User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.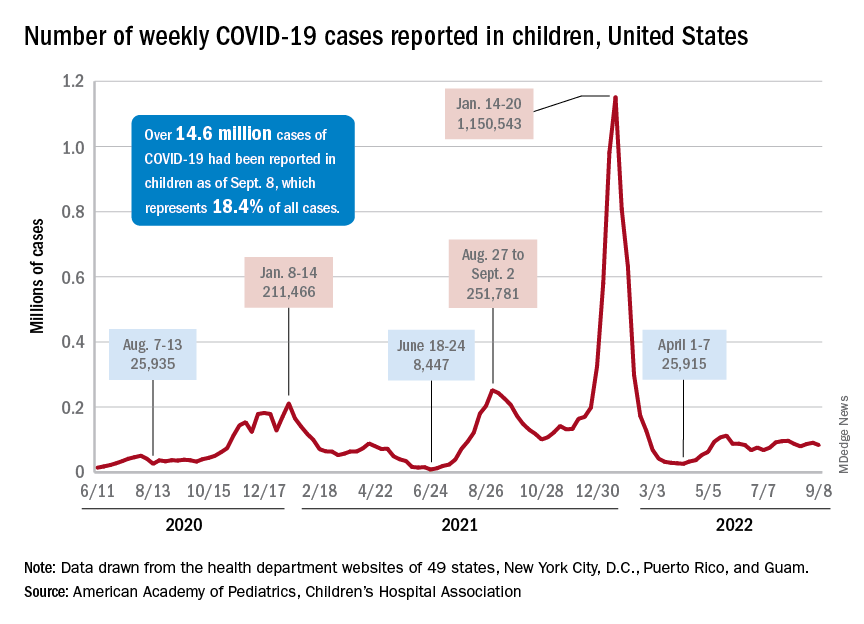
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
CDC warns of enterovirus strain linked to polio-like condition
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Lack of exercise linked to small heart, HFpEF
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Chronic lack of exercise – dubbed “exercise deficiency” – is associated with cardiac atrophy, reduced cardiac output and chamber size, and diminished cardiorespiratory fitness (CRF) in a subgroup of patients with heart failure with preserved ejection fraction (HFpEF), researchers say.
Increasing the physical activity levels of these sedentary individuals could be an effective preventive strategy, particularly for those who are younger and middle-aged, they suggest.
Thinking of HFpEF as an exercise deficiency syndrome leading to a small heart “flies in the face of decades of cardiovascular teaching, because traditionally, we’ve thought of heart failure as the big floppy heart,” Andre La Gerche, MBBS, PhD, of the Baker Heart and Diabetes Institute, Melbourne, told this news organization.
“While it is true that some people with HFpEF have thick, stiff hearts, we propose that another subset has a normal heart, except it’s small because it’s been underexercised,” he said.
The article, published online as part of a Focus Seminar series in the Journal of the American College of Cardiology, has “gone viral on social media,” Jason C. Kovacic, MBBS, PhD, of the Victor Chang Cardiac Research Institute, Darlinghurst, Australia, told this news organization.
Dr. Kovacic is a JACC section editor and the coordinating and senior author of the series, which covers other issues surrounding physical activity, both in athletes and the general public.
‘Coin-dropping moment’
To support their hypothesis that HFpEF is an exercise deficiency in certain patients, Dr. La Gerche and colleagues conducted a literature review that highlights the following points:
- There is a strong association between physical activity and both CRF and heart function.
- Exercise deficiency is a major risk factor for HFpEF in a subset of patients.
- Increasing physical activity is associated with greater cardiac mass, stroke volumes, cardiac output, and peak oxygen consumption.
- Physical inactivity leads to loss of heart muscle, reduced output and chamber size, and less ability to improve cardiac performance with exercise.
- Aging results in a smaller, stiffer heart; however, this effect is mitigated by regular exercise.
- Individuals who are sedentary throughout life cannot attenuate age-related reductions in heart size and have increasing chamber stiffness.
“When we explain it, it’s like a coin-dropping moment, because it’s actually a really simple concept,” Dr. La Gerche said. “A small heart has a small stroke volume. A patient with a small heart with a maximal stroke volume of 60 mL can generate a cardiac output of 9 L/min at a heart rate of 150 beats/min during exercise – an output that just isn’t enough. It’s like trying to drive a truck with a 50cc motorbike engine.”
“Plus,” Dr. La Gerche added, “exercise deficiency also sets the stage for comorbidities such as obesity, diabetes, and high blood pressure, all of which can ultimately lead to HFpEF.”
Considering HFpEF as an exercise deficiency syndrome has two clinical implications, Dr. La Gerche said. “First, it helps us understand the condition and diagnose more cases. For example, I think practitioners will start to recognize that breathlessness in some of their patients is associated with a small heart.”
“Second,” he said, “if it’s an exercise deficiency syndrome, the treatment is exercise. For most people, that means exercising regularly before the age of 60 to prevent HFpEF, because studies have found that after the age of 60, the heart is a bit fixed and harder to remodel. That doesn’t mean you shouldn’t try after 60 or that you won’t get benefit. But the real sweet spot is in middle age and younger.”
The bigger picture
The JACC Focus Seminar series starts with an article that underscores the benefits of regular physical activity. “The key is getting our patients to meet the guidelines: 150 to 300 minutes of moderate intensity exercise per week, or 75 to 250 minutes of vigorous activity per week,” Dr. Kovacic emphasized.
“Yes, we can give a statin to lower cholesterol. Yes, we can give a blood pressure medication to lower blood pressure. But when you prescribe exercise, you impact patients’ blood pressure, their cholesterol, their weight, their sense of well-being,” he said. “It cuts across so many different aspects of people’s lives that it’s important to underscore the value of exercise to everybody.”
That includes physicians, he affirmed. “It behooves all physicians to be leading by example. I would encourage those who are overweight or aren’t exercising as much as they should be to make the time to be healthy and to exercise. If you don’t, then bad health will force you to make the time to deal with bad health issues.”
Other articles in the series deal with the athlete’s heart. Christopher Semsarian, MBBS, PhD, MPH, University of Sydney, and colleagues discuss emerging data on hypertrophic cardiomyopathy and other genetic cardiovascular diseases, with the conclusion that it is probably okay for more athletes with these conditions to participate in recreational and competitive sports than was previously thought – another paradigm shift, according to Dr. Kovacic.
The final article addresses some of the challenges and controversies related to the athlete’s heart, including whether extreme exercise is associated with vulnerability to atrial fibrillation and other arrhythmias, and the impact of gender on the cardiac response to exercise, which can’t be determined now because of a paucity of data on women in sports.
Overall, Dr. Kovacic said, the series makes for “compelling” reading that should encourage readers to embark on their own studies to add to the data and support exercise prescription across the board.
No commercial funding or relevant conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Even mild COVID tied to vascular impairment
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
Hip fractures likely to double by 2050 as population ages
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
The annual incidence of hip fractures declined in most countries from 2005 to 2018, but this rate is projected to roughly double by 2050, according to a new study of 19 countries/regions.
The study by Chor-Wing Sing, PhD, and colleagues was presented at the annual meeting of the American Society of Bone and Mineral Research. The predicted increase in hip fractures is being driven by the aging population, with the population of those age 85 and older projected to increase 4.5-fold from 2010 to 2050, they note.
The researchers also estimate that from 2018 to 2050 the incidence of fractures will increase by 1.9-fold overall – more in men (2.4-fold) than in women (1.7-fold).
In addition, rates of use of osteoporosis drugs 1 year after a hip fracture were less than 50%, with less treatment in men. Men were also more likely than women to die within 1 year of a hip fracture.
The researchers conclude that “larger and more collaborative efforts among health care providers, policymakers, and patients are needed to prevent hip fractures and improve the treatment gap and post-fracture care, especially in men and the oldest old.”
Aging will fuel rise in hip fractures; more preventive treatment needed
“Even though there is a decreasing trend of hip fracture incidence in some countries, such a percentage decrease is insufficient to offset the percentage increase in the aging population,” senior co-author Ching-Lung Cheung, PhD, associate professor in the department of pharmacology and pharmacy at the University of Hong Kong, explained to this news organization.
The takeaways from the study are that “a greater effort on fracture prevention should be made to avoid the continuous increase in the number of hip fractures,” he said.
In addition, “although initiation of anti-osteoporosis medication after hip fracture is recommended in international guidelines, the 1-year treatment rate [was] well below 50% in most of the countries and regions studied. This indicates the treatment rate is far from optimal.”
“Our study also showed that the use of anti-osteoporosis medications following a hip fracture is lower in men than in women by 30% to 67%,” he said. “Thus, more attention should be paid to preventing and treating hip fractures in men.”
“The greater increase in the projected number of hip fractures in men than in women “could be [because] osteoporosis is commonly perceived as a ‘woman’s disease,’ ” he speculated.
Invited to comment, Juliet Compston, MD, who selected the study as one of the top clinical science highlight abstracts at the ASBMR meeting, agrees that “there is substantial room for improvement” in osteoporosis treatment rates following a hip fracture “in all the regions covered by the study.”
“In addition,” she continues, “the wide variations in treatment rates can provide important lessons about the most effective models of care for people who sustain a hip fracture: for example, fracture liaison services.”
Men suffer as osteoporosis perceived to be a ‘woman’s disease’
The even lower treatment rate in men than women is “concerning and likely reflects the mistaken perception that osteoporosis is predominantly a disease affecting women,” notes Dr. Compston, emeritus professor of bone medicine, University of Cambridge, United Kingdom.
Also invited to comment, Peter R. Ebeling, MD, outgoing president of the ASBMR, said that the projected doubling of hip fractures “is likely mainly due to aging of the population, with increasing lifespan for males in particular. However, increasing urbanization and decreasing weight-bearing exercise as a result are likely to also contribute in developing countries.”
“Unfortunately, despite the advances in treatments for osteoporosis over the last 25 years, osteoporosis treatment rates remain low, and osteoporosis remains undiagnosed in postmenopausal women and older men,” added Dr. Ebeling, from Monash University, Melbourne, who was not involved with the research.
“More targeted screening for osteoporosis would help,” he said, “as would treating patients for it following other minimal trauma fractures (vertebral, distal radius, and humerus, etc.), since if left untreated, about 50% of these patients will have hip fractures later in life.”
“Some countries may be doing better because they have health quality standards for hip fracture (for example, surgery within 24 hours, investigation, and treatment for osteoporosis). In other countries like Australia, bone density tests and treatment for osteoporosis are reimbursed, increasing their uptake.”
The public health implications of this study are “substantial” according to Dr. Compston. “People who have sustained a hip fracture are at high risk of subsequent fractures if untreated. There is a range of safe, cost-effective pharmacological therapies to reduce fracture rate, and wider use of these would have a major impact on the current and future burden imposed by hip fractures in the elderly population.”
Similarly, Dr. Ebeling noted that “prevention is important to save a huge health burden for patients and costs for society.”
“Patients with minimal trauma fractures (particularly hip or spinal fractures) should be investigated and treated for osteoporosis with care pathways established in the hospitals, reaching out to the community [fracture liaison services],” he said.
Support for these is being sought under Medicare in the United States, he noted, and bone densitometry reimbursement rates also need to be higher in the United States.
Projections for number of hip fractures to 2050
Previous international reviews of hip fractures have been based on heterogeneous data from more than 10 to 30 years ago, the researchers note.
They performed a retrospective cohort study using a common protocol across 19 countries/regions, as described in an article about the protocol published in BMJ Open.
They analyzed data from adults aged 50 and older who were hospitalized with a hip fracture to determine 1) the annual incidence of hip fractures in 2008-2015; 2) the uptake of drugs to treat osteoporosis at 1 year after a hip fracture; and 3) all-cause mortality at 1 year after a hip fracture.
In a second step, they estimated the number of hip fractures that would occur from 2030 to 2050, using World Bank population growth projections.
The data are from 20 health care databases from 19 countries/regions: Oceania (Australia, New Zealand), Asia (Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand), Northern Europe (Denmark, Finland, and U.K.), Western Europe (France, Germany, Italy, The Netherlands, and Spain), and North and South America (Canada, United States, and Brazil).
The population in Japan was under age 75. U.S. data are from two databases: Medicare (age ≥ 65) and Optum.
Most databases (13) covered 90%-100% of the national population, and the rest covered 5%-70% of the population.
From 2008 to 2015, the annual incidence of hip fractures declined in 11 countries/regions (Singapore, Denmark, Hong Kong, Taiwan, Finland, U.K., Italy, Spain, United States [Medicare], Canada, and New Zealand).
“One potential reason that some countries have seen relatively large declines in hip fractures is better osteoporosis management and post-fracture care,” said Dr. Sing in a press release issued by ASBMR. “Better fall-prevention programs and clearer guidelines for clinical care have likely made a difference.”
Hip fracture incidence increased in five countries (The Netherlands, South Korea, France, Germany, and Brazil) and was stable in four countries (Australia, Japan, Thailand, and United States [Optum]).
The United Kingdom had the highest rate of osteoporosis treatment at 1-year after a hip fracture (50.3%). Rates in the other countries/regions ranged from 11.5% to 37%.
Fewer men than women were receiving drugs for osteoporosis at 1 year (range 5.1% to 38.2% versus 15.0% to 54.7%).
From 2005 to 2018, rates of osteoporosis treatment at 1 year after a hip fracture declined in six countries, increased in four countries, and were stable in five countries.
All-cause mortality within 1 year of hip fracture was higher in men than in women (range 19.2% to 35.8% versus 12.1% to 25.4%).
“Among the studied countries and regions, the U.S. ranks fifth with the highest hip fracture incidence,” Dr. Cheung replied when specifically asked about this. “The risk of hip fracture is determined by multiple factors: for example, lifestyle, diet, genetics, as well as management of osteoporosis,” he noted.
“Denmark is the only country showing no projected increase, and it is because Denmark had a continuous and remarkable decrease in the incidence of hip fractures,” he added, which “can offset the number of hip fractures contributed by the population aging.”
The study was funded by Amgen. Dr. Sing and Dr. Cheung have reported no relevant financial relationships. One of the study authors is employed by Amgen.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Dermatoses often occur in people who wear face masks
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
Risk factors linked to post–COVID vaccination death identified
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
FROM JAMA NETWORK OPEN
FDA approves oral TYK2 inhibitor deucravacitinib for treating psoriasis
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.