastatic prostate cancer reveals distinct genomic patterns between non-Hispanic Black and White patients, with Black veterans showing higher odds of immunotherapy targets but lower odds of androgen receptor axis alterations. However, the rates of survival were similar despite the differences.
METHODOLOGY:
Researchers conducted a retrospective cohort study comparing alteration frequencies between 1784 non-Hispanic Black (35.6%) and 3,231 non-Hispanic White (64.4%) veterans who underwent NGS testing from January 23, 2019, to November 2, 2023.
- Analysis included DNA sequencing data from tissue or plasma biospecimens, including prostate biopsy specimens, radical prostatectomy specimens, and prostate cancer metastases, all sequenced with FoundationOne CDx or FoundationOne Liquid CDx platforms.
- Investigators examined pathogenic alterations in individual genes, actionable targets, and canonical prostate cancer pathways, while adjusting for NGS analyte and clinicopathologic covariates.
- Researchers evaluated associations between alteration frequency and race as well as survival through Cox proportional hazards modeling, stratified by race and adjusted for clinical factors.
TAKEAWAY:
Non-Hispanic Black race and ethnicity was associated with higher odds of genomic alterations in SPOP (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2-2.6) and immunotherapy targets (OR, 1.7; 95% CI, 1.1-2.5), including high microsatellite instability status (OR, 3.1; 95% CI, 1.1-9.4).
- Non-Hispanic Black veterans showed lower odds of genomic alterations in the AKT/PI3K pathway (OR, 0.6; 95% CI, 0.4-0.7), androgen receptor axis (OR, 0.7; 95% CI, 0.5-0.9), and tumor suppressor genes (OR, 0.7; 95% CI, 0.5-0.8).
- Tumor suppressor alterations were associated with shorter overall survival in both non-Hispanic Black (hazard ratio [HR], 1.54; 95% CI, 1.13-2.11) and non-Hispanic White (HR, 1.52; 95% CI, 1.25-1.85) veterans.
- CDK12 alterations significantly increased the hazard of death in non-Hispanic Black veterans (HR, 2.04; 95% CI, 1.13-3.67), while immunotherapy targets were associated with increased mortality in non-Hispanic White veterans (HR, 1.44; 95% CI, 1.02-2.02).
IN PRACTICE: " we did not identify any genomic alterations or biomarkers that should not be tested in PCa based on patient self-identified race. Ultimately, this work emphasizes that precision oncology enables the individualization of treatment decisions without having to rely on imprecise characteristics such as self-identified race.," wrote the study authors.
SOURCE: Isla P. Garraway, MD, PhD; Kosj Yamoah, MD, PhD; and Kara N. Maxwell, MD, PhD were co-senior authors. The article was published online on May 12 in JAMA Network Open.
LIMITATIONS: According to the authors, a lack of matched germline data for patients, complicated the interpretation of plasma results. In addition, survivorship bias may have inadvertently excluded the most aggressive metastatic prostate cancer phenotypes, as patients who did not live long enough to undergo NGS testing were not included. Results seen in the veteran population served by the Veterans Health Administration may not be generalizable to the broader population.
DISCLOSURES: The study received support from Challenge Award PCF22CHALO2 from the Prostate Cancer Foundation and the Veterans Affairs National Precision Oncology Program. Luca F. Valle, MD, reported receiving grant support from the Bristol Myers Squibb Foundation during the conduct of the study. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Are Your Patients With COPD Inhaling Eucalyptus Oil? Know the Risks
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hurricanes, Fires, Floods: A Rising Threat to Cancer Care
As Hurricane Helene approached western North Carolina, Martin Palmeri, MD, MBA, didn’t anticipate the storm would disrupt practice operations for more than a day or so.
But the massive rainfall and flooding damage last September proved to be far more challenging. Despite best efforts by the 13-physician practice, basic treatments for most patients were interrupted for about a week.
Flooding washed out some of the major roads leading to the main Asheville clinic and affiliated rural sites, limiting travel and slowing delivery of medications, intravenous (IV) fluids, and other supplies, Palmeri said. Some patients and employees weren’t initially reachable due to the loss of the internet and cell phone service. The storm-related fallout even forced patients to relocate elsewhere for weeks or longer.
During the storm, backup generators kept power on at the Asheville clinic, protecting chemotherapy and other refrigerated drugs, but the storm damaged the municipal water supply.
“Water was the number one thing — how do you get water to the office?” Palmeri said. “You can’t give someone an 8-hour infusion if they don’t have means of going to the toilet or having something to drink.”
Hurricanes. Wildfires. Heat waves. As climate-driven extreme weather has become more common, researchers, oncologists, and patients are increasingly being forced to consider the consequences of these disruptions.
Along with preventing patients and providers from reaching treatment sites, experts said, extreme weather can undercut patients’ health and care in other ways. Patients with more limited lung capacity following lung cancer surgery, for instance, may struggle with breathing during wildfires. Extreme heat can prove risky for patients already dehydrated or weakened by treatment-related side effects. Power outages and severe flooding can affect vital infrastructure, disrupting operations at facilities that manufacture essential drugs. Power outages can also impede radiotherapy, which requires machines powered by electricity.
“Any of these [weather] events can disrupt this critical cancer care continuum among a population of people that already are very vulnerable,” said Joan Casey, PhD, an environmental epidemiologist and associate professor at the University of Washington in Seattle.
Extreme Weather and Cancer Survival
For patients with cancer, survival often relies on highly regimented protocols, which may require surgery plus frequent visits for radiation, chemotherapy, or immunotherapy that can last months, said Eric Bernicker, MD, a Colorado oncologist and lead author of a 2023 American Society of Clinical Oncology position statement about the impact of climate change on cancer care.
Interruptions to care, regardless of the cause, can lead to worse outcomes for patients, Bernicker said. “If you’re in the middle of your post-lumpectomy radiation and your radiation center shuts for 2 weeks,” he said, “that is not good.”
Research indicated that even short treatment disruptions can affect outcomes for patients with cancer and that delays caused by extreme weather — which may last for weeks — can affect survival for these patients.
One analysis, published in JAMA Oncology in 2023, found that patients exposed to wildfire within the first year after potentially curative lung cancer surgery had worse survival outcomes than those who weren’t exposed during their recovery.
In another study, patients with lung cancer who had their radiation interrupted when a hurricane struck had a 19% greater risk of dying overall compared with similar patients who were not affected. Another analysis found that patients with breast cancer who were partway through treatment when Hurricane Katrina hit the Louisiana coastline had a significantly greater risk of dying over a 10-year period compared with patients who lived elsewhere.
The potential threats to survival highlighted the impacts of extreme weather on carefully orchestrated systems of care that place patients facing already fragile situations in impossible binds, Casey said.
Douglas Flora, MD, a Kentucky oncologist and president-elect of the Association of Cancer Care Centers, Rockville, Maryland, agreed.
“We’ve seen this with an increasing frequency over the last several years,” Flora said. “It’s one thing if it’s routine follow-up or surveillance care, but many cancer patients’ survivals are directly related to not having interruptions in their care.”
Challenging Realities
Following Helene, the most pressing issue was the lack of water, Palmeri said.
The lack of reliable clean water created challenges for patients receiving radiation or chemotherapy infusions, which can cause vomiting and diarrhea that leave patients dehydrated. Toilets were also unusable.
Even when the city of Asheville said the water was likely safe enough to bathe in, local leaders still reported potential risks from bacteria and other contaminants in the water, Palmeri said. Those with a fragile immune system or breaks in the skin “could get serious and life-threatening infections,” he explained.
To make matters worse, damage to a North Carolina facility manufacturing IV fluids left the United States in shortage for months. IV fluids are key not only for providing hydration but also for easing nausea, fatigue, and other issues caused by cancer therapies.
With wildfires, as occurred in southern California early this year, patients undergoing cancer treatment might feel they have no option but to remain near home to continue getting care, Casey said. “It’s restricting their agency in the kinds of choices that they have to make during these severe weather events.”
Meanwhile, thick wildfire smoke can confine patients to their homes, said Lawrence Wagman, MD, a surgical oncologist and a regional medical director at the City of Hope network, who described its main facility in Duarte, California, coming within a dozen miles of the Eaton fire. “One of the biggest problems was so much smoke in the air,” he said. “And the air quality was so low that it was, in many ways, dangerous for patients to travel.”
“These fires were so aggressive, and they kept popping up,” Wagman said. Plus, the emotional strain of looming wildfires persisted for both patients and cancer clinicians for weeks on end, he added.
For those who evacuate, the logistics can be complex.
Not only are cancer treatment plans highly structured, but switching care to another facility is far from easy, Bernicker said. The new facility will likely need to submit a treatment plan and get insurance coverage before moving forward.
“I’m not saying that takes forever,” he said. “But what I’m saying is that it’s not like you just roll in and they hang the [infusion] bag.”
Neither is a shelter typically an option for patients during treatment, said Seth Berkowitz, a licensed clinical social worker and director of Strategic Healthcare Partnerships at The Leukemia & Lymphoma Society. “They have to have a place to go that’s safe and germ-free.”
In western North Carolina, the strain on already ill patients and their caregivers could be overwhelming, Palmeri said. He recounted how the husband of one patient with advanced cancer died after the storm came through.
“He tried to go out there with a chainsaw to clear a way out so that they could get out of their house in case he needed to take her to the hospital,” Palmeri said. “And he had a heart attack there in the driveway.”
Rebuilding and Planning Ahead
Experts are only at the early stages of grasping the magnitude of extreme weather on cancer care and developing strategies to curtail care gaps and potential harm to patients, said Katie Lichter, MD, a radiation oncologist at the University of California San Francisco, who studies extreme weather and cancer treatment.
“How does it impact health care delivery services at every step, from prevention to screening to treatment and survivorship?” Lichter asked. “We’re just starting to understand and to even quantify that,” she said, which included identifying patients who are most vulnerable. She worries, in particular, about patients living in rural areas who already travel longer distances and often face more difficulties accessing care.
The gap between research and reality still looms large. A recent analysis, led by Lichter, looked at 176 California radiation oncology clinics and found that all of them were located within 25 miles of a wildfire that had occurred within the prior 5 years. Yet among the 51 clinics that responded to a 2022 survey,just 47% reported that their clinic had a wildfire emergency preparedness plan.
The American Cancer Society does provide some guidance on how patients can prepare for a weather-related crisis, including having extra supplies of medications or special equipment on hand.
Still, providers are often in reaction mode when extreme weather strikes.
Without adequate clean water after Helene, leaders at Palmeri’s practice moved swiftly, purchasing 40,000-50,000 bottles of water and bringing in porta potties from elsewhere.
“I think we were able to get things up and going very quickly,” said Palmeri, who noted that full services resumed about 10 days after the storm. “For most patients, missing a week of treatment would not do a disservice to their well-being or outcome.”
Going forward, to provide a more comprehensive strategy, Lichter is working with colleagues to develop clinical tool kits to help oncology practices and patients prepare for severe weather events, such as outlining backup treatment contingency plans, ensuring early medication refills, and boosting communication with patient alert systems.
Clinicians are also implementing their own strategies. To limit communication gaps during power outages, Palmeri said that, since Helene, his practice has made sure that their clinic sites, physicians, and other key people now have cell phone service through satellite via Starlink.
“No one has phone books anymore,” he said, so cancer clinicians should keep crucial contact information on paper, such as details about businesses that distribute water and porta potties, given that online searches may not be feasible.
Clinicians should also advise patients to keep a hard copy of recent medical findings handy, including medications and lab results, in case they arrive at an emergency room far from home and physicians can’t access their electronic health record, Bernicker said.
When there is enough advance warning of an approaching weather event, clinicians can help patients keep at least a week’s worth of medication on hand for symptom-related issues, such as nausea or pain, as well as antibiotics so patients don’t have to seek out emergency care during the crisis, Bernicker said. However, Bernicker noted, some insurers may be reluctant to fill certain prescriptions in advance, like those for opioids.
Making headway on more robust preparedness strategies may be slowed. As of March, the National Institutes of Health will no longer fund research about the health effects of climate change.
Bernicker hoped that such cutbacks would be rolled back. What’s on the line, he stressed, is maintaining the highest quality of care for patients with cancer.
“We really are in a golden age of oncology therapeutics,” he said. “We have patients living longer than anyone would have predicted 20 or 25 years ago. But all those advances are contingent on people having access to their centers and not having that interrupted.”
A version of this article first appeared on Medscape.com.
As Hurricane Helene approached western North Carolina, Martin Palmeri, MD, MBA, didn’t anticipate the storm would disrupt practice operations for more than a day or so.
But the massive rainfall and flooding damage last September proved to be far more challenging. Despite best efforts by the 13-physician practice, basic treatments for most patients were interrupted for about a week.
Flooding washed out some of the major roads leading to the main Asheville clinic and affiliated rural sites, limiting travel and slowing delivery of medications, intravenous (IV) fluids, and other supplies, Palmeri said. Some patients and employees weren’t initially reachable due to the loss of the internet and cell phone service. The storm-related fallout even forced patients to relocate elsewhere for weeks or longer.
During the storm, backup generators kept power on at the Asheville clinic, protecting chemotherapy and other refrigerated drugs, but the storm damaged the municipal water supply.
“Water was the number one thing — how do you get water to the office?” Palmeri said. “You can’t give someone an 8-hour infusion if they don’t have means of going to the toilet or having something to drink.”
Hurricanes. Wildfires. Heat waves. As climate-driven extreme weather has become more common, researchers, oncologists, and patients are increasingly being forced to consider the consequences of these disruptions.
Along with preventing patients and providers from reaching treatment sites, experts said, extreme weather can undercut patients’ health and care in other ways. Patients with more limited lung capacity following lung cancer surgery, for instance, may struggle with breathing during wildfires. Extreme heat can prove risky for patients already dehydrated or weakened by treatment-related side effects. Power outages and severe flooding can affect vital infrastructure, disrupting operations at facilities that manufacture essential drugs. Power outages can also impede radiotherapy, which requires machines powered by electricity.
“Any of these [weather] events can disrupt this critical cancer care continuum among a population of people that already are very vulnerable,” said Joan Casey, PhD, an environmental epidemiologist and associate professor at the University of Washington in Seattle.
Extreme Weather and Cancer Survival
For patients with cancer, survival often relies on highly regimented protocols, which may require surgery plus frequent visits for radiation, chemotherapy, or immunotherapy that can last months, said Eric Bernicker, MD, a Colorado oncologist and lead author of a 2023 American Society of Clinical Oncology position statement about the impact of climate change on cancer care.
Interruptions to care, regardless of the cause, can lead to worse outcomes for patients, Bernicker said. “If you’re in the middle of your post-lumpectomy radiation and your radiation center shuts for 2 weeks,” he said, “that is not good.”
Research indicated that even short treatment disruptions can affect outcomes for patients with cancer and that delays caused by extreme weather — which may last for weeks — can affect survival for these patients.
One analysis, published in JAMA Oncology in 2023, found that patients exposed to wildfire within the first year after potentially curative lung cancer surgery had worse survival outcomes than those who weren’t exposed during their recovery.
In another study, patients with lung cancer who had their radiation interrupted when a hurricane struck had a 19% greater risk of dying overall compared with similar patients who were not affected. Another analysis found that patients with breast cancer who were partway through treatment when Hurricane Katrina hit the Louisiana coastline had a significantly greater risk of dying over a 10-year period compared with patients who lived elsewhere.
The potential threats to survival highlighted the impacts of extreme weather on carefully orchestrated systems of care that place patients facing already fragile situations in impossible binds, Casey said.
Douglas Flora, MD, a Kentucky oncologist and president-elect of the Association of Cancer Care Centers, Rockville, Maryland, agreed.
“We’ve seen this with an increasing frequency over the last several years,” Flora said. “It’s one thing if it’s routine follow-up or surveillance care, but many cancer patients’ survivals are directly related to not having interruptions in their care.”
Challenging Realities
Following Helene, the most pressing issue was the lack of water, Palmeri said.
The lack of reliable clean water created challenges for patients receiving radiation or chemotherapy infusions, which can cause vomiting and diarrhea that leave patients dehydrated. Toilets were also unusable.
Even when the city of Asheville said the water was likely safe enough to bathe in, local leaders still reported potential risks from bacteria and other contaminants in the water, Palmeri said. Those with a fragile immune system or breaks in the skin “could get serious and life-threatening infections,” he explained.
To make matters worse, damage to a North Carolina facility manufacturing IV fluids left the United States in shortage for months. IV fluids are key not only for providing hydration but also for easing nausea, fatigue, and other issues caused by cancer therapies.
With wildfires, as occurred in southern California early this year, patients undergoing cancer treatment might feel they have no option but to remain near home to continue getting care, Casey said. “It’s restricting their agency in the kinds of choices that they have to make during these severe weather events.”
Meanwhile, thick wildfire smoke can confine patients to their homes, said Lawrence Wagman, MD, a surgical oncologist and a regional medical director at the City of Hope network, who described its main facility in Duarte, California, coming within a dozen miles of the Eaton fire. “One of the biggest problems was so much smoke in the air,” he said. “And the air quality was so low that it was, in many ways, dangerous for patients to travel.”
“These fires were so aggressive, and they kept popping up,” Wagman said. Plus, the emotional strain of looming wildfires persisted for both patients and cancer clinicians for weeks on end, he added.
For those who evacuate, the logistics can be complex.
Not only are cancer treatment plans highly structured, but switching care to another facility is far from easy, Bernicker said. The new facility will likely need to submit a treatment plan and get insurance coverage before moving forward.
“I’m not saying that takes forever,” he said. “But what I’m saying is that it’s not like you just roll in and they hang the [infusion] bag.”
Neither is a shelter typically an option for patients during treatment, said Seth Berkowitz, a licensed clinical social worker and director of Strategic Healthcare Partnerships at The Leukemia & Lymphoma Society. “They have to have a place to go that’s safe and germ-free.”
In western North Carolina, the strain on already ill patients and their caregivers could be overwhelming, Palmeri said. He recounted how the husband of one patient with advanced cancer died after the storm came through.
“He tried to go out there with a chainsaw to clear a way out so that they could get out of their house in case he needed to take her to the hospital,” Palmeri said. “And he had a heart attack there in the driveway.”
Rebuilding and Planning Ahead
Experts are only at the early stages of grasping the magnitude of extreme weather on cancer care and developing strategies to curtail care gaps and potential harm to patients, said Katie Lichter, MD, a radiation oncologist at the University of California San Francisco, who studies extreme weather and cancer treatment.
“How does it impact health care delivery services at every step, from prevention to screening to treatment and survivorship?” Lichter asked. “We’re just starting to understand and to even quantify that,” she said, which included identifying patients who are most vulnerable. She worries, in particular, about patients living in rural areas who already travel longer distances and often face more difficulties accessing care.
The gap between research and reality still looms large. A recent analysis, led by Lichter, looked at 176 California radiation oncology clinics and found that all of them were located within 25 miles of a wildfire that had occurred within the prior 5 years. Yet among the 51 clinics that responded to a 2022 survey,just 47% reported that their clinic had a wildfire emergency preparedness plan.
The American Cancer Society does provide some guidance on how patients can prepare for a weather-related crisis, including having extra supplies of medications or special equipment on hand.
Still, providers are often in reaction mode when extreme weather strikes.
Without adequate clean water after Helene, leaders at Palmeri’s practice moved swiftly, purchasing 40,000-50,000 bottles of water and bringing in porta potties from elsewhere.
“I think we were able to get things up and going very quickly,” said Palmeri, who noted that full services resumed about 10 days after the storm. “For most patients, missing a week of treatment would not do a disservice to their well-being or outcome.”
Going forward, to provide a more comprehensive strategy, Lichter is working with colleagues to develop clinical tool kits to help oncology practices and patients prepare for severe weather events, such as outlining backup treatment contingency plans, ensuring early medication refills, and boosting communication with patient alert systems.
Clinicians are also implementing their own strategies. To limit communication gaps during power outages, Palmeri said that, since Helene, his practice has made sure that their clinic sites, physicians, and other key people now have cell phone service through satellite via Starlink.
“No one has phone books anymore,” he said, so cancer clinicians should keep crucial contact information on paper, such as details about businesses that distribute water and porta potties, given that online searches may not be feasible.
Clinicians should also advise patients to keep a hard copy of recent medical findings handy, including medications and lab results, in case they arrive at an emergency room far from home and physicians can’t access their electronic health record, Bernicker said.
When there is enough advance warning of an approaching weather event, clinicians can help patients keep at least a week’s worth of medication on hand for symptom-related issues, such as nausea or pain, as well as antibiotics so patients don’t have to seek out emergency care during the crisis, Bernicker said. However, Bernicker noted, some insurers may be reluctant to fill certain prescriptions in advance, like those for opioids.
Making headway on more robust preparedness strategies may be slowed. As of March, the National Institutes of Health will no longer fund research about the health effects of climate change.
Bernicker hoped that such cutbacks would be rolled back. What’s on the line, he stressed, is maintaining the highest quality of care for patients with cancer.
“We really are in a golden age of oncology therapeutics,” he said. “We have patients living longer than anyone would have predicted 20 or 25 years ago. But all those advances are contingent on people having access to their centers and not having that interrupted.”
A version of this article first appeared on Medscape.com.
As Hurricane Helene approached western North Carolina, Martin Palmeri, MD, MBA, didn’t anticipate the storm would disrupt practice operations for more than a day or so.
But the massive rainfall and flooding damage last September proved to be far more challenging. Despite best efforts by the 13-physician practice, basic treatments for most patients were interrupted for about a week.
Flooding washed out some of the major roads leading to the main Asheville clinic and affiliated rural sites, limiting travel and slowing delivery of medications, intravenous (IV) fluids, and other supplies, Palmeri said. Some patients and employees weren’t initially reachable due to the loss of the internet and cell phone service. The storm-related fallout even forced patients to relocate elsewhere for weeks or longer.
During the storm, backup generators kept power on at the Asheville clinic, protecting chemotherapy and other refrigerated drugs, but the storm damaged the municipal water supply.
“Water was the number one thing — how do you get water to the office?” Palmeri said. “You can’t give someone an 8-hour infusion if they don’t have means of going to the toilet or having something to drink.”
Hurricanes. Wildfires. Heat waves. As climate-driven extreme weather has become more common, researchers, oncologists, and patients are increasingly being forced to consider the consequences of these disruptions.
Along with preventing patients and providers from reaching treatment sites, experts said, extreme weather can undercut patients’ health and care in other ways. Patients with more limited lung capacity following lung cancer surgery, for instance, may struggle with breathing during wildfires. Extreme heat can prove risky for patients already dehydrated or weakened by treatment-related side effects. Power outages and severe flooding can affect vital infrastructure, disrupting operations at facilities that manufacture essential drugs. Power outages can also impede radiotherapy, which requires machines powered by electricity.
“Any of these [weather] events can disrupt this critical cancer care continuum among a population of people that already are very vulnerable,” said Joan Casey, PhD, an environmental epidemiologist and associate professor at the University of Washington in Seattle.
Extreme Weather and Cancer Survival
For patients with cancer, survival often relies on highly regimented protocols, which may require surgery plus frequent visits for radiation, chemotherapy, or immunotherapy that can last months, said Eric Bernicker, MD, a Colorado oncologist and lead author of a 2023 American Society of Clinical Oncology position statement about the impact of climate change on cancer care.
Interruptions to care, regardless of the cause, can lead to worse outcomes for patients, Bernicker said. “If you’re in the middle of your post-lumpectomy radiation and your radiation center shuts for 2 weeks,” he said, “that is not good.”
Research indicated that even short treatment disruptions can affect outcomes for patients with cancer and that delays caused by extreme weather — which may last for weeks — can affect survival for these patients.
One analysis, published in JAMA Oncology in 2023, found that patients exposed to wildfire within the first year after potentially curative lung cancer surgery had worse survival outcomes than those who weren’t exposed during their recovery.
In another study, patients with lung cancer who had their radiation interrupted when a hurricane struck had a 19% greater risk of dying overall compared with similar patients who were not affected. Another analysis found that patients with breast cancer who were partway through treatment when Hurricane Katrina hit the Louisiana coastline had a significantly greater risk of dying over a 10-year period compared with patients who lived elsewhere.
The potential threats to survival highlighted the impacts of extreme weather on carefully orchestrated systems of care that place patients facing already fragile situations in impossible binds, Casey said.
Douglas Flora, MD, a Kentucky oncologist and president-elect of the Association of Cancer Care Centers, Rockville, Maryland, agreed.
“We’ve seen this with an increasing frequency over the last several years,” Flora said. “It’s one thing if it’s routine follow-up or surveillance care, but many cancer patients’ survivals are directly related to not having interruptions in their care.”
Challenging Realities
Following Helene, the most pressing issue was the lack of water, Palmeri said.
The lack of reliable clean water created challenges for patients receiving radiation or chemotherapy infusions, which can cause vomiting and diarrhea that leave patients dehydrated. Toilets were also unusable.
Even when the city of Asheville said the water was likely safe enough to bathe in, local leaders still reported potential risks from bacteria and other contaminants in the water, Palmeri said. Those with a fragile immune system or breaks in the skin “could get serious and life-threatening infections,” he explained.
To make matters worse, damage to a North Carolina facility manufacturing IV fluids left the United States in shortage for months. IV fluids are key not only for providing hydration but also for easing nausea, fatigue, and other issues caused by cancer therapies.
With wildfires, as occurred in southern California early this year, patients undergoing cancer treatment might feel they have no option but to remain near home to continue getting care, Casey said. “It’s restricting their agency in the kinds of choices that they have to make during these severe weather events.”
Meanwhile, thick wildfire smoke can confine patients to their homes, said Lawrence Wagman, MD, a surgical oncologist and a regional medical director at the City of Hope network, who described its main facility in Duarte, California, coming within a dozen miles of the Eaton fire. “One of the biggest problems was so much smoke in the air,” he said. “And the air quality was so low that it was, in many ways, dangerous for patients to travel.”
“These fires were so aggressive, and they kept popping up,” Wagman said. Plus, the emotional strain of looming wildfires persisted for both patients and cancer clinicians for weeks on end, he added.
For those who evacuate, the logistics can be complex.
Not only are cancer treatment plans highly structured, but switching care to another facility is far from easy, Bernicker said. The new facility will likely need to submit a treatment plan and get insurance coverage before moving forward.
“I’m not saying that takes forever,” he said. “But what I’m saying is that it’s not like you just roll in and they hang the [infusion] bag.”
Neither is a shelter typically an option for patients during treatment, said Seth Berkowitz, a licensed clinical social worker and director of Strategic Healthcare Partnerships at The Leukemia & Lymphoma Society. “They have to have a place to go that’s safe and germ-free.”
In western North Carolina, the strain on already ill patients and their caregivers could be overwhelming, Palmeri said. He recounted how the husband of one patient with advanced cancer died after the storm came through.
“He tried to go out there with a chainsaw to clear a way out so that they could get out of their house in case he needed to take her to the hospital,” Palmeri said. “And he had a heart attack there in the driveway.”
Rebuilding and Planning Ahead
Experts are only at the early stages of grasping the magnitude of extreme weather on cancer care and developing strategies to curtail care gaps and potential harm to patients, said Katie Lichter, MD, a radiation oncologist at the University of California San Francisco, who studies extreme weather and cancer treatment.
“How does it impact health care delivery services at every step, from prevention to screening to treatment and survivorship?” Lichter asked. “We’re just starting to understand and to even quantify that,” she said, which included identifying patients who are most vulnerable. She worries, in particular, about patients living in rural areas who already travel longer distances and often face more difficulties accessing care.
The gap between research and reality still looms large. A recent analysis, led by Lichter, looked at 176 California radiation oncology clinics and found that all of them were located within 25 miles of a wildfire that had occurred within the prior 5 years. Yet among the 51 clinics that responded to a 2022 survey,just 47% reported that their clinic had a wildfire emergency preparedness plan.
The American Cancer Society does provide some guidance on how patients can prepare for a weather-related crisis, including having extra supplies of medications or special equipment on hand.
Still, providers are often in reaction mode when extreme weather strikes.
Without adequate clean water after Helene, leaders at Palmeri’s practice moved swiftly, purchasing 40,000-50,000 bottles of water and bringing in porta potties from elsewhere.
“I think we were able to get things up and going very quickly,” said Palmeri, who noted that full services resumed about 10 days after the storm. “For most patients, missing a week of treatment would not do a disservice to their well-being or outcome.”
Going forward, to provide a more comprehensive strategy, Lichter is working with colleagues to develop clinical tool kits to help oncology practices and patients prepare for severe weather events, such as outlining backup treatment contingency plans, ensuring early medication refills, and boosting communication with patient alert systems.
Clinicians are also implementing their own strategies. To limit communication gaps during power outages, Palmeri said that, since Helene, his practice has made sure that their clinic sites, physicians, and other key people now have cell phone service through satellite via Starlink.
“No one has phone books anymore,” he said, so cancer clinicians should keep crucial contact information on paper, such as details about businesses that distribute water and porta potties, given that online searches may not be feasible.
Clinicians should also advise patients to keep a hard copy of recent medical findings handy, including medications and lab results, in case they arrive at an emergency room far from home and physicians can’t access their electronic health record, Bernicker said.
When there is enough advance warning of an approaching weather event, clinicians can help patients keep at least a week’s worth of medication on hand for symptom-related issues, such as nausea or pain, as well as antibiotics so patients don’t have to seek out emergency care during the crisis, Bernicker said. However, Bernicker noted, some insurers may be reluctant to fill certain prescriptions in advance, like those for opioids.
Making headway on more robust preparedness strategies may be slowed. As of March, the National Institutes of Health will no longer fund research about the health effects of climate change.
Bernicker hoped that such cutbacks would be rolled back. What’s on the line, he stressed, is maintaining the highest quality of care for patients with cancer.
“We really are in a golden age of oncology therapeutics,” he said. “We have patients living longer than anyone would have predicted 20 or 25 years ago. But all those advances are contingent on people having access to their centers and not having that interrupted.”
A version of this article first appeared on Medscape.com.
Many Early-Onset Cancers Increasing, Particularly in Women
Rates of certain cancers in the United States — including breast, colorectal, and thyroid cancers — increased between 2010 and 2019 among patients aged less than 50 years, while overall cancer incidence and mortality rates did not increase, a new study found.
Among the more than two million cases of early-onset cancer diagnosed during this period, 63.2% were in women, researchers reported recently in Cancer Discovery.
Breast cancer, thyroid cancer, and melanoma were the most common early-onset cancers in women. Among men, the most common were colorectal cancer, testicular cancer, and melanoma.
Researchers from the National Cancer Institute analyzed cancer incidence data from the United States Cancer Statistics database for 2010-2019 and national death certificate data from the National Center for Health Statistics from 2010 to 2022. The team excluded incidence data from 2020 and 2021, which was artificially low due to COVID.
The researchers divided the data according to age groups: The early-onset age groups were 15-29, 30-39, and 40-49 years, and the late-onset groups were 50-59, 60-69, and 70-79 years. The team also estimated the expected number of early-onset cases in 2019 by multiplying 2010 age-specific cancer incidence rates by population counts for 2019.
First author Meredith Shiels, of the National Cancer Institute, and colleagues found that the largest absolute increase in incidence of early-onset cancers, compared with expected incidence, were for breast (n = 4834 additional cancers), colorectal (n = 2099), kidney (n = 1793), and uterine cancers (n = 1209). These diagnoses accounted for 80% of the additional cancer diagnoses in 2019 vs 2010.
Looking at increases by age group, Shiels and colleagues reported that 1.9% of all cancers occurred in overall early-onset cohort 15- to 49-year-olds (age-standardized incidence rate of 39.8 per 100,000), and the incidence was greater in the older cohorts: 3.6% for 30- to 39-year-olds (123.5 per 100,000) and 8.8% for 40- to 49-year-olds (293.9 per 100,000).
Overall, 14 of 33 cancer types significantly increased in incidence in at least one early-onset age group. Among these 14 cancer types, five — melanoma, plasma cell neoplasms, cervical cancer, stomach cancer, and cancer of the bones and joints — showed increases only in early-onset age groups, not in late-onset age groups. For example, between 2010 and 2019, cervical cancer rates increased by 1.39% per year among 30- to 39-year-olds, melanoma rates increased by 0.82% per year among 40- to 49-year-olds, and stomach cancer rates increased by 1.38% per year.
The remaining nine cancer types increased in at least one early-onset and one late-onset group. These included female breast, colorectal, kidney, testicular, uterine, pancreatic cancers as well as precursor B-cell non-Hodgkin lymphoma, diffuse large B-cell lymphoma, and mycosis fungoides/Sézary syndrome.
For four of the 14 cancer types with increasing incidence rates — testicular cancer, uterine cancer, colorectal cancer, and cancer of the bones and joints — mortality also increased in at least one early-onset age group, whereas the remaining 10 cancer types increased in incidence without an increase in mortality for any age group.
Shiels and her colleagues aren’t the first to address the rising incidence of early-onset cancers. In a keynote lecture at the European Society of Medical Oncology (ESMO) 2024 Annual Meeting, Irit Ben-Aharon, MD, PhD, from the Rambam Health Care Campus in Haifa, Israel, noted that from 1990-2019, the global incidence of early-onset cancer increased by 79%.
Although the current study doesn’t identify drivers of rising cancer rates in younger patients, “descriptive data like these provide a critical starting point for understanding the drivers of rising rates of cancer in early-onset age groups and could translate to effective cancer prevention and early detection efforts,” Shiels said in a press release. For instance, “recent guidelines have lowered the age of initiation for breast and colorectal cancer screening based, at least partially, on observations that rates for these cancers are increasing at younger ages.”
This study is “a great step forward” toward understanding the increasing incidence of early-onset cancers, agreed Shuji Ogino, MD, PhD, from Harvard Medical School and Brigham and Women’s Hospital in Boston, who wasn’t involved in the research.
The investigators provide new details, particularly by breaking down the early- and late-onset age groups into subcategories and by comparing incidence and mortality rates, Ogino noted.
“Mortality is a great endpoint because if the increased in early incidence is just an effect of [increased] screening we won’t see a mortality increase,” Ogino said. But “we need more data and some way to tease out the screening effect.” Plus, he added, “we need more mechanistic studies and tissue-based analyses to determine if early-onset cancers that are increasing in incidence are a different beast, rather than just an earlier beast.”
This study was funded by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health and the Institute of Cancer Research. Shiels declared no conflicts of interest.
version of this article first appeared on Medscape.com.
Rates of certain cancers in the United States — including breast, colorectal, and thyroid cancers — increased between 2010 and 2019 among patients aged less than 50 years, while overall cancer incidence and mortality rates did not increase, a new study found.
Among the more than two million cases of early-onset cancer diagnosed during this period, 63.2% were in women, researchers reported recently in Cancer Discovery.
Breast cancer, thyroid cancer, and melanoma were the most common early-onset cancers in women. Among men, the most common were colorectal cancer, testicular cancer, and melanoma.
Researchers from the National Cancer Institute analyzed cancer incidence data from the United States Cancer Statistics database for 2010-2019 and national death certificate data from the National Center for Health Statistics from 2010 to 2022. The team excluded incidence data from 2020 and 2021, which was artificially low due to COVID.
The researchers divided the data according to age groups: The early-onset age groups were 15-29, 30-39, and 40-49 years, and the late-onset groups were 50-59, 60-69, and 70-79 years. The team also estimated the expected number of early-onset cases in 2019 by multiplying 2010 age-specific cancer incidence rates by population counts for 2019.
First author Meredith Shiels, of the National Cancer Institute, and colleagues found that the largest absolute increase in incidence of early-onset cancers, compared with expected incidence, were for breast (n = 4834 additional cancers), colorectal (n = 2099), kidney (n = 1793), and uterine cancers (n = 1209). These diagnoses accounted for 80% of the additional cancer diagnoses in 2019 vs 2010.
Looking at increases by age group, Shiels and colleagues reported that 1.9% of all cancers occurred in overall early-onset cohort 15- to 49-year-olds (age-standardized incidence rate of 39.8 per 100,000), and the incidence was greater in the older cohorts: 3.6% for 30- to 39-year-olds (123.5 per 100,000) and 8.8% for 40- to 49-year-olds (293.9 per 100,000).
Overall, 14 of 33 cancer types significantly increased in incidence in at least one early-onset age group. Among these 14 cancer types, five — melanoma, plasma cell neoplasms, cervical cancer, stomach cancer, and cancer of the bones and joints — showed increases only in early-onset age groups, not in late-onset age groups. For example, between 2010 and 2019, cervical cancer rates increased by 1.39% per year among 30- to 39-year-olds, melanoma rates increased by 0.82% per year among 40- to 49-year-olds, and stomach cancer rates increased by 1.38% per year.
The remaining nine cancer types increased in at least one early-onset and one late-onset group. These included female breast, colorectal, kidney, testicular, uterine, pancreatic cancers as well as precursor B-cell non-Hodgkin lymphoma, diffuse large B-cell lymphoma, and mycosis fungoides/Sézary syndrome.
For four of the 14 cancer types with increasing incidence rates — testicular cancer, uterine cancer, colorectal cancer, and cancer of the bones and joints — mortality also increased in at least one early-onset age group, whereas the remaining 10 cancer types increased in incidence without an increase in mortality for any age group.
Shiels and her colleagues aren’t the first to address the rising incidence of early-onset cancers. In a keynote lecture at the European Society of Medical Oncology (ESMO) 2024 Annual Meeting, Irit Ben-Aharon, MD, PhD, from the Rambam Health Care Campus in Haifa, Israel, noted that from 1990-2019, the global incidence of early-onset cancer increased by 79%.
Although the current study doesn’t identify drivers of rising cancer rates in younger patients, “descriptive data like these provide a critical starting point for understanding the drivers of rising rates of cancer in early-onset age groups and could translate to effective cancer prevention and early detection efforts,” Shiels said in a press release. For instance, “recent guidelines have lowered the age of initiation for breast and colorectal cancer screening based, at least partially, on observations that rates for these cancers are increasing at younger ages.”
This study is “a great step forward” toward understanding the increasing incidence of early-onset cancers, agreed Shuji Ogino, MD, PhD, from Harvard Medical School and Brigham and Women’s Hospital in Boston, who wasn’t involved in the research.
The investigators provide new details, particularly by breaking down the early- and late-onset age groups into subcategories and by comparing incidence and mortality rates, Ogino noted.
“Mortality is a great endpoint because if the increased in early incidence is just an effect of [increased] screening we won’t see a mortality increase,” Ogino said. But “we need more data and some way to tease out the screening effect.” Plus, he added, “we need more mechanistic studies and tissue-based analyses to determine if early-onset cancers that are increasing in incidence are a different beast, rather than just an earlier beast.”
This study was funded by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health and the Institute of Cancer Research. Shiels declared no conflicts of interest.
version of this article first appeared on Medscape.com.
Rates of certain cancers in the United States — including breast, colorectal, and thyroid cancers — increased between 2010 and 2019 among patients aged less than 50 years, while overall cancer incidence and mortality rates did not increase, a new study found.
Among the more than two million cases of early-onset cancer diagnosed during this period, 63.2% were in women, researchers reported recently in Cancer Discovery.
Breast cancer, thyroid cancer, and melanoma were the most common early-onset cancers in women. Among men, the most common were colorectal cancer, testicular cancer, and melanoma.
Researchers from the National Cancer Institute analyzed cancer incidence data from the United States Cancer Statistics database for 2010-2019 and national death certificate data from the National Center for Health Statistics from 2010 to 2022. The team excluded incidence data from 2020 and 2021, which was artificially low due to COVID.
The researchers divided the data according to age groups: The early-onset age groups were 15-29, 30-39, and 40-49 years, and the late-onset groups were 50-59, 60-69, and 70-79 years. The team also estimated the expected number of early-onset cases in 2019 by multiplying 2010 age-specific cancer incidence rates by population counts for 2019.
First author Meredith Shiels, of the National Cancer Institute, and colleagues found that the largest absolute increase in incidence of early-onset cancers, compared with expected incidence, were for breast (n = 4834 additional cancers), colorectal (n = 2099), kidney (n = 1793), and uterine cancers (n = 1209). These diagnoses accounted for 80% of the additional cancer diagnoses in 2019 vs 2010.
Looking at increases by age group, Shiels and colleagues reported that 1.9% of all cancers occurred in overall early-onset cohort 15- to 49-year-olds (age-standardized incidence rate of 39.8 per 100,000), and the incidence was greater in the older cohorts: 3.6% for 30- to 39-year-olds (123.5 per 100,000) and 8.8% for 40- to 49-year-olds (293.9 per 100,000).
Overall, 14 of 33 cancer types significantly increased in incidence in at least one early-onset age group. Among these 14 cancer types, five — melanoma, plasma cell neoplasms, cervical cancer, stomach cancer, and cancer of the bones and joints — showed increases only in early-onset age groups, not in late-onset age groups. For example, between 2010 and 2019, cervical cancer rates increased by 1.39% per year among 30- to 39-year-olds, melanoma rates increased by 0.82% per year among 40- to 49-year-olds, and stomach cancer rates increased by 1.38% per year.
The remaining nine cancer types increased in at least one early-onset and one late-onset group. These included female breast, colorectal, kidney, testicular, uterine, pancreatic cancers as well as precursor B-cell non-Hodgkin lymphoma, diffuse large B-cell lymphoma, and mycosis fungoides/Sézary syndrome.
For four of the 14 cancer types with increasing incidence rates — testicular cancer, uterine cancer, colorectal cancer, and cancer of the bones and joints — mortality also increased in at least one early-onset age group, whereas the remaining 10 cancer types increased in incidence without an increase in mortality for any age group.
Shiels and her colleagues aren’t the first to address the rising incidence of early-onset cancers. In a keynote lecture at the European Society of Medical Oncology (ESMO) 2024 Annual Meeting, Irit Ben-Aharon, MD, PhD, from the Rambam Health Care Campus in Haifa, Israel, noted that from 1990-2019, the global incidence of early-onset cancer increased by 79%.
Although the current study doesn’t identify drivers of rising cancer rates in younger patients, “descriptive data like these provide a critical starting point for understanding the drivers of rising rates of cancer in early-onset age groups and could translate to effective cancer prevention and early detection efforts,” Shiels said in a press release. For instance, “recent guidelines have lowered the age of initiation for breast and colorectal cancer screening based, at least partially, on observations that rates for these cancers are increasing at younger ages.”
This study is “a great step forward” toward understanding the increasing incidence of early-onset cancers, agreed Shuji Ogino, MD, PhD, from Harvard Medical School and Brigham and Women’s Hospital in Boston, who wasn’t involved in the research.
The investigators provide new details, particularly by breaking down the early- and late-onset age groups into subcategories and by comparing incidence and mortality rates, Ogino noted.
“Mortality is a great endpoint because if the increased in early incidence is just an effect of [increased] screening we won’t see a mortality increase,” Ogino said. But “we need more data and some way to tease out the screening effect.” Plus, he added, “we need more mechanistic studies and tissue-based analyses to determine if early-onset cancers that are increasing in incidence are a different beast, rather than just an earlier beast.”
This study was funded by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health and the Institute of Cancer Research. Shiels declared no conflicts of interest.
version of this article first appeared on Medscape.com.
Collins Lays Out Plans to Reduce VA by 15% in Congressional Hearings
Collins Lays Out Plans to Reduce VA by 15% in Senate Hearing
US Department of Veterans Affairs (VA) Secretary Doug Collins testified in US House of Representatives and US Senate committees hearings that bringing staff numbers down to fiscal year 2019 figures was simply a goal: “Our goal, as we look at it, as everything goes forward, is a 15% decrease,” he told the senators. “It’s a goal. You have to start somewhere.”
“It’s a process we’re going through and I’m not going to work out a process in front of a committee or anywhere else,” Collins testified in the Senate on May 6, adding that it would be “incompetence” or “malpractice” to do so before time. “[When] we’re doing something as large as we are in an organization as sensitive on this Hill, it would not be right for us to do that in public. It would not be right for us to just come out and say here’s everything that we got and then have everybody scared because in the end it may not be the final decision.”
“We’re going to come to the best possible decision we can for the veterans in this country so they can have a VA system that actually works,” Collins argued in the Senate. “The VA’s been an issue for a long time. We’re trying to not make it an issue anymore.”
Collins later told a House committee on May 15 that VA was conducting a thorough review of department structure and staffing across the enterpise. "Our goal is to increase productivity and efficiency and to eliminate waste and bureaucracy improving health care delivery and benefits to our veterans. We are going to maintain VA essential jobs like doctors and nurses and claims processors" but eliminate positions it deemed "nonmission-critical" and consolidating areas of "overlap and waste."
Senate ranking member Richard Blumenthal (D-CT) and Chairman Jerry Moran (R-KS) both placed an emphasis on accountability for responsible resizing at the hearing.
“The department is at a critical juncture,” Moran said. “Perhaps that’s always true, and I want to hear from you that the changes under way at the VA are backed by data, informed by veteran demand, focused on improving outcomes for men and women the VA serves, and will be carried out in close coordination with this committee, as well as with veterans, VA staff, and veteran organizations.” Moran stressed that cutting should be about right-sizing, done carefully, and while treating people “with gratitude and respect.”
Blumenthal was more direct in his criticism of the approach: “You cannot slash and trash the VA without eliminating those essential positions which provide access and availability of health care. It simply cannot be done,” he told Collins.
In response, Collins replied, “You have stated on several occasions already that I am saying we are going to fire 83,000 employees. That is wrong.” Collins insisted that the VA was “looking at a goal of how many employees we have and how many employees that are actually working in the front line taking care. I have doctors and nurses right now that do not see patients. Is that helping veteran health care?”
Collins defended the actions of the VA and spoke about challenges he was “constantly fighting” in the early weeks of his tenure. “We’ve been hit by a barrage of false rumors, innuendo, disinformation, speculation implying firing doctors and nurses, and forcing staff to work in closets and showers and that there’s chaos in the department, none of which have been backed up. Why? Because we canceled some contracts that worked for the VA that we should be doing in-house and we let go of less than one half of one percent of nonmission critical employees.”
The Trump Administration offered federal employees the option of resigning, which purportedly will go toward meeting the 15% target. NPR reported that VA employees have since shared data showing that 11,273 agency employees nationwide have applied for deferred resignation. Most of those employees are nurses (about 1300), medical support assistants (about 800), and social workers (about 300).
Collins stressed that the aim of restructuring was to protect veterans’ health care. By getting rid of DEI initiatives, the VA saved $14 million, which he said was redirected to veterans with disabilities who need prosthetics.
Sen. Bernie Sanders (D-VT) addressed concerns about the existing shortage of clinicians at the VA, asking Collins what he was doing to bring in more doctors, nurses, and social workers. In addition to moving doctors and nurses from nonpatient care to patient care, Collins said, he planned to work with Congress to make salaries more competitive.
But money and adding more employees are not always the solution, Collins said. For example, he said, the VA has been spending $588 million a year veteran suicide research, its top clinical priority. Yet, he said there has not been a significant decrease in veteran suicide rates since 2008.
The most recent VA suicide report, released in 2024, indicates suicide rates have remained steady since 2001. However, in 2022, the number of suicides among veterans (6407) was actually lower than in 12 of the previous 14 years.
According to media reports, congressional lawmakers, and union officials, Veteran Crisis Line (VCL) staff were among the 2400 probationary employees fired in February. In a Feb. 20 video, Collins accused Democrats of spreading lies and insisted no one who answered the phone was fired.
Later, in a letter to senators, Collins admitted that 24 VCL support staff were “erroneously” sent termination notices. The firings were later reversed, Collins said, and all VCL employees had been reinstated at the same position they previously held. “Ensuring the VCL is always accessible 24/7 is one of the department’s top priorities,” Collins insisted.
Collins shared his approval of keeping and expanding VA programs and studies on psychedelic treatments for patients with posttraumatic stress disorder and traumatic brain injury. He also spoke to the proposed 2026 budget calling for a $5.4 billion increase for the VA. If approved, that money would be targeted for medical care and homelessness.
US Department of Veterans Affairs (VA) Secretary Doug Collins testified in US House of Representatives and US Senate committees hearings that bringing staff numbers down to fiscal year 2019 figures was simply a goal: “Our goal, as we look at it, as everything goes forward, is a 15% decrease,” he told the senators. “It’s a goal. You have to start somewhere.”
“It’s a process we’re going through and I’m not going to work out a process in front of a committee or anywhere else,” Collins testified in the Senate on May 6, adding that it would be “incompetence” or “malpractice” to do so before time. “[When] we’re doing something as large as we are in an organization as sensitive on this Hill, it would not be right for us to do that in public. It would not be right for us to just come out and say here’s everything that we got and then have everybody scared because in the end it may not be the final decision.”
“We’re going to come to the best possible decision we can for the veterans in this country so they can have a VA system that actually works,” Collins argued in the Senate. “The VA’s been an issue for a long time. We’re trying to not make it an issue anymore.”
Collins later told a House committee on May 15 that VA was conducting a thorough review of department structure and staffing across the enterpise. "Our goal is to increase productivity and efficiency and to eliminate waste and bureaucracy improving health care delivery and benefits to our veterans. We are going to maintain VA essential jobs like doctors and nurses and claims processors" but eliminate positions it deemed "nonmission-critical" and consolidating areas of "overlap and waste."
Senate ranking member Richard Blumenthal (D-CT) and Chairman Jerry Moran (R-KS) both placed an emphasis on accountability for responsible resizing at the hearing.
“The department is at a critical juncture,” Moran said. “Perhaps that’s always true, and I want to hear from you that the changes under way at the VA are backed by data, informed by veteran demand, focused on improving outcomes for men and women the VA serves, and will be carried out in close coordination with this committee, as well as with veterans, VA staff, and veteran organizations.” Moran stressed that cutting should be about right-sizing, done carefully, and while treating people “with gratitude and respect.”
Blumenthal was more direct in his criticism of the approach: “You cannot slash and trash the VA without eliminating those essential positions which provide access and availability of health care. It simply cannot be done,” he told Collins.
In response, Collins replied, “You have stated on several occasions already that I am saying we are going to fire 83,000 employees. That is wrong.” Collins insisted that the VA was “looking at a goal of how many employees we have and how many employees that are actually working in the front line taking care. I have doctors and nurses right now that do not see patients. Is that helping veteran health care?”
Collins defended the actions of the VA and spoke about challenges he was “constantly fighting” in the early weeks of his tenure. “We’ve been hit by a barrage of false rumors, innuendo, disinformation, speculation implying firing doctors and nurses, and forcing staff to work in closets and showers and that there’s chaos in the department, none of which have been backed up. Why? Because we canceled some contracts that worked for the VA that we should be doing in-house and we let go of less than one half of one percent of nonmission critical employees.”
The Trump Administration offered federal employees the option of resigning, which purportedly will go toward meeting the 15% target. NPR reported that VA employees have since shared data showing that 11,273 agency employees nationwide have applied for deferred resignation. Most of those employees are nurses (about 1300), medical support assistants (about 800), and social workers (about 300).
Collins stressed that the aim of restructuring was to protect veterans’ health care. By getting rid of DEI initiatives, the VA saved $14 million, which he said was redirected to veterans with disabilities who need prosthetics.
Sen. Bernie Sanders (D-VT) addressed concerns about the existing shortage of clinicians at the VA, asking Collins what he was doing to bring in more doctors, nurses, and social workers. In addition to moving doctors and nurses from nonpatient care to patient care, Collins said, he planned to work with Congress to make salaries more competitive.
But money and adding more employees are not always the solution, Collins said. For example, he said, the VA has been spending $588 million a year veteran suicide research, its top clinical priority. Yet, he said there has not been a significant decrease in veteran suicide rates since 2008.
The most recent VA suicide report, released in 2024, indicates suicide rates have remained steady since 2001. However, in 2022, the number of suicides among veterans (6407) was actually lower than in 12 of the previous 14 years.
According to media reports, congressional lawmakers, and union officials, Veteran Crisis Line (VCL) staff were among the 2400 probationary employees fired in February. In a Feb. 20 video, Collins accused Democrats of spreading lies and insisted no one who answered the phone was fired.
Later, in a letter to senators, Collins admitted that 24 VCL support staff were “erroneously” sent termination notices. The firings were later reversed, Collins said, and all VCL employees had been reinstated at the same position they previously held. “Ensuring the VCL is always accessible 24/7 is one of the department’s top priorities,” Collins insisted.
Collins shared his approval of keeping and expanding VA programs and studies on psychedelic treatments for patients with posttraumatic stress disorder and traumatic brain injury. He also spoke to the proposed 2026 budget calling for a $5.4 billion increase for the VA. If approved, that money would be targeted for medical care and homelessness.
US Department of Veterans Affairs (VA) Secretary Doug Collins testified in US House of Representatives and US Senate committees hearings that bringing staff numbers down to fiscal year 2019 figures was simply a goal: “Our goal, as we look at it, as everything goes forward, is a 15% decrease,” he told the senators. “It’s a goal. You have to start somewhere.”
“It’s a process we’re going through and I’m not going to work out a process in front of a committee or anywhere else,” Collins testified in the Senate on May 6, adding that it would be “incompetence” or “malpractice” to do so before time. “[When] we’re doing something as large as we are in an organization as sensitive on this Hill, it would not be right for us to do that in public. It would not be right for us to just come out and say here’s everything that we got and then have everybody scared because in the end it may not be the final decision.”
“We’re going to come to the best possible decision we can for the veterans in this country so they can have a VA system that actually works,” Collins argued in the Senate. “The VA’s been an issue for a long time. We’re trying to not make it an issue anymore.”
Collins later told a House committee on May 15 that VA was conducting a thorough review of department structure and staffing across the enterpise. "Our goal is to increase productivity and efficiency and to eliminate waste and bureaucracy improving health care delivery and benefits to our veterans. We are going to maintain VA essential jobs like doctors and nurses and claims processors" but eliminate positions it deemed "nonmission-critical" and consolidating areas of "overlap and waste."
Senate ranking member Richard Blumenthal (D-CT) and Chairman Jerry Moran (R-KS) both placed an emphasis on accountability for responsible resizing at the hearing.
“The department is at a critical juncture,” Moran said. “Perhaps that’s always true, and I want to hear from you that the changes under way at the VA are backed by data, informed by veteran demand, focused on improving outcomes for men and women the VA serves, and will be carried out in close coordination with this committee, as well as with veterans, VA staff, and veteran organizations.” Moran stressed that cutting should be about right-sizing, done carefully, and while treating people “with gratitude and respect.”
Blumenthal was more direct in his criticism of the approach: “You cannot slash and trash the VA without eliminating those essential positions which provide access and availability of health care. It simply cannot be done,” he told Collins.
In response, Collins replied, “You have stated on several occasions already that I am saying we are going to fire 83,000 employees. That is wrong.” Collins insisted that the VA was “looking at a goal of how many employees we have and how many employees that are actually working in the front line taking care. I have doctors and nurses right now that do not see patients. Is that helping veteran health care?”
Collins defended the actions of the VA and spoke about challenges he was “constantly fighting” in the early weeks of his tenure. “We’ve been hit by a barrage of false rumors, innuendo, disinformation, speculation implying firing doctors and nurses, and forcing staff to work in closets and showers and that there’s chaos in the department, none of which have been backed up. Why? Because we canceled some contracts that worked for the VA that we should be doing in-house and we let go of less than one half of one percent of nonmission critical employees.”
The Trump Administration offered federal employees the option of resigning, which purportedly will go toward meeting the 15% target. NPR reported that VA employees have since shared data showing that 11,273 agency employees nationwide have applied for deferred resignation. Most of those employees are nurses (about 1300), medical support assistants (about 800), and social workers (about 300).
Collins stressed that the aim of restructuring was to protect veterans’ health care. By getting rid of DEI initiatives, the VA saved $14 million, which he said was redirected to veterans with disabilities who need prosthetics.
Sen. Bernie Sanders (D-VT) addressed concerns about the existing shortage of clinicians at the VA, asking Collins what he was doing to bring in more doctors, nurses, and social workers. In addition to moving doctors and nurses from nonpatient care to patient care, Collins said, he planned to work with Congress to make salaries more competitive.
But money and adding more employees are not always the solution, Collins said. For example, he said, the VA has been spending $588 million a year veteran suicide research, its top clinical priority. Yet, he said there has not been a significant decrease in veteran suicide rates since 2008.
The most recent VA suicide report, released in 2024, indicates suicide rates have remained steady since 2001. However, in 2022, the number of suicides among veterans (6407) was actually lower than in 12 of the previous 14 years.
According to media reports, congressional lawmakers, and union officials, Veteran Crisis Line (VCL) staff were among the 2400 probationary employees fired in February. In a Feb. 20 video, Collins accused Democrats of spreading lies and insisted no one who answered the phone was fired.
Later, in a letter to senators, Collins admitted that 24 VCL support staff were “erroneously” sent termination notices. The firings were later reversed, Collins said, and all VCL employees had been reinstated at the same position they previously held. “Ensuring the VCL is always accessible 24/7 is one of the department’s top priorities,” Collins insisted.
Collins shared his approval of keeping and expanding VA programs and studies on psychedelic treatments for patients with posttraumatic stress disorder and traumatic brain injury. He also spoke to the proposed 2026 budget calling for a $5.4 billion increase for the VA. If approved, that money would be targeted for medical care and homelessness.
Collins Lays Out Plans to Reduce VA by 15% in Senate Hearing
Collins Lays Out Plans to Reduce VA by 15% in Senate Hearing
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).
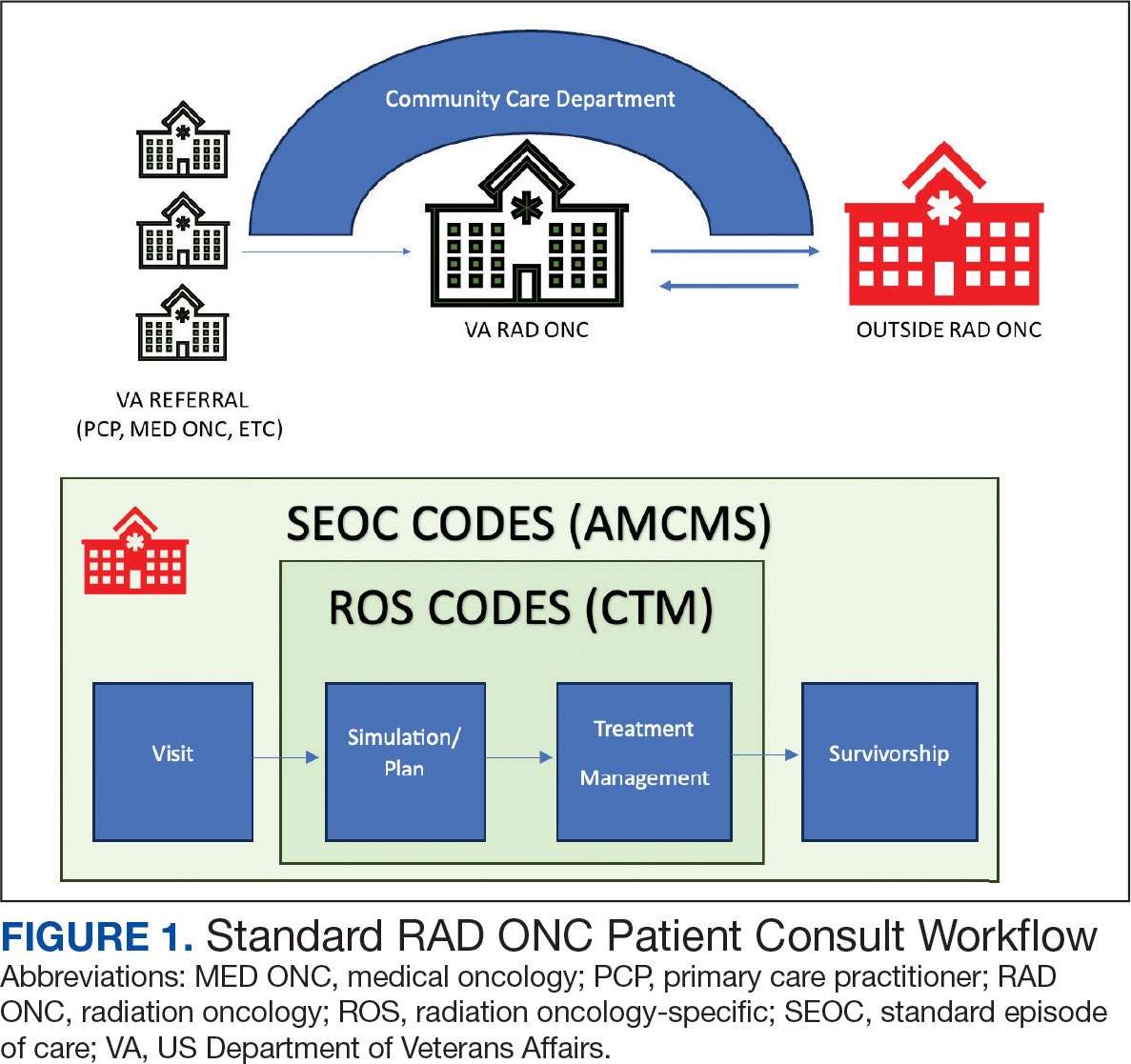
VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6
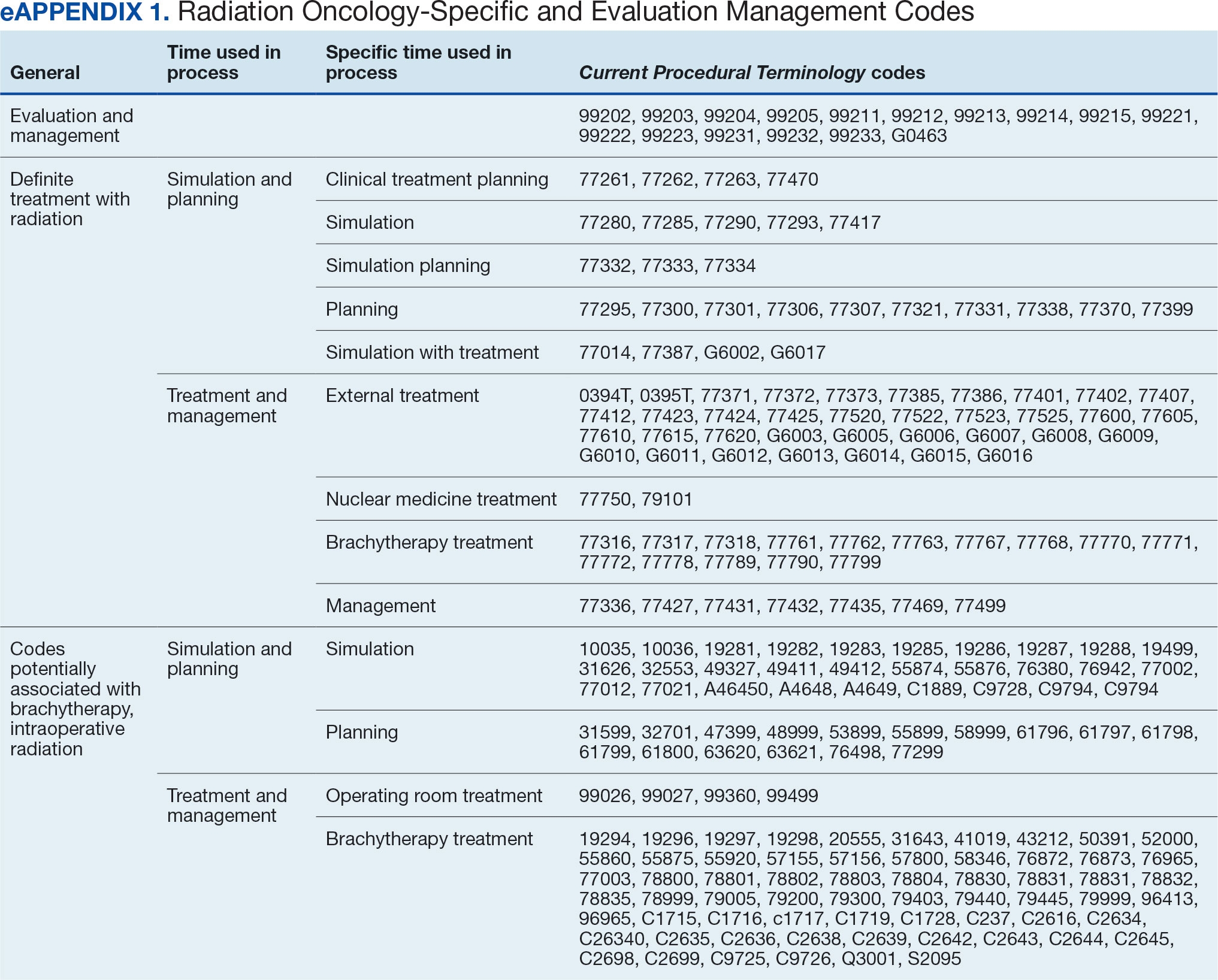
Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).
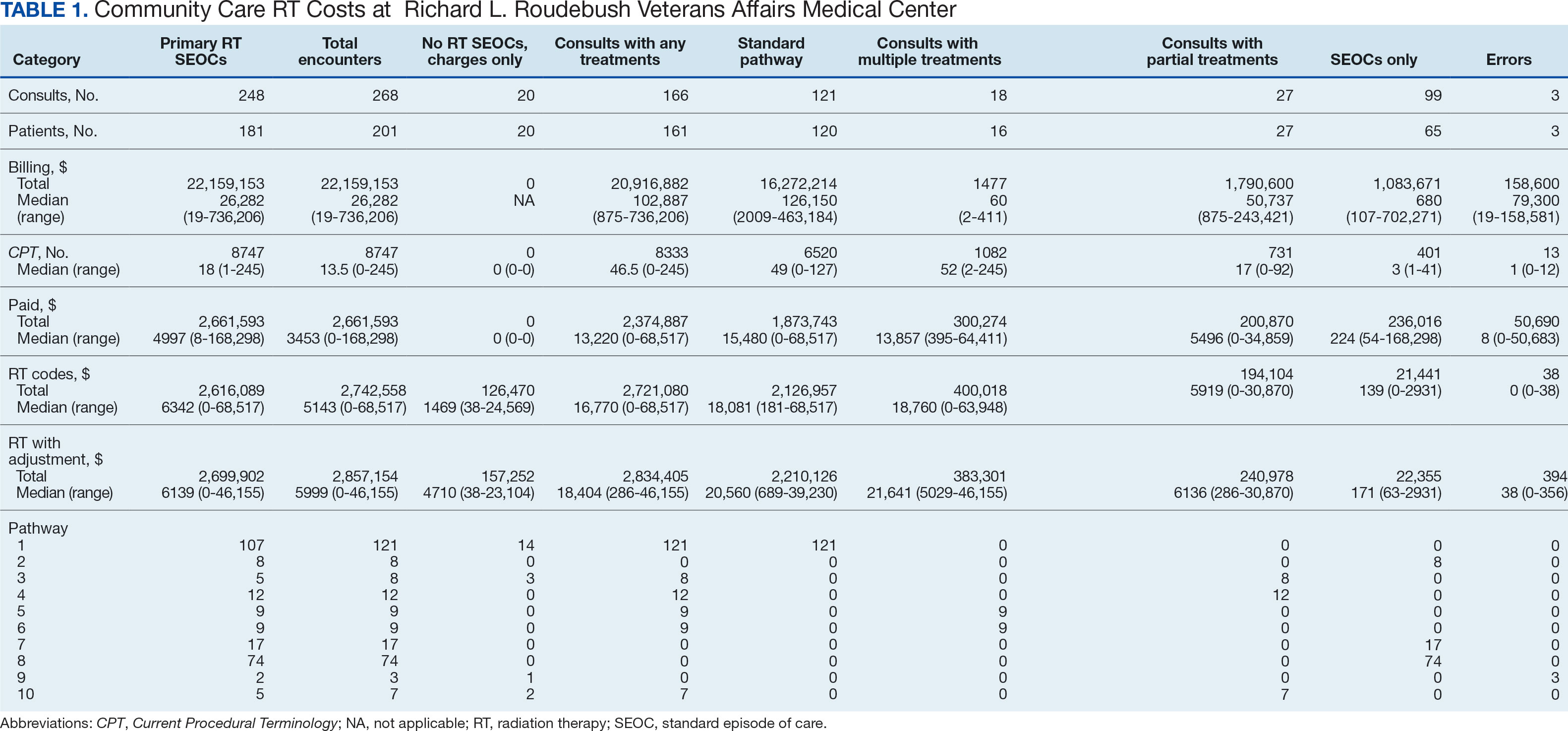
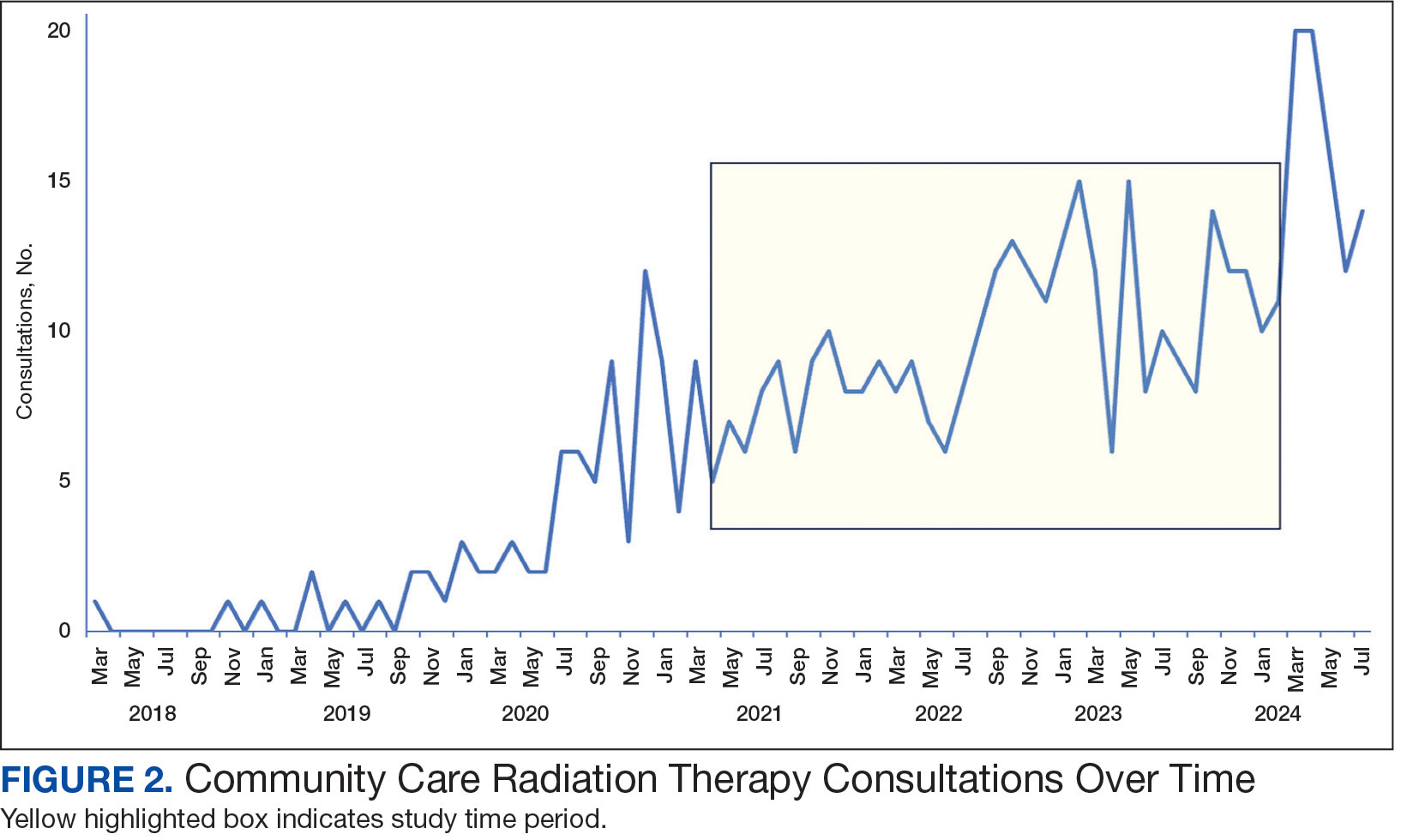
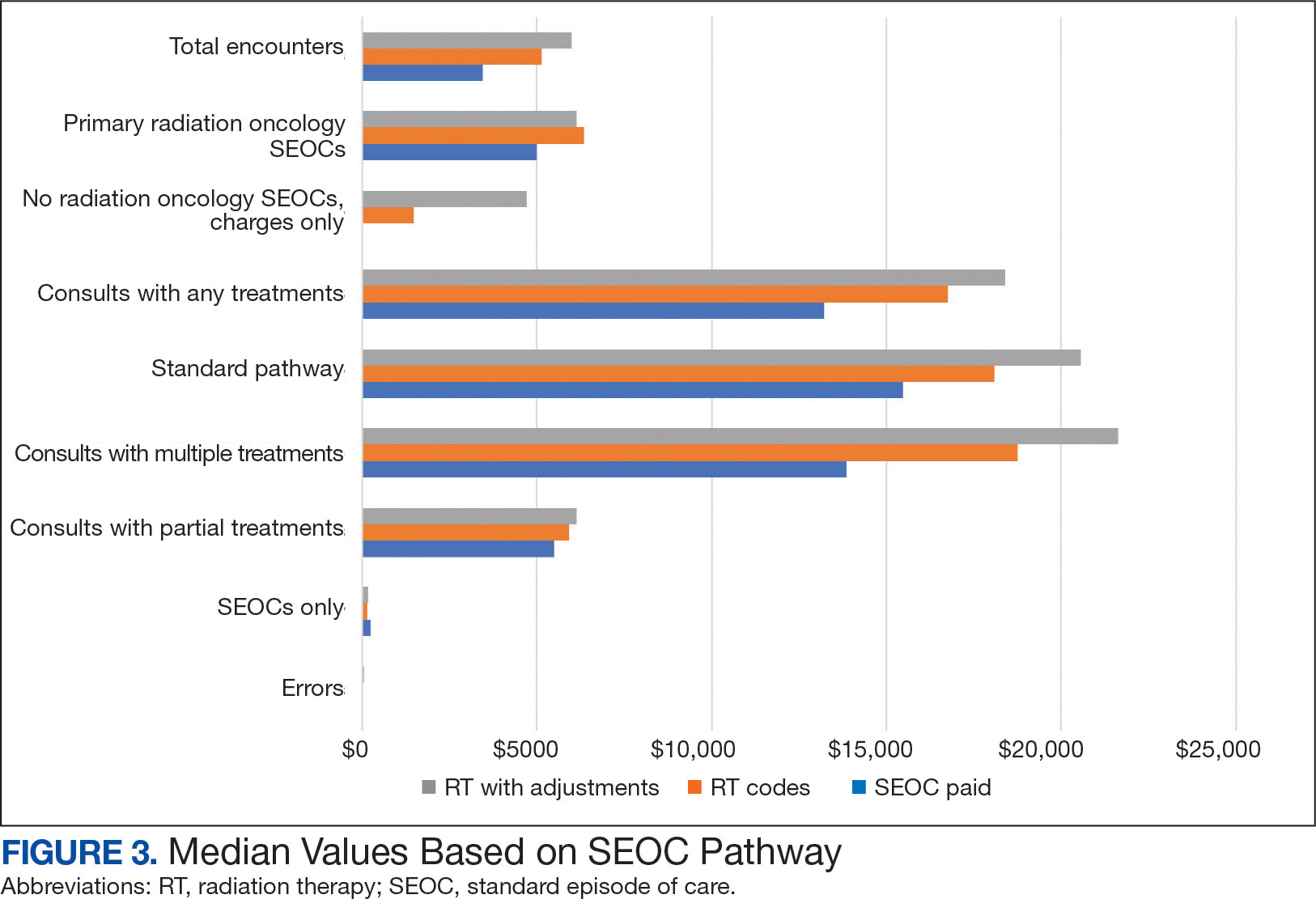
After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).
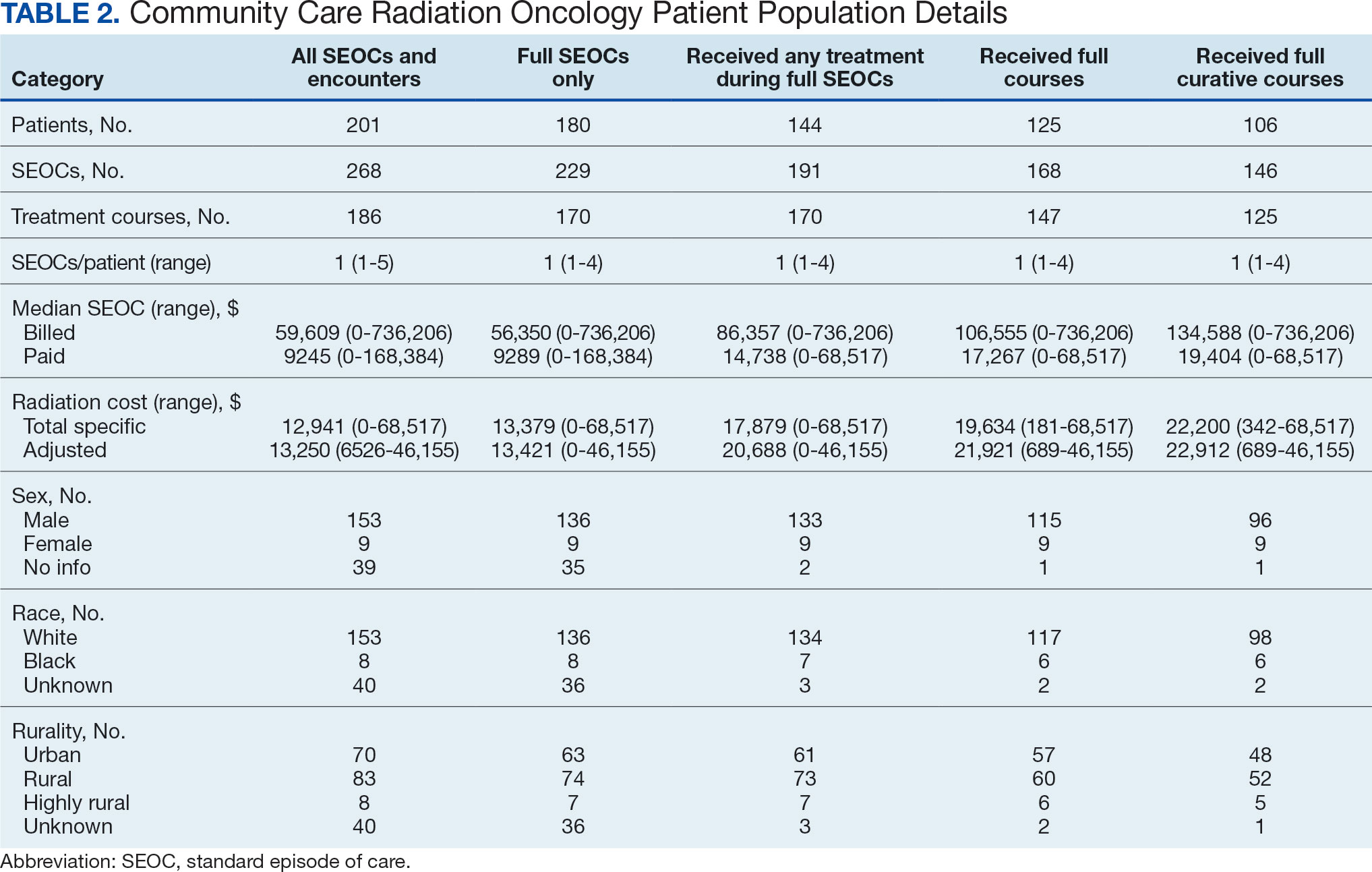
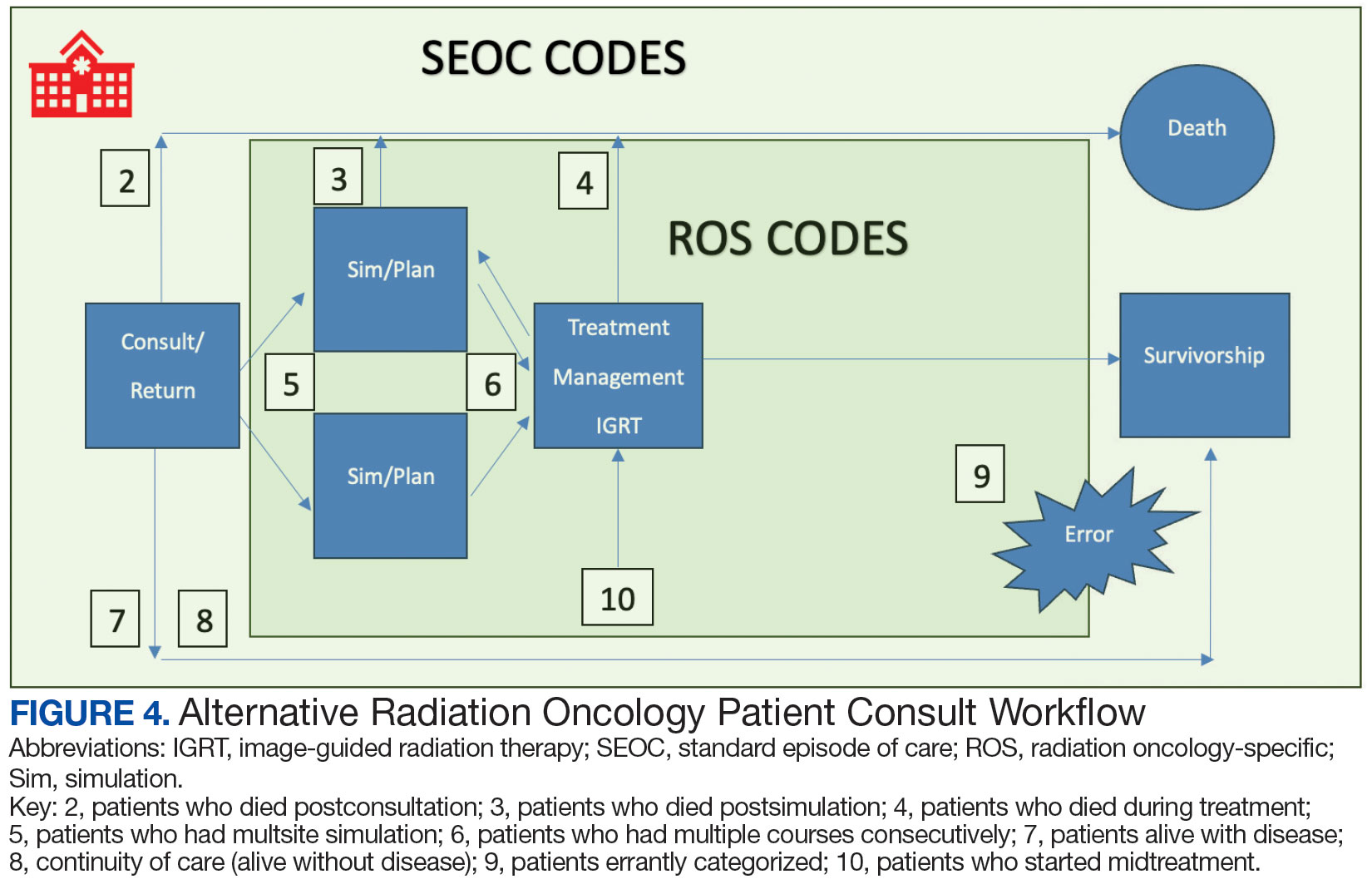
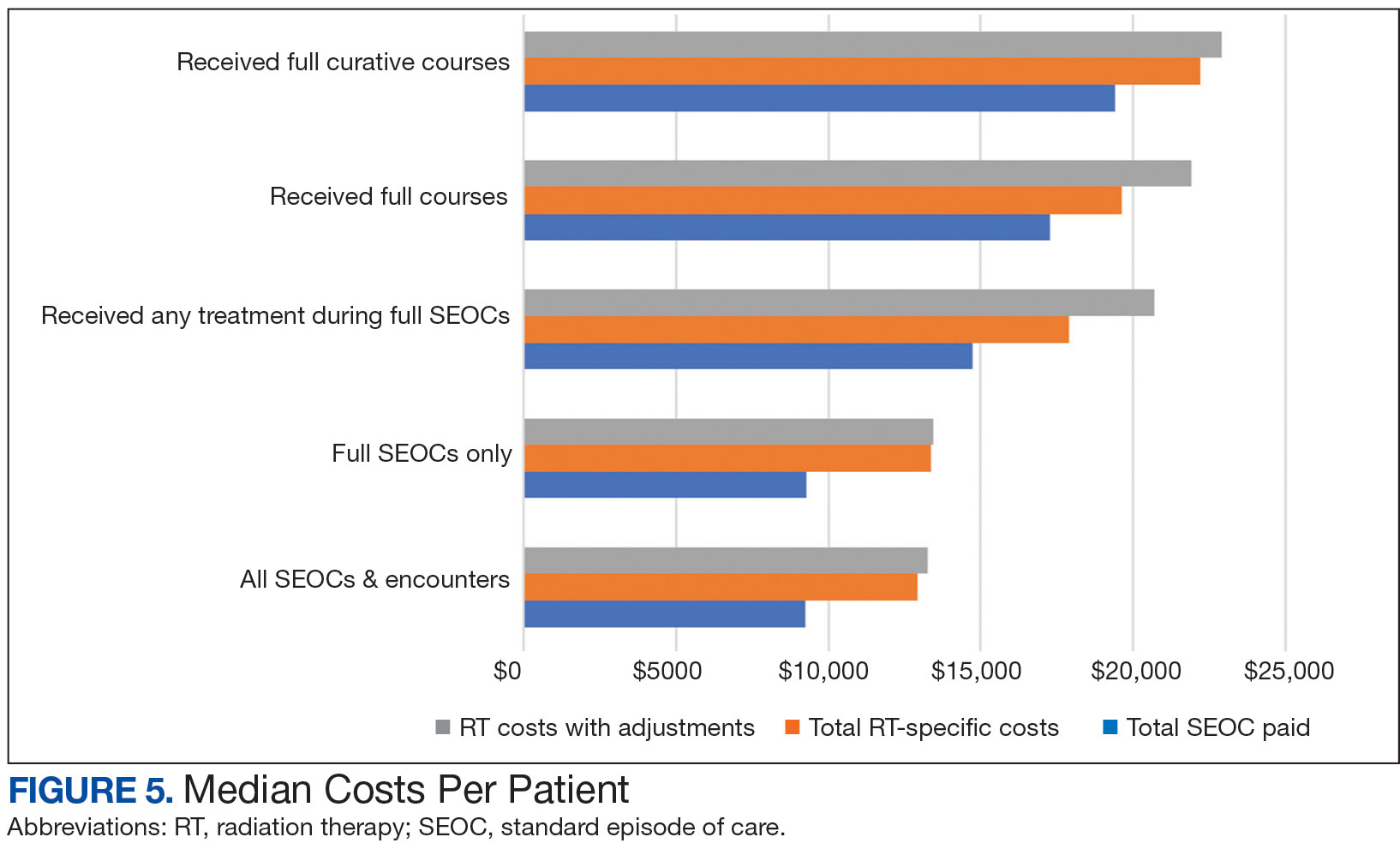
Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.
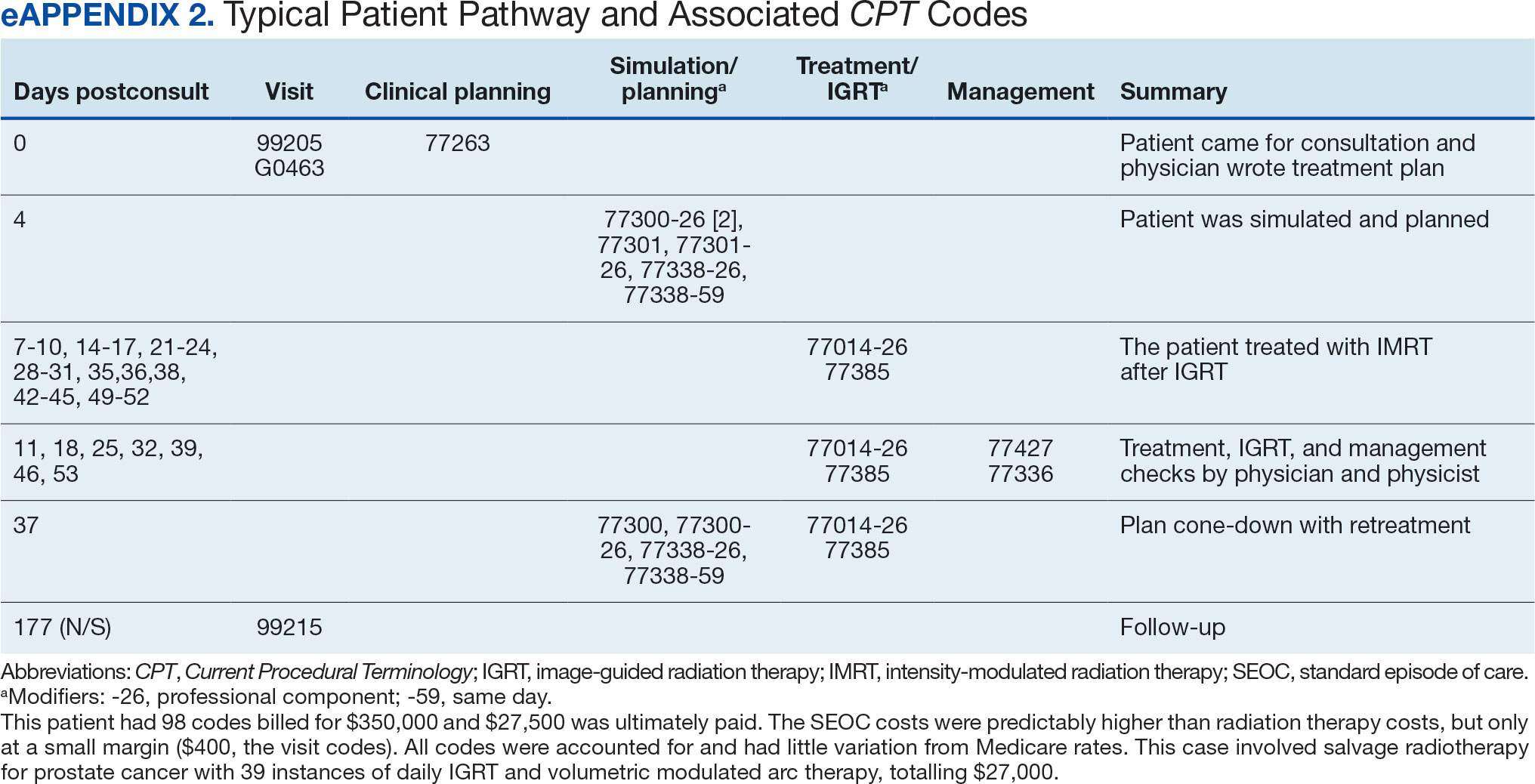

Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).
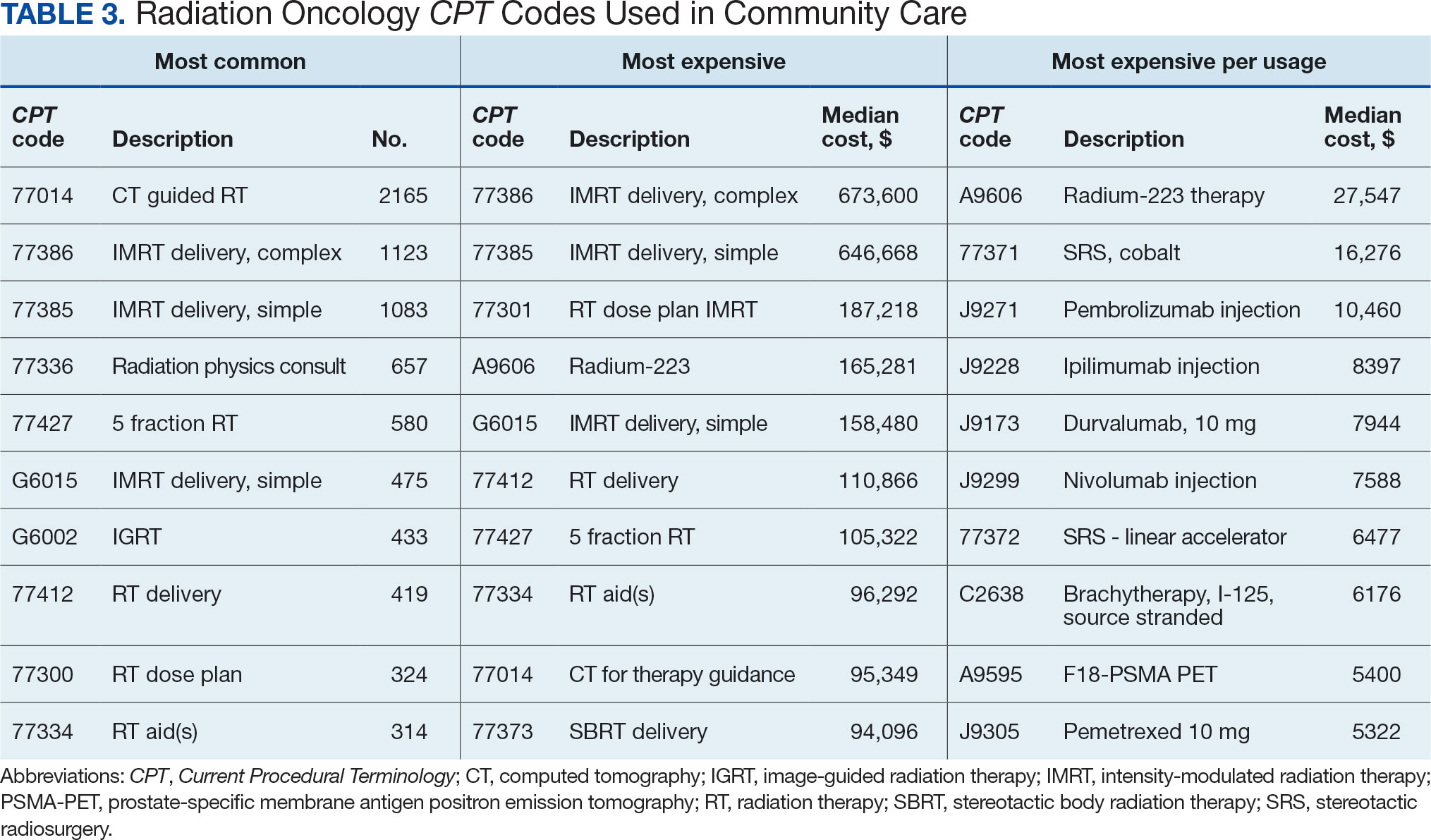
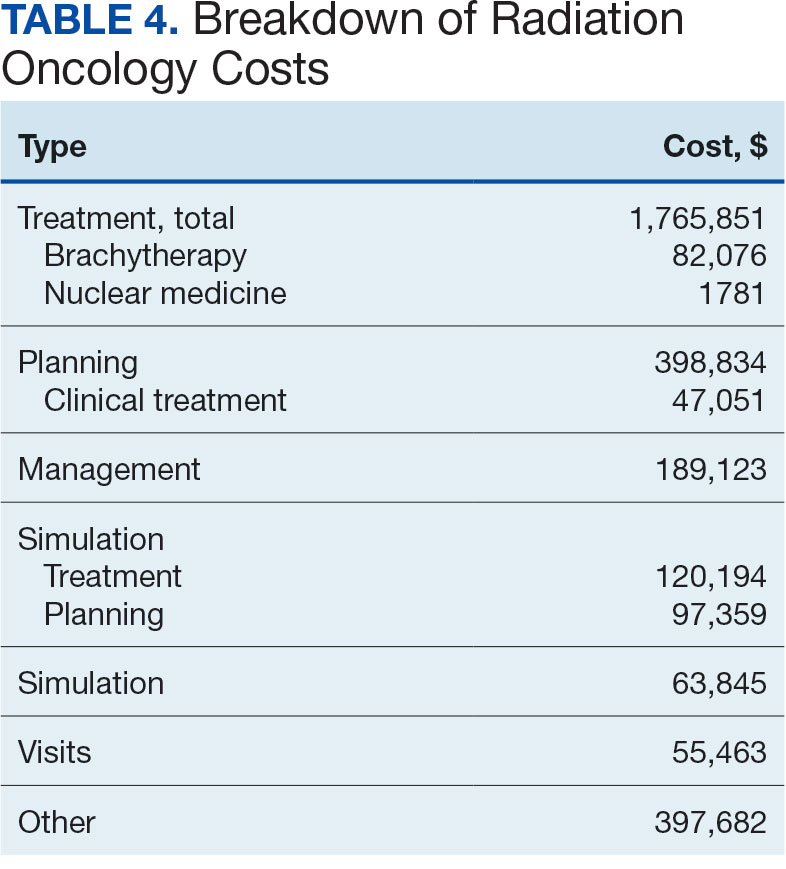

DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center