User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
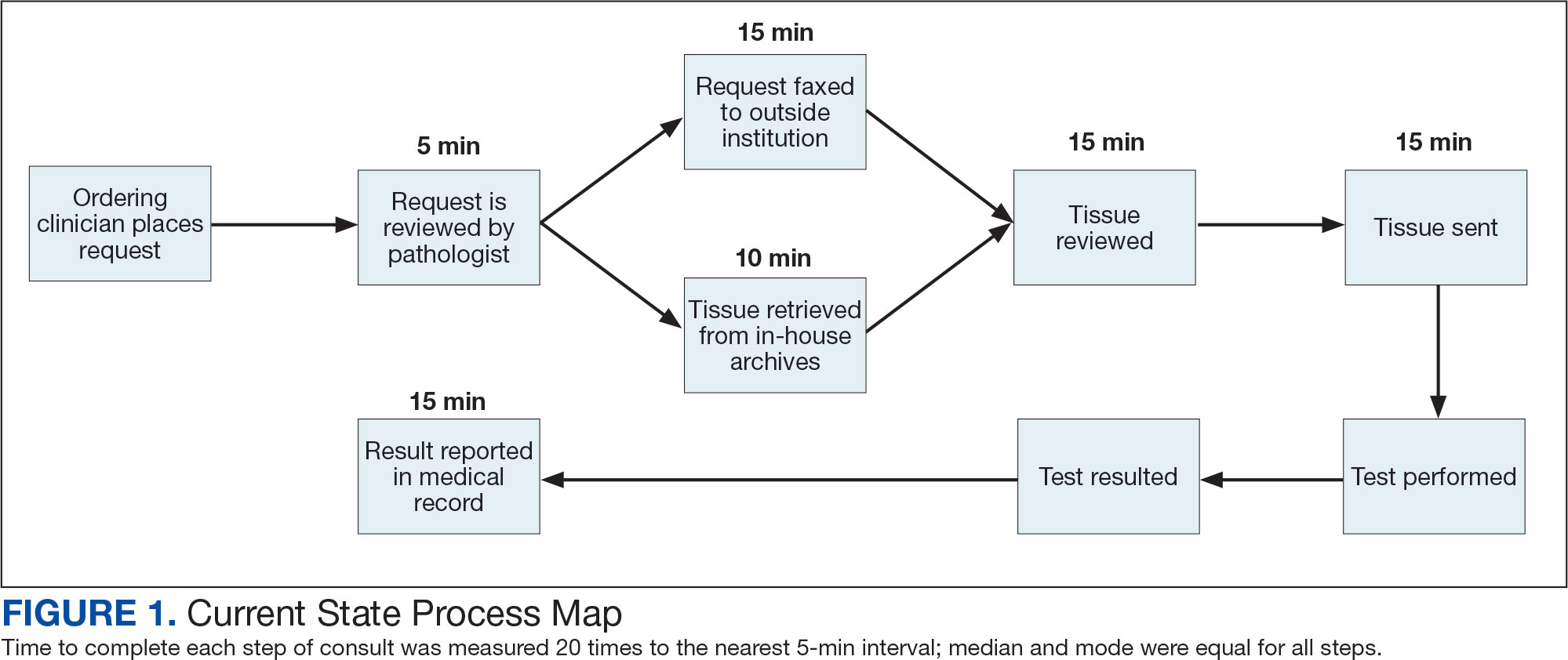
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.
The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.
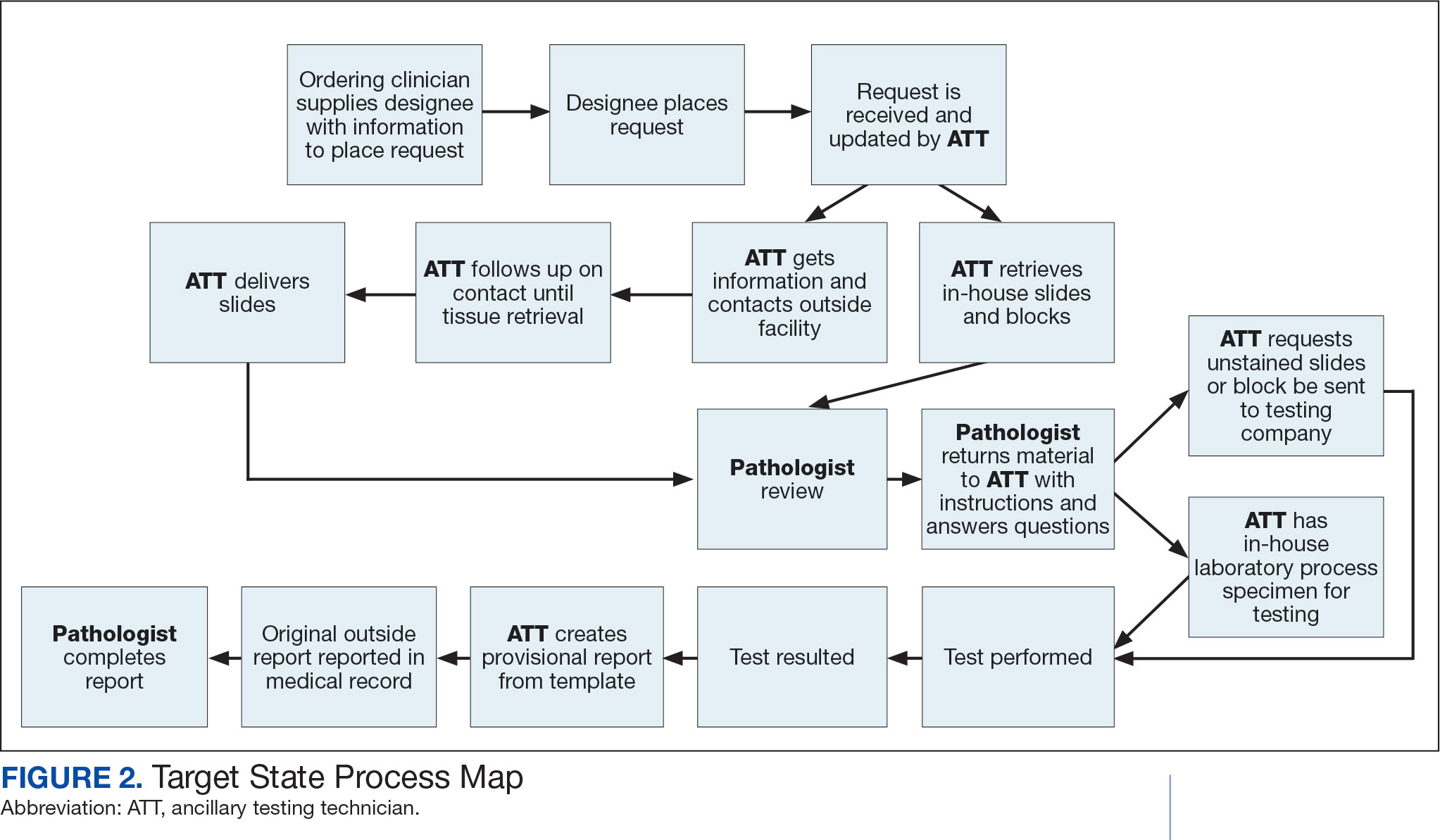
The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
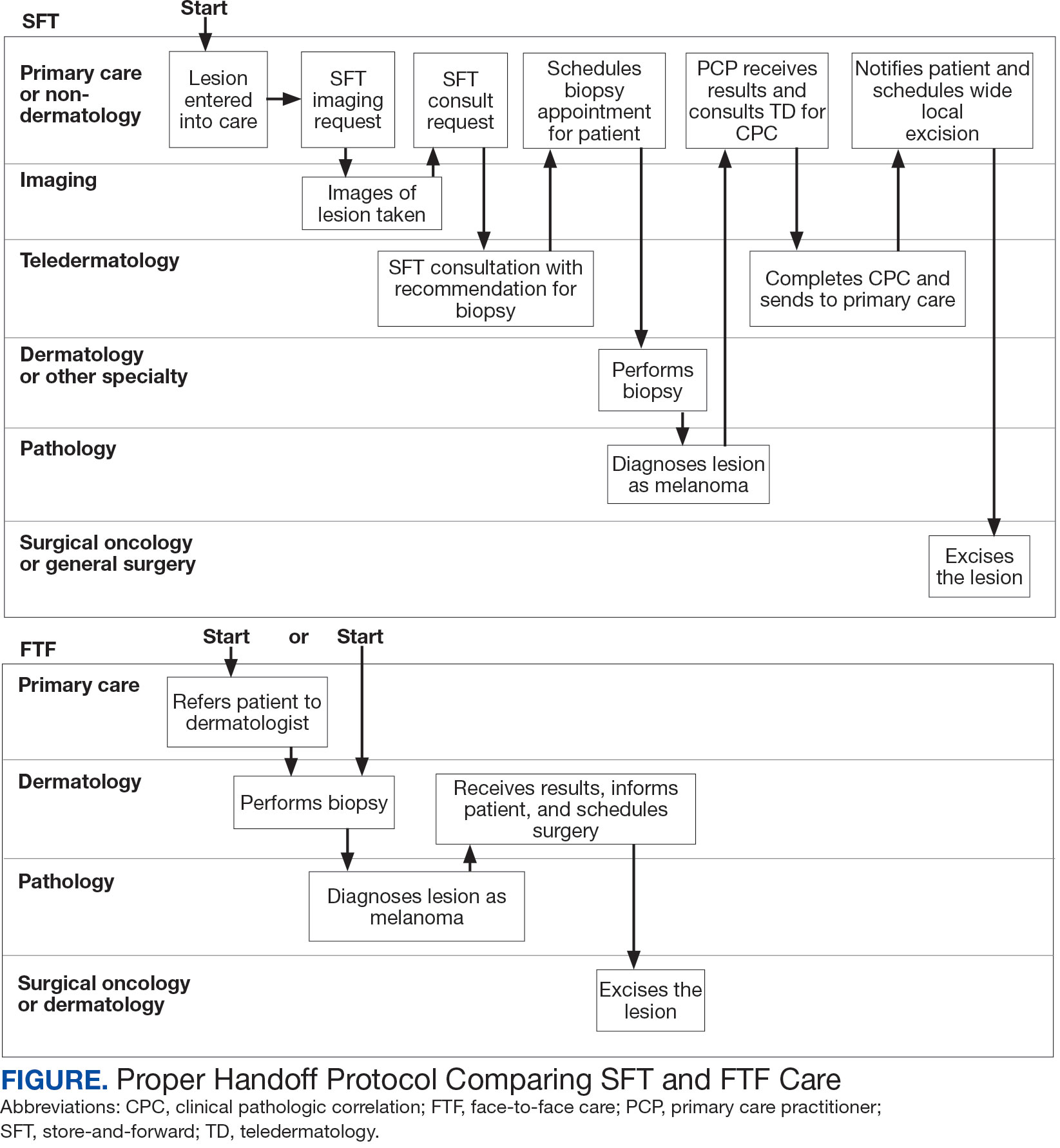
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).
This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.
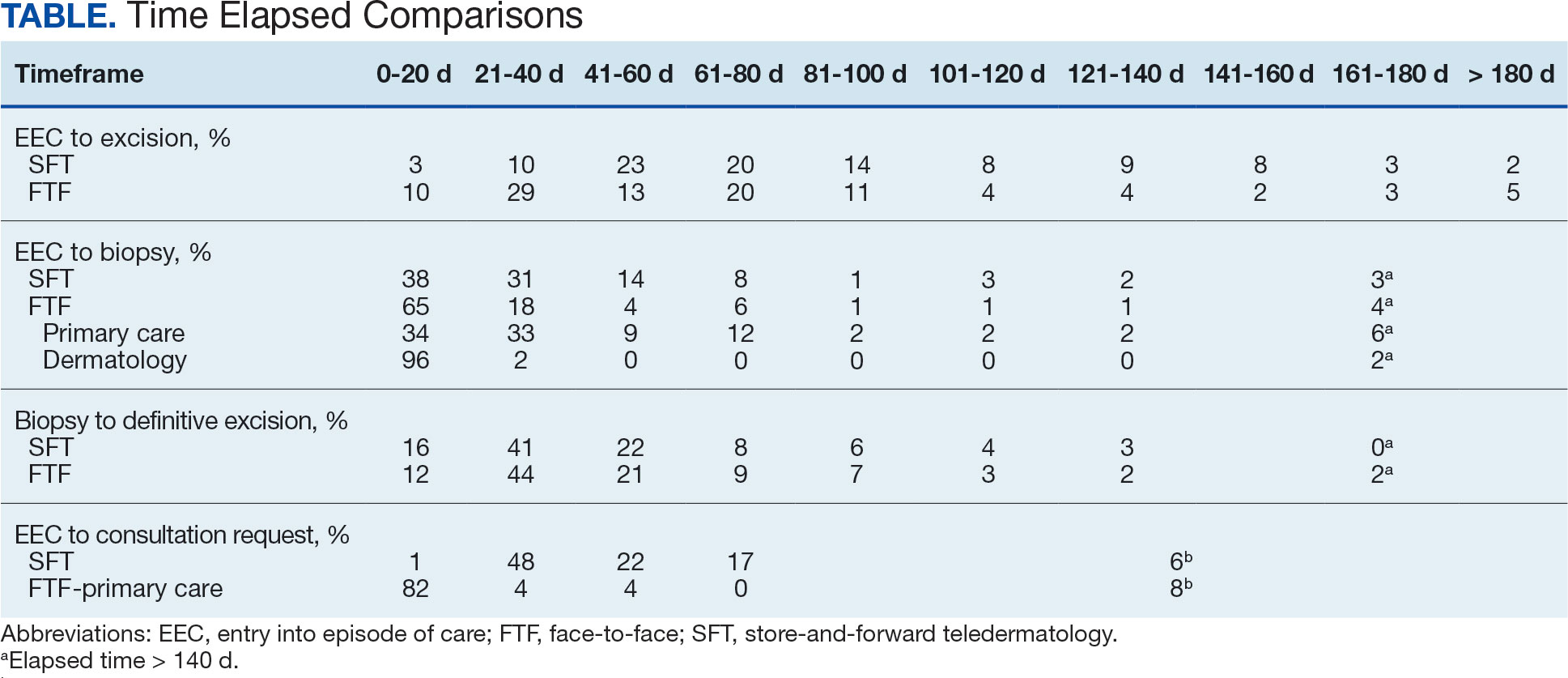
EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Study Investigates Non-Hodgkin Lymphoma in Air Force Missileers
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
Exercise Can Help Protect Against Cancer Fatigue, Depression
Lingering fatigue and depression are more common among women than men cancer survivors and often lead to a decrease in recreational physical activities in all patients, new data showed.
However, moderate physical activity was linked to an almost 50% lower risk for cancer-related fatigue, and both moderate and vigorous physical activity were associated with a two- to fivefold reduced risk for depression among cancer survivors, according to the analysis presented at the American Association for Cancer Research (AACR) Annual Meeting 2025.
The findings “highlight the importance of providing special attention and tailored interventions such as exercise programs, support groups, and mind-body behavioral techniques for vulnerable groups to help effectively manage fatigue and improve participation in recreational activities as they are an essential aspect of quality of life,” Simo Du, MD, a resident at NYC Health + Hospitals and Jacobi Medical Center/Albert Einstein College of Medicine, New York City, said in a news release.
Du noted that, during her residency, cancer-related fatigue was a common complaint among patients, affecting “not just their daily activities but also their overall quality of life and mental health, making tasks like climbing stairs, doing groceries, or laundry overwhelming.”
Cancer-related fatigue affects more than 80% of patients who receive chemotherapy or radiation therapy, while depression affects around 25% of patients. Unlike typical fatigue, cancer-related fatigue can linger for weeks, months, or even years after treatment, Du explained.
Despite its high prevalence, cancer-related fatigue remains “overlooked and undertreated,” she noted during a conference press briefing. In addition, cancer-related fatigue can affect men and women differently.
To investigate further, Du and her colleagues analyzed National Health and Nutrition Examination Survey (NHANES) data from 1552 cancer survivors (736 men and 816 women).
After adjusting for age, race, socioeconomic status, and comorbidities, women cancer survivors were more likely to experience fatigue (odds ratio [OR], 1.54; P < .017) and depression (OR, 1.32; P = .341) related to their cancer compared with men cancer survivors.
Du said there are likely multiple reasons behind the sex differences observed.
Women may, for instance, be more likely to experience side effects from chemotherapy, radiation, and long-term use of endocrine treatments because of slower drug clearance, which can lead to higher concentrations and a stronger immune response that may heighten inflammatory side effects.
In multivariate logistic regression analysis, cancer-related fatigue (OR, 1.93; P = .002) and depression (OR, 2.28; P = .011) were both strongly associated with reduced moderate recreational activities, such as brisk walking, biking, golfing, and light yard work.
The data also showed a protective role for physical activity. For patients who engaged in moderate physical activity, their risk for cancer-related fatigue (OR, 0.52; P = .002) and depression (OR, 0.41; P = .006) was significantly reduced, Du reported.
For depression (but not cancer-related fatigue), “the higher the intensity of physical activity, the higher the protective effects, with almost 4-5 times the reduction of the depression,” Du noted.
Although the NHANES uses standardized protocols designed to minimize biases, Du said a limitation of the current study is the use of self-reported data and the fact that women could potentially overreport fatigue symptoms and men could potentially underreport symptoms of depression.
Looking ahead, Du and her colleagues are planning studies to assess the effectiveness of tailored interventions on cancer-related fatigue and explore the connection between cancer-related fatigue and different mechanisms, such as inflammatory markers, to see if gender modifies the association.
Commenting on the study for this news organization, Jennifer Ligibel, MD, a senior physician at the Dana-Farber Cancer Institute in Boston, said that, because the dataset is cross-sectional, it is unclear whether people who were more tired weren’t exercising or if people who weren’t exercising were more tired.
However, Ligibel explained, a huge body of literature has demonstrated that exercise is “the most efficient remedy for fatigue,” and it likely helps with depression too.
In fact, in a recent survey of patients with cancer conducted by the American Society for Clinical Oncology, slightly more than half of patients reported that their oncologist talked about exercise and diet during clinic visits, Ligibel said. Provider recommendations for exercise and diet were associated with positive changes in these behaviors.
“Roughly half of oncologists now give exercise advice; that figure is a lot more than it was a few years ago, but it’s still not universal,” Ligibel said.
The study had no specific funding. Du and Ligibel had no disclosures.
A version of this article first appeared on Medscape.com.
Lingering fatigue and depression are more common among women than men cancer survivors and often lead to a decrease in recreational physical activities in all patients, new data showed.
However, moderate physical activity was linked to an almost 50% lower risk for cancer-related fatigue, and both moderate and vigorous physical activity were associated with a two- to fivefold reduced risk for depression among cancer survivors, according to the analysis presented at the American Association for Cancer Research (AACR) Annual Meeting 2025.
The findings “highlight the importance of providing special attention and tailored interventions such as exercise programs, support groups, and mind-body behavioral techniques for vulnerable groups to help effectively manage fatigue and improve participation in recreational activities as they are an essential aspect of quality of life,” Simo Du, MD, a resident at NYC Health + Hospitals and Jacobi Medical Center/Albert Einstein College of Medicine, New York City, said in a news release.
Du noted that, during her residency, cancer-related fatigue was a common complaint among patients, affecting “not just their daily activities but also their overall quality of life and mental health, making tasks like climbing stairs, doing groceries, or laundry overwhelming.”
Cancer-related fatigue affects more than 80% of patients who receive chemotherapy or radiation therapy, while depression affects around 25% of patients. Unlike typical fatigue, cancer-related fatigue can linger for weeks, months, or even years after treatment, Du explained.
Despite its high prevalence, cancer-related fatigue remains “overlooked and undertreated,” she noted during a conference press briefing. In addition, cancer-related fatigue can affect men and women differently.
To investigate further, Du and her colleagues analyzed National Health and Nutrition Examination Survey (NHANES) data from 1552 cancer survivors (736 men and 816 women).
After adjusting for age, race, socioeconomic status, and comorbidities, women cancer survivors were more likely to experience fatigue (odds ratio [OR], 1.54; P < .017) and depression (OR, 1.32; P = .341) related to their cancer compared with men cancer survivors.
Du said there are likely multiple reasons behind the sex differences observed.
Women may, for instance, be more likely to experience side effects from chemotherapy, radiation, and long-term use of endocrine treatments because of slower drug clearance, which can lead to higher concentrations and a stronger immune response that may heighten inflammatory side effects.
In multivariate logistic regression analysis, cancer-related fatigue (OR, 1.93; P = .002) and depression (OR, 2.28; P = .011) were both strongly associated with reduced moderate recreational activities, such as brisk walking, biking, golfing, and light yard work.
The data also showed a protective role for physical activity. For patients who engaged in moderate physical activity, their risk for cancer-related fatigue (OR, 0.52; P = .002) and depression (OR, 0.41; P = .006) was significantly reduced, Du reported.
For depression (but not cancer-related fatigue), “the higher the intensity of physical activity, the higher the protective effects, with almost 4-5 times the reduction of the depression,” Du noted.
Although the NHANES uses standardized protocols designed to minimize biases, Du said a limitation of the current study is the use of self-reported data and the fact that women could potentially overreport fatigue symptoms and men could potentially underreport symptoms of depression.
Looking ahead, Du and her colleagues are planning studies to assess the effectiveness of tailored interventions on cancer-related fatigue and explore the connection between cancer-related fatigue and different mechanisms, such as inflammatory markers, to see if gender modifies the association.
Commenting on the study for this news organization, Jennifer Ligibel, MD, a senior physician at the Dana-Farber Cancer Institute in Boston, said that, because the dataset is cross-sectional, it is unclear whether people who were more tired weren’t exercising or if people who weren’t exercising were more tired.
However, Ligibel explained, a huge body of literature has demonstrated that exercise is “the most efficient remedy for fatigue,” and it likely helps with depression too.
In fact, in a recent survey of patients with cancer conducted by the American Society for Clinical Oncology, slightly more than half of patients reported that their oncologist talked about exercise and diet during clinic visits, Ligibel said. Provider recommendations for exercise and diet were associated with positive changes in these behaviors.
“Roughly half of oncologists now give exercise advice; that figure is a lot more than it was a few years ago, but it’s still not universal,” Ligibel said.
The study had no specific funding. Du and Ligibel had no disclosures.
A version of this article first appeared on Medscape.com.
Lingering fatigue and depression are more common among women than men cancer survivors and often lead to a decrease in recreational physical activities in all patients, new data showed.
However, moderate physical activity was linked to an almost 50% lower risk for cancer-related fatigue, and both moderate and vigorous physical activity were associated with a two- to fivefold reduced risk for depression among cancer survivors, according to the analysis presented at the American Association for Cancer Research (AACR) Annual Meeting 2025.
The findings “highlight the importance of providing special attention and tailored interventions such as exercise programs, support groups, and mind-body behavioral techniques for vulnerable groups to help effectively manage fatigue and improve participation in recreational activities as they are an essential aspect of quality of life,” Simo Du, MD, a resident at NYC Health + Hospitals and Jacobi Medical Center/Albert Einstein College of Medicine, New York City, said in a news release.
Du noted that, during her residency, cancer-related fatigue was a common complaint among patients, affecting “not just their daily activities but also their overall quality of life and mental health, making tasks like climbing stairs, doing groceries, or laundry overwhelming.”
Cancer-related fatigue affects more than 80% of patients who receive chemotherapy or radiation therapy, while depression affects around 25% of patients. Unlike typical fatigue, cancer-related fatigue can linger for weeks, months, or even years after treatment, Du explained.
Despite its high prevalence, cancer-related fatigue remains “overlooked and undertreated,” she noted during a conference press briefing. In addition, cancer-related fatigue can affect men and women differently.
To investigate further, Du and her colleagues analyzed National Health and Nutrition Examination Survey (NHANES) data from 1552 cancer survivors (736 men and 816 women).
After adjusting for age, race, socioeconomic status, and comorbidities, women cancer survivors were more likely to experience fatigue (odds ratio [OR], 1.54; P < .017) and depression (OR, 1.32; P = .341) related to their cancer compared with men cancer survivors.
Du said there are likely multiple reasons behind the sex differences observed.
Women may, for instance, be more likely to experience side effects from chemotherapy, radiation, and long-term use of endocrine treatments because of slower drug clearance, which can lead to higher concentrations and a stronger immune response that may heighten inflammatory side effects.
In multivariate logistic regression analysis, cancer-related fatigue (OR, 1.93; P = .002) and depression (OR, 2.28; P = .011) were both strongly associated with reduced moderate recreational activities, such as brisk walking, biking, golfing, and light yard work.
The data also showed a protective role for physical activity. For patients who engaged in moderate physical activity, their risk for cancer-related fatigue (OR, 0.52; P = .002) and depression (OR, 0.41; P = .006) was significantly reduced, Du reported.
For depression (but not cancer-related fatigue), “the higher the intensity of physical activity, the higher the protective effects, with almost 4-5 times the reduction of the depression,” Du noted.
Although the NHANES uses standardized protocols designed to minimize biases, Du said a limitation of the current study is the use of self-reported data and the fact that women could potentially overreport fatigue symptoms and men could potentially underreport symptoms of depression.
Looking ahead, Du and her colleagues are planning studies to assess the effectiveness of tailored interventions on cancer-related fatigue and explore the connection between cancer-related fatigue and different mechanisms, such as inflammatory markers, to see if gender modifies the association.
Commenting on the study for this news organization, Jennifer Ligibel, MD, a senior physician at the Dana-Farber Cancer Institute in Boston, said that, because the dataset is cross-sectional, it is unclear whether people who were more tired weren’t exercising or if people who weren’t exercising were more tired.
However, Ligibel explained, a huge body of literature has demonstrated that exercise is “the most efficient remedy for fatigue,” and it likely helps with depression too.
In fact, in a recent survey of patients with cancer conducted by the American Society for Clinical Oncology, slightly more than half of patients reported that their oncologist talked about exercise and diet during clinic visits, Ligibel said. Provider recommendations for exercise and diet were associated with positive changes in these behaviors.
“Roughly half of oncologists now give exercise advice; that figure is a lot more than it was a few years ago, but it’s still not universal,” Ligibel said.
The study had no specific funding. Du and Ligibel had no disclosures.
A version of this article first appeared on Medscape.com.
FROM AACR 2025
Single Antiplatelet After TAVR Lowers Risk
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
FROM SCAI 2025
Rethinking the Scalpel: Advancing Non-Surgical Strategies for Early Breast Cancer
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breast cancer is the most common cancer in women worldwide and a leading cause of cancer-related deaths. The most common form of breast cancer is invasive ductal carcinoma, which accounts for 75%-80% of breast cancers. The second most common form is invasive lobular carcinoma, which accounts for 10%-15% of cases.
Surgical treatment of breast cancer involves removal and pathological staging of the cancerous tissue. Breast-conserving surgery and mastectomy are two surgical treatment options for patients with breast cancer. Breast-conserving surgery, which involves resection of the tumor and the surrounding margin of healthy tissue to achieve clean margins, is usually combined with radiotherapy. Mastectomy is considered in patients with relative and absolute contraindications to breast-conserving therapeutic options (eg, patients with a genetic predisposition to breast cancer, tumors > 5 cm, extensive margins, prior radiation to breast or chest wall, first-trimester pregnancy, extensive ductal carcinoma in situ, inflammatory breast cancer). Although surgical treatment of breast cancer is widely used, there have been calls to minimize unnecessary invasive surgical interventions in patients with early-stage breast cancer.
Reassessing the Role of Surgery in the Early Stages
Some surgical procedures, including axillary lymph node dissection (ALND) and contralateral prophylactic mastectomy (CPM), once considered standard treatment for early-stage breast cancer, are now being recognized as unnecessary in most cases of early-stage breast cancer without sentinel node metastases. Although ALND, which involves removal of all lymphatic tissue in the axilla, has been used for decades in the surgical management of early-stage breast cancer, this intervention typically results in lymphedema and significant morbidity.
Contralateral prophylactic mastectomy is a surgical option chosen by some women with early-stage unilateral breast cancer. However, this procedure is considered controversial in this patient population since evidence shows no survival advantage with CPM. A large-scale survey by Jagsi et al of female patients with in situ or early-stage breast cancer concluded that CPM was more common in patients who were White, had a higher level of education, and had private health insurance. In the study, 598 of the 1569 patients without an identified mutation or high genetic risk reported that a surgeon recommended against CPM. Of this group, only 1.9% underwent CPM. In contrast, of the 746 patients who reported that they did not receive any recommendation from a surgeon, 19% underwent CPM.
Re-excision and mastectomy are considered in patients with early-stage breast cancer when clear margins are not achieved with breast-conserving surgery. To prevent unnecessary reoperations and mastectomies, the 2013 invasive cancer margin consensus guideline by the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology, defined adequate margins in breast-conserving surgery in invasive breast cancer as “no ink on tumor.” The guideline is endorsed by the American Society of Breast Surgeons, ASTRO, and the St Gallen Consensus Conference.
A Shift in Practice: Moving Away From Routine Node Dissection
Based on findings from multiple clinical trials, experts recommend sentinel lymph node biopsy (SLNB) over ANLD and omit axillary surgery in certain patients. Findings from ACOSOG Z1071, SENTINA, and SN FNAC prospective multi-institutional trials support the use of SLNB as the initial diagnostic procedure. Sentinel lobe biopsy involves removal and evaluation of the first lymph node which receives lymphatic drainage from the breast cancer site. Negative biopsy findings on SLNB can avoid ALND as it is less likely that metastasis has occurred.
Although SLNB is preferred in younger patients with early-stage breast cancer, it is not routinely recommended for women aged ≥ 70 years of age with clinically node-negative, early-stage, HR-positive and HER2-negative breast cancer. This recommendation is based on study findings showing no difference in survival of women aged > 70 years with HR-positive clinical stage I breast cancer who did and did not undergo axillary evaluation.
The Z0011 trial by the American College of Surgeons Oncology Group found SLNB alone was not inferior to ALND regarding overall and disease-free survival in patients with clinically node-negative cancer undergoing breast conservation surgery and radiation therapy.
SLNB: A Less Invasive Alternative to ALND
Compared to SLNB, ALND is associated with more morbidity, physical symptoms, and poorer quality of life. A systemic review by Bakri et al evaluating the impact of ALND vs SLNB found higher rates of lymphedema, pain, reduced strength, and range of motion in patients who underwent ALND. In addition, an analysis of the National Cancer Database by Cocco et al found that patients with limited CN+ T1-2 breast cancer had favorable survival outcomes after undergoing SLNB and regional node irradiation vs ALND.
Rethinking First Steps: Non-Surgical Strategies
While surgical intervention with or without radiation therapy remains a primary treatment in early-stage breast cancer, there is an increased emphasis on de-escalation to minimize surgery and consider nonsurgical options in this patient population. A neoadjuvant systemic therapeutic approach by Kuerer et al for HER2-positive breast cancer and triple-negative breast cancer yielded a pathological complete response in 62% of patients. This multicenter, single-arm, phase 2 trial evaluated patients with HER2-positive breast cancer and a residual breast lesion < 2 cm or unicentric cT1-2N0-1M0 triple-negative breast cancer. Patients in the study underwent radiotherapy alone after excluding invasive in-situ disease.
The Clinician’s Role in Shaping Conservative Surgical Approaches
De-escalating surgery in breast cancer should involve acknowledging the patient’s fears and misperceptions regarding the risks of cancer recurrence that can lead them to opt for more invasive surgical treatments. Patients may not or fully regard the long-term effects of electing an invasive procedure in the absence of clinical indications. For example, patients undergoing more invasive interventions may experience worse body image and quality of life.
Clinicians may also not adequately estimate other harms associated with unnecessary surgical interventions. Providing clinicians with data that focuses on the psychological outcomes and satisfaction of patients post surgery may help them to better interpret and consider patient values and wishes and minimize future unnecessary surgeries.
Breast cancer remains one of the best-studied cancers with multiple high-quality randomized controlled trials supporting de-escalation of surgery. De-escalation of breast cancer surgery has been successful in multiple ways, including the implementation of ALND in early-stage breast cancer. However, other options such as CPM remain common. Proper patient and physician education involving data from clinical trials and reports of patient satisfaction may further decrease unnecessary surgical interventions.
Nameera Temkar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Veterans and Nonveterans Show Similar Mammogram Rates
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A national survey of 8996 females reveals comparable mammography screening rates between those who identify as veterans (57.9%) and nonveterans (55.2%).
METHODOLOGY:
Researchers analyzed data from the 2019 National Health Interview Survey, a cross-sectional national survey tracking health information.
Female respondents aged 40 to 74 years without history of breast cancer were included in the analysis.
Analysis evaluated the association between screening and veteran status through logistic regression, adjusting for potential confounders.
Survey procedures accounted for complex sampling design to obtain valid estimates for the civilian, noninstitutionalized US population.
TAKEAWAY:
Analysis included 8996 female survey respondents, including 169 veterans (1.9%) and 320 (3.2%) reported having military health coverage.
Mammography screening rates within the last year were comparable between veterans (57.9%) and nonveterans (55.2%).
Veteran status showed no significant association with differences in mammography screening percentages (P = .96).
Among insured participants, military health insurance demonstrated no significant association with mammography screening percentages (P = .13).
The authors suggest that radiology practices should design proactive outreach strategies to address the needs of the growing number of female veterans who may face increased breast cancer risk due to military environmental exposures.
IN PRACTICE: “Although the results from our study demonstrate comparable mammography screening percentages, veterans may face additional risk factors for breast cancer due to occupational,” the authors argue.
SOURCE: This summary is based on a preprint published online in the Journal of the American College of Radiology: Milton A, Miles R, Gettle LM, Van Geertruyden P, Narayan AK. Utilization of Mammography Screening in Female Veterans: Cross-Sectional Survey Results from the National Health Interview Survey. J Am Coll Radiol. Published online April 24, 2025. doi:10.1016/j.jacr.2025.04.017
LIMITATIONS: The study relied on self-reported adherence data, which could overestimate screening percentages. Data collection occurred prior to updated United States Preventive Services Task Force guidelines recommending routine mammography screening for women starting at age 40 years every 2 years. The relatively small number of female veteran respondents limited the precision of population estimates. Additionally, the data were collected before the COVID-19 pandemic, which has been associated with reduced mammographic screening, particularly in medically underserved populations.
DISCLOSURES: Anand Narayan disclosed receiving financial support from Susan G. Komen Breast Cancer Foundation and National Academy of Medicine. The study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The remaining authors reported no potential conflicts of interest. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
UK Funds AI Blood Test for Early Cancer Detection
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
A clinical trial of a promising blood test that could offer faster and more accurate diagnoses for common cancers has received funding from the Department of Health and Social Care (DHSC).
The miONCO-Dx test detects cancer at an early stage by analysing microRNA expression in blood.
It uses artificial intelligence to identify the presence and origin of the disease.
The test was developed by Xgenera, a University of Southampton spinout, in collaboration with the National Institute for Health and Care Research.
Initial analysis of data from more than 20,000 patients showed that the test detected 12 of the most common and lethal cancers at an early stage and with over 99% accuracy.
Bowel Cancer Among Key Targets
Bowel cancer, the fourth most common cancer in the United Kingdom, is a principal target for the test.
Around 44,000 people in the UK are diagnosed with bowel cancer each year. At stage 1, approximately 90% of people survive for 5 years or more, but this drops to around 10% at stage 4.
Wes Streeting, Secretary of State for Health and Social Care, said in a press release, “The key to surviving cancer is catching it as early as possible, so this government is taking the urgent action needed to make sure that happens.”
£2.4 Million Awarded for Clinical Trial
The DHSC has awarded Xgenera £2.4 million to advance development of the test, which has now been refined into a cheaper, faster, and more scalable version.
The funding will support a clinical trial involving 8000 patients. The DHSC described this as “a formal and significant step towards bringing the test closer to patients by ensuring it is fit for purpose in the NHS.”
The trial will be run by Cancer Research UK Southampton Clinical Trials Unit.
Potential for NHS Use
Dr Victoria Goss, head of early diagnosis and translational research at the trials unit, said in a press release, “A reliable test such as this could have the potential to see a major shift in cancer screening, making it easier and cheaper to provide on the NHS, cutting health inequalities, and ultimately reducing the number of people who die from the disease.”
Xgenera co-founder Dr Andy Shapanis, a research fellow at the University of Southampton, said that the new study would evaluate the useability, accuracy, and cost-effectiveness of the test for use within the NHS in future.
“The hope is that if the test is shown to be successful in the early diagnosis of the 12 cancers we have currently identified biomarkers for, then it could be expanded to look at over 50 other cancers in the future,” he said.
Comparison With Other Tests
The miONCO-Dx test follows other attempts at multicancer early detection, such as the Galleri test from Grail, which is already being trialled in the NHS.
Galleri screens for altered DNA methylation patterns in blood and claims to detect more than 50 types of cancer. It raised hopes for earlier diagnosis, less invasive treatment, and potential cost savings.
However, critics have raised concerns about low detection rates in early-stage cancers, high false-positive rates, imprecise cancer origin analysis, cost, and unproven mortality gains. Questions have also been expressed about possible political influence in its selection for NHS trials.
A Broader Screening Platform
Xgenera co-founder Professor Paul Skipp, director of the Centre for Proteomic Research at the University of Southampton, said earlier this year that the miONCO-Dx test was “a real game-changer.”
The test can detect lung, breast, prostate, pancreatic, colorectal, ovarian, liver, brain, oesophageal, bladder, and gastric cancer and bone and soft-tissue sarcoma. It works by identifying imbalances in microRNAs, a class of small noncoding RNAs with functions in posttranscriptional regulation of gene expression, influencing cellular activities including cell growth, differentiation, development, and apoptosis.
The presence of microRNA imbalances can be identified from just 10-15 drops of blood, across all stages of tumour growth.
In comparison, according to Skipp, screening is only available currently for three types of cancer in the UK, and each test targets a single type.
Xgenera has also received external investment from the innovation investment companies Qantx, Empirical Ventures, and Ascension Ventures to further develop the test.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.
A version of this article first appeared on Medscape.com.
Can a Polygenic Risk Score Turn the Tide on Prostate Cancer Screening?
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Incorporating a polygenic risk score into prostate cancer screening could enhance the detection of clinically significant prostate cancer that conventional screening may miss, according to results of the BARCODE 1 clinical trial conducted in the United Kingdom.
The study found that about 72% of participants with high polygenic risk scores were diagnosed with clinically significant prostate cancers, which would not have been detected with prostate-specific antigen (PSA) testing or MRI.
“With this test, it could be possible to turn the tide on prostate cancer,” study author Ros Eeles, PhD, professor of oncogenetics at The Institute of Cancer Research, London, England, said in a statement following the publication of the analysis in The New England Journal of Medicine.
Prostate cancer remains the second most commonly diagnosed cancer among men. As a screening tool, PSA testing has been criticized for leading to a high rate of false positive results and overdiagnosis — defined as a screen-detected cancer that would take longer to progress to clinical cancer than the patient’s lifetime. Both issues can result in overtreatment.
Given prostate cancer’s high heritability and the proliferation of genome-wide association studies identifying common genetic variants, there has been growing interest in using polygenic risk scores to improve risk stratification and guide screening.
“Building on decades of research into the genetic markers of prostate cancer, our study shows that the theory does work in practice — we can identify men at risk of aggressive cancers who need further tests and spare the men who are at lower risk from unnecessary treatments,” said Eeles.
An Adjunct to Screening?
The BARCODE 1 study, conducted in the United Kingdom, tested the clinical utility of a polygenic risk score as an adjunct to screening.
The researchers recruited men aged 55-69 years from primary care centers in the United Kingdom. Using germline DNA extracted from saliva, they derived polygenic risk scores from 130 genetic variants known to be associated with an increased risk for prostate cancer.
Among a total of 6393 men who had their scores calculated, 745 (12%) had a score in the top 10% of genetic risk (≥ 90th percentile) and were invited to undergo further screening.
Of these, 468 (63%) accepted the invite and underwent multiparametric MRI and transperineal prostate biopsy, irrespective of the PSA level. Overall, 187 (40%) were diagnosed with prostate cancer following biopsy. Of the 187 men with prostate cancer, 55% (n = 103) had disease classified as intermediate or high risk (Gleason score ≥ 7) per National Comprehensive Cancer Network criteria and therefore warranted further treatment.
Researchers then compared screening that incorporated polygenic risk scores with standard screening with PSA levels and MRI.
When participants’ risk was stratified by their polygenic risk score, 103 patients (55%) with prostate cancer could be classified as intermediate or higher risk, thus warranting treatment. Overall, 74 (71.8%) of those cancers would have been missed using the standard diagnostic pathway in the United Kingdom, which requires patients to have a high PSA level (> 3.0 μg/L) as well as a positive MRI result. These 74 patients either had PSA levels ≤ 3.0 μg/L or negative MRIs, which would mean these patients would typically fall below the action threshold for further testing.
Of the 103 participants warranting treatment, 40 of these men would have been classified as unfavorable intermediate, high, or very high risk, which would require radical treatment. Among this group, roughly 43% would have been missed using the UK diagnostic pathway.
However, the investigators estimated a rate of overdiagnosis with the use of polygenic risk scores of 16%-21%, similar to the overdiagnosis estimates in two prior PSA-based screening studies, signaling that the addition of polygenic risk scores does not necessarily reduce the risk for overdiagnosis.
Overall, “this study is the strongest evidence to date on the clinical utility of a polygenic score for prostate cancer screening,” commented Michael Inouye, professor of systems genomics & population health, University of Cambridge, Cambridge, England, in a statement from the UK nonprofit Science Media Centre (SMC).
“I suspect we will look back on this as a landmark study that really made the clinical case for polygenic scores as a new tool that moved health systems from disease management to early detection and prevention,” said Inouye, who was not involved in the study.
However, other experts were more cautious about the findings.
Dusko Ilic, MD, professor of stem cell sciences, King’s College London, London, England, said the results are “promising, especially in identifying significant cancers that would otherwise be missed,” but cautioned that “there is no direct evidence yet that using [polygenic risk scores] improves long-term outcomes such as mortality or quality-adjusted life years.”
“Modeling suggests benefit, but empirical confirmation is needed,” Ilic said in the SMC statement.
The hope is that the recently launched TRANSFORM trial will help answer some of these outstanding questions.
The current study suggests that polygenic risk scores for prostate cancer “would be a useful component of a multimodality screening program that assesses age, family history of prostate cancer, PSA, and MRI results as triage tools before biopsy is recommended,” David Hunter, MPH, ScD, with Harvard T. H. Chan School of Public Health, Boston, and University of Oxford, Oxford, England, wrote in an editorial accompanying the study.
“To make this integrated program a reality, however, changes to infrastructure would be needed to make running and analyzing a regulated genome array as easy as requesting a PSA level or ordering an MRI. Clearly, we are far from that future,” Hunter cautioned.
“A possible first step that would require less infrastructure could be to order a polygenic risk score only for men with a positive PSA result, then use the polygenic risk score to determine who should undergo an MRI, and then use all the information to determine whether biopsy is recommended,” Hunter said.
In his view, the current study is a “first step on a long road to evaluating new components of any disease screening pathway.”
The research received funding from the European Research Council, the Bob Willis Fund, Cancer Research UK, the Peacock Trust, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Disclosures for authors and editorialists are available with the original article. Inouye and Ilic reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Early Warning Signs: Catching Gastric Cancer in Time
Hello. I’m Dr. David Johnson, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School and Old Dominion University in Norfolk, Virginia.
The American College of Gastroenterology (ACG) recently issued a clinical guideline on the diagnosis and management of gastric premalignant conditions.
Coincidentally, earlier this year, the ACG and the American Society for Gastrointestinal Endoscopy (ASGE) collaborated to issue recommendations on quality indicators for upper endoscopy.
In this overview, I’ll focus on gastric premalignant conditions while drawing upon some of the quality indicators from ACG/ASGE. Together, these publications highlight several things that we may be overlooking and clearly need to do better at addressing, given the emerging data in this area.
Gastric Premalignant Conditions: Increased Risk for Progression
Gastric premalignant conditions are common and include atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps.
The increased risk for progression to gastric adenocarcinoma in the United States is quite striking. It also reveals an important cancer disparity in certain high-risk groups.
The incidence rates of gastric cancer are two- to 13-fold greater in non-White individuals, particularly among immigrants from high-risk areas. The rates exceed those for esophageal cancer and approach those for colorectal cancer. We have had a dramatic oversight in recognizing the risk for gastric carcinoma in underserved areas.
Gastric carcinoma is not a good diagnosis. The 5-year survival rate is 36%. Approximately 40% of cases are metastatic at the time of diagnosis; only about 15% are caught at a curable early stage.
These rates are in far contrast to Asia and areas that do programmatic screening, where the numbers are dramatically reduced. However, in the United States, we’re simply not there yet.
Endoscopic Evaluation
There are three phases of endoscopic evaluation for gastric premalignant conditions on which I’d like to focus: pre-endoscopy, intra-endoscopy, and post-endoscopy.
Pre-endoscopy — that is, before you even put in the endoscope — it’s important to assess patients’ potential risk for certain conditions, regardless of the reason for performing the endoscopy.
Gastric carcinoma is in the top eight leading causes of cancer death in the United States, with the risk being particularly high in Hispanics and Asians.
According to 2020 data, 40 million people living in the United States were born in another country, with over 70% coming from areas that have a high incidence of gastric carcinoma.
Accounting for the increased risk in these groups will become even more important. By 2065, it is projected that Asian and Hispanic individuals — the immigrant groups with the highest risk for gastric cancer — will comprise 70% of the US population. Therefore, we need to do a better job on pre-endoscopic assessment if we want to address this major cancer disparity in the United States.
However, it’s a potentially fixable problem because, in most cases, gastric carcinoma is preceded by a typical asymptomatic precancerous cascade known as the “Correa’s cascade.” It is analogous to what we see in Barrett’s esophagus or even in colorectal neoplasia as it evolves.
This histologic cascade is important to recognize in patients before they get to the stage of progression. Doing so is highly dependent on the assessment prior to the endoscopy.
You should also be thinking about high-risk groups before a pre-endoscopy evaluation.
During intra-endoscopy, it’s important to plan your actions while performing the procedure, even if you are not specifically screening for gastric carcinoma. We must be held accountable to established recommendations, standards, and best practices for accurately defining observations, determining appropriate actions, and effectively implementing screening protocols.
A high-quality evaluation of the gastric mucosa is critically important, as an estimated 5%-11% of neoplastic lesions are missed on upper endoscopy completed within 3 years of a gastric cancer diagnosis. With less-experienced endoscopists, the rate of missed neoplastic lesions may be as high as 25%.
A quality endoscopy begins (as it would in the colon) with mucosal cleaning. This may not be achieved by water alone and requires the use of mucolytic and defoaming agents, such as simethicone or N-acetylcysteine, which can be inordinately helpful.
Complete mucosal evaluation entails using insufflation to adequately distend the gastric folds.
Atrophic gastritis is observed in a high percentage of patients, and I’m always on the lookout for it when performing endoscopies. One way to identify atrophic gastritis is to look for the loss of gastric folds, which has an approximate sensitivity of 67% and specificity of 85%. The increased visibility of the submucosal venules is another sign of atrophic gastritis, although its sensitivity and specificity are relatively lower at 48% and 87%, respectively.
Full photo documentation of the gastric mucosa should be included. It is an important part of the intra-endoscopy procedure and also is mentioned as part of the recently published quality indicators for upper endoscopy from ACG/ASGE.
Recognizing which patients require a biopsy is important. Appropriate biopsy samples should be obtained in patients with recognizable abnormalities. We’re often looking for changes that may be quite subtle. Dysplasia and even adenocarcinoma in the stomach sometimes just cannot be appreciated based on pure endoscopic evaluation.
When it comes to the biopsy protocol, the recommendation is to follow the Sydney system, which was developed in the 1990s. Simply put, this protocol calls for the biopsy samples to be obtained and placed in two separate jars. You look at the greater and lesser curvature of the antrum and the incisura (jar one), and the greater and lesser curvature of the corpus (jar two) in any identified incidental focused biopsy. This is what we do in the colon and the esophagus.
You should do this after you appropriately clean the mucosa and distend the gastric lumen, ideally using CO2, to avoid the retention of gas.
Time is another important quality measure. This is similar to the colon, where we see that withdrawal times have increased from 6 minutes to 8-9 minutes. In the stomach, there were initial data about a minimum of 2-3 minutes. However, more recent data in the ACG guideline and the ACG/ASGE quality indicators indicate that detection rates increase after mucosal clearance and dissension when the gastric evaluation is 6-7 minutes. These are numbers we need to pay attention to. The ACG guideline notes that a 2- to 3-minute withdrawal time for upper endoscopy is substandard and not acceptable. If you’re still at that level, then you’re below the standard of care.
Again, consider time and photo documentation while examining four to six specific areas of both the greater and lesser curvature of the antrum and corpus, and the incisura angularis. It is ideal to use high-definition endoscopy along with the available virtual imaging enhancers. This is the basic requirement for achieving high-quality assessments.
When it comes to post-endoscopy, it’s essential to talk to your pathologist about using validated instruments of reporting what gastric precancerous lesions involve. There are two different systems: the Operative Link for Gastritis Assessment (OLGA) and the Operative Link on Gastric Intestinal Metaplasia (OLGIM). Although not routinely used in the United States, they are the best recommendations we have for validated histologic scoring systems.
Your pathologist needs to be up to speed on understanding how to report the extent of intestinal metaplasia (complete, incomplete, and mixed) and identifying the areas of focality or abnormalities. It defines what the subsequent surveillance recommendations should be.
Know What’s Required
Let’s review what’s required when it comes to diagnosing and managing gastric premalignant conditions.
First, pre-endoscopy must involve the assessment of risk. Before we put in the endoscope, it’s critical to know, regardless of why the patient is there, who needs to be assessed for gastric premalignant conditions.
Second, during the intra-endoscopy phase, don’t forget the assessments and documentation. If you’re assessing for gastric intestinal metaplasia, use the Sydney system.
Time is a big component. Withdrawal times are discussed much more frequently now, with the takeaway being that longer is better. Aiming for 6-7 minutes is best, according to recommendations from international endoscopy societies and quality measures from the United States. The ACG guideline says that withdrawal times of 2-3 minutes are not best practice and fall short of standard of care.
For post-endoscopy, talk to your pathologist to ensure better communication and understanding. Make sure that you have a quality gastrointestinal pathologist interpreting these lesions so patients can either enter surveillance or discontinue surveillance after potentially two exams negative for evidence of progressive change.
To do better, we need to understand these guidelines, which are all applicable now.
I’m Dr. David Johnson. Thanks for listening. See you next time.
David A. Johnson, MD, a regular contributor to Medscape, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease.
He has disclosed the relevant financial relationships: Advisor to ISOTHRIVE.
A version of this article first appeared on Medscape.com.
Hello. I’m Dr. David Johnson, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School and Old Dominion University in Norfolk, Virginia.
The American College of Gastroenterology (ACG) recently issued a clinical guideline on the diagnosis and management of gastric premalignant conditions.
Coincidentally, earlier this year, the ACG and the American Society for Gastrointestinal Endoscopy (ASGE) collaborated to issue recommendations on quality indicators for upper endoscopy.
In this overview, I’ll focus on gastric premalignant conditions while drawing upon some of the quality indicators from ACG/ASGE. Together, these publications highlight several things that we may be overlooking and clearly need to do better at addressing, given the emerging data in this area.
Gastric Premalignant Conditions: Increased Risk for Progression
Gastric premalignant conditions are common and include atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps.
The increased risk for progression to gastric adenocarcinoma in the United States is quite striking. It also reveals an important cancer disparity in certain high-risk groups.
The incidence rates of gastric cancer are two- to 13-fold greater in non-White individuals, particularly among immigrants from high-risk areas. The rates exceed those for esophageal cancer and approach those for colorectal cancer. We have had a dramatic oversight in recognizing the risk for gastric carcinoma in underserved areas.
Gastric carcinoma is not a good diagnosis. The 5-year survival rate is 36%. Approximately 40% of cases are metastatic at the time of diagnosis; only about 15% are caught at a curable early stage.
These rates are in far contrast to Asia and areas that do programmatic screening, where the numbers are dramatically reduced. However, in the United States, we’re simply not there yet.
Endoscopic Evaluation
There are three phases of endoscopic evaluation for gastric premalignant conditions on which I’d like to focus: pre-endoscopy, intra-endoscopy, and post-endoscopy.
Pre-endoscopy — that is, before you even put in the endoscope — it’s important to assess patients’ potential risk for certain conditions, regardless of the reason for performing the endoscopy.
Gastric carcinoma is in the top eight leading causes of cancer death in the United States, with the risk being particularly high in Hispanics and Asians.
According to 2020 data, 40 million people living in the United States were born in another country, with over 70% coming from areas that have a high incidence of gastric carcinoma.
Accounting for the increased risk in these groups will become even more important. By 2065, it is projected that Asian and Hispanic individuals — the immigrant groups with the highest risk for gastric cancer — will comprise 70% of the US population. Therefore, we need to do a better job on pre-endoscopic assessment if we want to address this major cancer disparity in the United States.
However, it’s a potentially fixable problem because, in most cases, gastric carcinoma is preceded by a typical asymptomatic precancerous cascade known as the “Correa’s cascade.” It is analogous to what we see in Barrett’s esophagus or even in colorectal neoplasia as it evolves.
This histologic cascade is important to recognize in patients before they get to the stage of progression. Doing so is highly dependent on the assessment prior to the endoscopy.
You should also be thinking about high-risk groups before a pre-endoscopy evaluation.
During intra-endoscopy, it’s important to plan your actions while performing the procedure, even if you are not specifically screening for gastric carcinoma. We must be held accountable to established recommendations, standards, and best practices for accurately defining observations, determining appropriate actions, and effectively implementing screening protocols.
A high-quality evaluation of the gastric mucosa is critically important, as an estimated 5%-11% of neoplastic lesions are missed on upper endoscopy completed within 3 years of a gastric cancer diagnosis. With less-experienced endoscopists, the rate of missed neoplastic lesions may be as high as 25%.
A quality endoscopy begins (as it would in the colon) with mucosal cleaning. This may not be achieved by water alone and requires the use of mucolytic and defoaming agents, such as simethicone or N-acetylcysteine, which can be inordinately helpful.
Complete mucosal evaluation entails using insufflation to adequately distend the gastric folds.
Atrophic gastritis is observed in a high percentage of patients, and I’m always on the lookout for it when performing endoscopies. One way to identify atrophic gastritis is to look for the loss of gastric folds, which has an approximate sensitivity of 67% and specificity of 85%. The increased visibility of the submucosal venules is another sign of atrophic gastritis, although its sensitivity and specificity are relatively lower at 48% and 87%, respectively.
Full photo documentation of the gastric mucosa should be included. It is an important part of the intra-endoscopy procedure and also is mentioned as part of the recently published quality indicators for upper endoscopy from ACG/ASGE.
Recognizing which patients require a biopsy is important. Appropriate biopsy samples should be obtained in patients with recognizable abnormalities. We’re often looking for changes that may be quite subtle. Dysplasia and even adenocarcinoma in the stomach sometimes just cannot be appreciated based on pure endoscopic evaluation.
When it comes to the biopsy protocol, the recommendation is to follow the Sydney system, which was developed in the 1990s. Simply put, this protocol calls for the biopsy samples to be obtained and placed in two separate jars. You look at the greater and lesser curvature of the antrum and the incisura (jar one), and the greater and lesser curvature of the corpus (jar two) in any identified incidental focused biopsy. This is what we do in the colon and the esophagus.
You should do this after you appropriately clean the mucosa and distend the gastric lumen, ideally using CO2, to avoid the retention of gas.
Time is another important quality measure. This is similar to the colon, where we see that withdrawal times have increased from 6 minutes to 8-9 minutes. In the stomach, there were initial data about a minimum of 2-3 minutes. However, more recent data in the ACG guideline and the ACG/ASGE quality indicators indicate that detection rates increase after mucosal clearance and dissension when the gastric evaluation is 6-7 minutes. These are numbers we need to pay attention to. The ACG guideline notes that a 2- to 3-minute withdrawal time for upper endoscopy is substandard and not acceptable. If you’re still at that level, then you’re below the standard of care.
Again, consider time and photo documentation while examining four to six specific areas of both the greater and lesser curvature of the antrum and corpus, and the incisura angularis. It is ideal to use high-definition endoscopy along with the available virtual imaging enhancers. This is the basic requirement for achieving high-quality assessments.
When it comes to post-endoscopy, it’s essential to talk to your pathologist about using validated instruments of reporting what gastric precancerous lesions involve. There are two different systems: the Operative Link for Gastritis Assessment (OLGA) and the Operative Link on Gastric Intestinal Metaplasia (OLGIM). Although not routinely used in the United States, they are the best recommendations we have for validated histologic scoring systems.
Your pathologist needs to be up to speed on understanding how to report the extent of intestinal metaplasia (complete, incomplete, and mixed) and identifying the areas of focality or abnormalities. It defines what the subsequent surveillance recommendations should be.
Know What’s Required
Let’s review what’s required when it comes to diagnosing and managing gastric premalignant conditions.
First, pre-endoscopy must involve the assessment of risk. Before we put in the endoscope, it’s critical to know, regardless of why the patient is there, who needs to be assessed for gastric premalignant conditions.
Second, during the intra-endoscopy phase, don’t forget the assessments and documentation. If you’re assessing for gastric intestinal metaplasia, use the Sydney system.
Time is a big component. Withdrawal times are discussed much more frequently now, with the takeaway being that longer is better. Aiming for 6-7 minutes is best, according to recommendations from international endoscopy societies and quality measures from the United States. The ACG guideline says that withdrawal times of 2-3 minutes are not best practice and fall short of standard of care.
For post-endoscopy, talk to your pathologist to ensure better communication and understanding. Make sure that you have a quality gastrointestinal pathologist interpreting these lesions so patients can either enter surveillance or discontinue surveillance after potentially two exams negative for evidence of progressive change.
To do better, we need to understand these guidelines, which are all applicable now.
I’m Dr. David Johnson. Thanks for listening. See you next time.
David A. Johnson, MD, a regular contributor to Medscape, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease.
He has disclosed the relevant financial relationships: Advisor to ISOTHRIVE.
A version of this article first appeared on Medscape.com.
Hello. I’m Dr. David Johnson, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School and Old Dominion University in Norfolk, Virginia.
The American College of Gastroenterology (ACG) recently issued a clinical guideline on the diagnosis and management of gastric premalignant conditions.
Coincidentally, earlier this year, the ACG and the American Society for Gastrointestinal Endoscopy (ASGE) collaborated to issue recommendations on quality indicators for upper endoscopy.
In this overview, I’ll focus on gastric premalignant conditions while drawing upon some of the quality indicators from ACG/ASGE. Together, these publications highlight several things that we may be overlooking and clearly need to do better at addressing, given the emerging data in this area.
Gastric Premalignant Conditions: Increased Risk for Progression
Gastric premalignant conditions are common and include atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps.
The increased risk for progression to gastric adenocarcinoma in the United States is quite striking. It also reveals an important cancer disparity in certain high-risk groups.
The incidence rates of gastric cancer are two- to 13-fold greater in non-White individuals, particularly among immigrants from high-risk areas. The rates exceed those for esophageal cancer and approach those for colorectal cancer. We have had a dramatic oversight in recognizing the risk for gastric carcinoma in underserved areas.
Gastric carcinoma is not a good diagnosis. The 5-year survival rate is 36%. Approximately 40% of cases are metastatic at the time of diagnosis; only about 15% are caught at a curable early stage.
These rates are in far contrast to Asia and areas that do programmatic screening, where the numbers are dramatically reduced. However, in the United States, we’re simply not there yet.
Endoscopic Evaluation
There are three phases of endoscopic evaluation for gastric premalignant conditions on which I’d like to focus: pre-endoscopy, intra-endoscopy, and post-endoscopy.
Pre-endoscopy — that is, before you even put in the endoscope — it’s important to assess patients’ potential risk for certain conditions, regardless of the reason for performing the endoscopy.
Gastric carcinoma is in the top eight leading causes of cancer death in the United States, with the risk being particularly high in Hispanics and Asians.
According to 2020 data, 40 million people living in the United States were born in another country, with over 70% coming from areas that have a high incidence of gastric carcinoma.
Accounting for the increased risk in these groups will become even more important. By 2065, it is projected that Asian and Hispanic individuals — the immigrant groups with the highest risk for gastric cancer — will comprise 70% of the US population. Therefore, we need to do a better job on pre-endoscopic assessment if we want to address this major cancer disparity in the United States.
However, it’s a potentially fixable problem because, in most cases, gastric carcinoma is preceded by a typical asymptomatic precancerous cascade known as the “Correa’s cascade.” It is analogous to what we see in Barrett’s esophagus or even in colorectal neoplasia as it evolves.
This histologic cascade is important to recognize in patients before they get to the stage of progression. Doing so is highly dependent on the assessment prior to the endoscopy.
You should also be thinking about high-risk groups before a pre-endoscopy evaluation.
During intra-endoscopy, it’s important to plan your actions while performing the procedure, even if you are not specifically screening for gastric carcinoma. We must be held accountable to established recommendations, standards, and best practices for accurately defining observations, determining appropriate actions, and effectively implementing screening protocols.
A high-quality evaluation of the gastric mucosa is critically important, as an estimated 5%-11% of neoplastic lesions are missed on upper endoscopy completed within 3 years of a gastric cancer diagnosis. With less-experienced endoscopists, the rate of missed neoplastic lesions may be as high as 25%.
A quality endoscopy begins (as it would in the colon) with mucosal cleaning. This may not be achieved by water alone and requires the use of mucolytic and defoaming agents, such as simethicone or N-acetylcysteine, which can be inordinately helpful.
Complete mucosal evaluation entails using insufflation to adequately distend the gastric folds.
Atrophic gastritis is observed in a high percentage of patients, and I’m always on the lookout for it when performing endoscopies. One way to identify atrophic gastritis is to look for the loss of gastric folds, which has an approximate sensitivity of 67% and specificity of 85%. The increased visibility of the submucosal venules is another sign of atrophic gastritis, although its sensitivity and specificity are relatively lower at 48% and 87%, respectively.
Full photo documentation of the gastric mucosa should be included. It is an important part of the intra-endoscopy procedure and also is mentioned as part of the recently published quality indicators for upper endoscopy from ACG/ASGE.
Recognizing which patients require a biopsy is important. Appropriate biopsy samples should be obtained in patients with recognizable abnormalities. We’re often looking for changes that may be quite subtle. Dysplasia and even adenocarcinoma in the stomach sometimes just cannot be appreciated based on pure endoscopic evaluation.
When it comes to the biopsy protocol, the recommendation is to follow the Sydney system, which was developed in the 1990s. Simply put, this protocol calls for the biopsy samples to be obtained and placed in two separate jars. You look at the greater and lesser curvature of the antrum and the incisura (jar one), and the greater and lesser curvature of the corpus (jar two) in any identified incidental focused biopsy. This is what we do in the colon and the esophagus.
You should do this after you appropriately clean the mucosa and distend the gastric lumen, ideally using CO2, to avoid the retention of gas.
Time is another important quality measure. This is similar to the colon, where we see that withdrawal times have increased from 6 minutes to 8-9 minutes. In the stomach, there were initial data about a minimum of 2-3 minutes. However, more recent data in the ACG guideline and the ACG/ASGE quality indicators indicate that detection rates increase after mucosal clearance and dissension when the gastric evaluation is 6-7 minutes. These are numbers we need to pay attention to. The ACG guideline notes that a 2- to 3-minute withdrawal time for upper endoscopy is substandard and not acceptable. If you’re still at that level, then you’re below the standard of care.
Again, consider time and photo documentation while examining four to six specific areas of both the greater and lesser curvature of the antrum and corpus, and the incisura angularis. It is ideal to use high-definition endoscopy along with the available virtual imaging enhancers. This is the basic requirement for achieving high-quality assessments.
When it comes to post-endoscopy, it’s essential to talk to your pathologist about using validated instruments of reporting what gastric precancerous lesions involve. There are two different systems: the Operative Link for Gastritis Assessment (OLGA) and the Operative Link on Gastric Intestinal Metaplasia (OLGIM). Although not routinely used in the United States, they are the best recommendations we have for validated histologic scoring systems.
Your pathologist needs to be up to speed on understanding how to report the extent of intestinal metaplasia (complete, incomplete, and mixed) and identifying the areas of focality or abnormalities. It defines what the subsequent surveillance recommendations should be.
Know What’s Required
Let’s review what’s required when it comes to diagnosing and managing gastric premalignant conditions.
First, pre-endoscopy must involve the assessment of risk. Before we put in the endoscope, it’s critical to know, regardless of why the patient is there, who needs to be assessed for gastric premalignant conditions.
Second, during the intra-endoscopy phase, don’t forget the assessments and documentation. If you’re assessing for gastric intestinal metaplasia, use the Sydney system.
Time is a big component. Withdrawal times are discussed much more frequently now, with the takeaway being that longer is better. Aiming for 6-7 minutes is best, according to recommendations from international endoscopy societies and quality measures from the United States. The ACG guideline says that withdrawal times of 2-3 minutes are not best practice and fall short of standard of care.
For post-endoscopy, talk to your pathologist to ensure better communication and understanding. Make sure that you have a quality gastrointestinal pathologist interpreting these lesions so patients can either enter surveillance or discontinue surveillance after potentially two exams negative for evidence of progressive change.
To do better, we need to understand these guidelines, which are all applicable now.
I’m Dr. David Johnson. Thanks for listening. See you next time.
David A. Johnson, MD, a regular contributor to Medscape, is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease.
He has disclosed the relevant financial relationships: Advisor to ISOTHRIVE.
A version of this article first appeared on Medscape.com.

