User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
NAMS affirms value of hormone therapy for menopausal women
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
FROM MENOPAUSE
Concerns that low LDL-C alters cognitive function challenged in novel analysis
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Is prostasin a clue to diabetes/cancer link?
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
FROM DIABETOLOGIA
Treatments explored to ease postviral symptoms of ME/CFS and long COVID
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
FROM IACFSME 2022
Social isolation, loneliness tied to death, MI, stroke: AHA
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Neuropathy drives hypoglycemia cluelessness in T1D
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.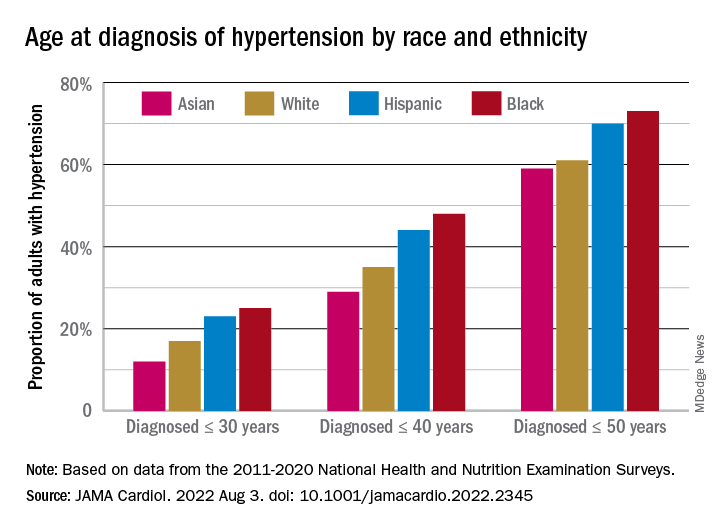
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Why exercise doesn’t help people with long COVID
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.










