User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Eat more dairy, less red meat to prevent type 2 diabetes
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
USPSTF recommends anxiety screening in adults younger than 65
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
Children with sickle cell anemia not getting treatments, screening
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
FROM THE MMWR
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.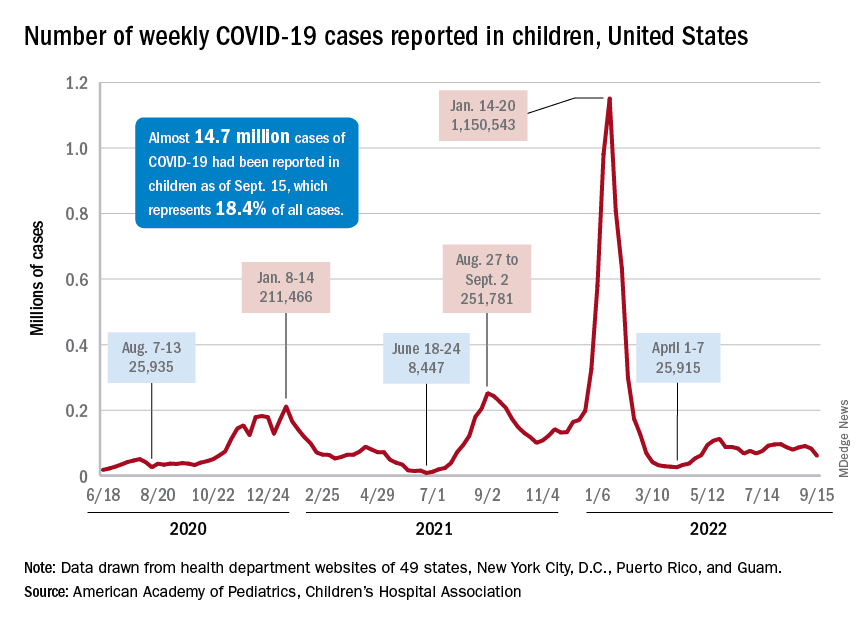
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
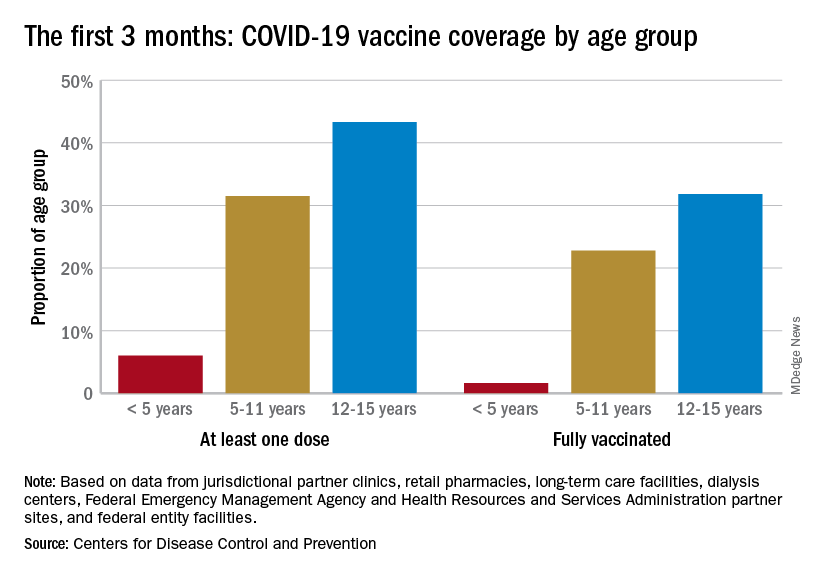
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
Eighty percent of U.S. maternal deaths are preventable: Study
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
More than 80% of U.S. maternal deaths across a 2-year period were due to preventable causes, according to a new CDC report.
Black mothers made up about a third of deaths, and more than 90% of deaths among Indigenous mothers were preventable.
“It’s significant. It’s staggering. It’s heartbreaking,” Allison Bryant, MD, a high-risk pregnancy specialist and senior medical director for health equity at Massachusetts General Hospital, told USA Today.
“It just means that we have so much work to do,” she said.
In the report, CDC researchers looked at pregnancy-related deaths between 2017 to 2019 based on numbers from maternal mortality review committees, which are multidisciplinary groups in 36 states that investigate the circumstances around maternal deaths.
Of the 1,018 deaths during the 2-year period, 839 occurred up to a year after delivery. About 22% of deaths happened during pregnancy, and 25% happened on the day of delivery or within a week after delivery. But 53% occurred more than 7 days after delivery.
Mental health conditions, such as overdoses and deaths by suicide, were the top underlying cause, followed by hemorrhage, or extreme bleeding. About a quarter of deaths were due to mental health conditions, followed by 14% due to hemorrhage and 13% due to heart problems. The rest were related to infection, embolism, cardiomyopathy, and high blood pressure-related disorders.
The analysis included a section on maternal deaths for American Indian and Alaska Native mothers, who are more than twice as likely as White mothers to die but are often undercounted in health data due to misclassification. More than 90% of their deaths were preventable between 2017 to 2019, with most due to mental health conditions and hemorrhage.
“It’s incredibly distressful,” Brian Thompson, MD, of the Oneida Nation and assistant professor of obstetrics and gynecology at Upstate Medical University, New York, told USA Today.
Dr. Thompson is working with the National Indian Health Board to create the first national tribal review committee for maternal deaths.
“It really needs to be looked at and examined why that is the case if essentially all of them are preventable,” he said.
Black mothers were also three times as likely as White mothers to die and more likely to die from heart problems. Hispanic mothers, who made up 14% of deaths, were more likely to die from mental health conditions.
Some of the deaths, such as hemorrhage, should be highly preventable. Existing toolkits for clinicians provide evidence-based guidelines to prevent and treat excessive bleeding.
“No pregnant person should be passing away from a hemorrhage,” Andrea Jackson, MD, division chief of obstetrics and gynecology at the University of California, San Francisco, told USA Today.
“We have the tools in the United States, and we know how to deal with it,” she said. “That was really disheartening to see.”
What’s more, the new CDC report highlights the need for more mental health resources during pregnancy and the postpartum period – up to a year or more after delivery – including improvements in access to care, diagnosis, and treatment.
“These are things that need to happen systemically,” LeThenia Baker, MD, an obstetrician and gynecologist at Wellstar Health, Georgia, told USA Today.
“It can’t just be a few practices here or there who are adopting best practices,” she said. “It has to be a systemic change.”
A version of this article first appeared on WebMD.com.
Ketamine linked to reduced suicidal thoughts, depression, anxiety
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a retrospective chart review analysis, which included more than 400 participants with TRD, illustrate that ketamine is a safe and rapid treatment in a real-world patient population, lead author Patrick A. Oliver, MD, founder and medical director, MindPeace Clinics, Richmond, Va., told this news organization.
The effect was perhaps most notable for reducing suicidal ideation, he said.
“In 2 weeks, we can take somebody from being suicidal to nonsuicidal. It’s a total game changer,” Dr. Oliver added.
Every year in the United States, about 12 million individuals think about suicide, 3.2 million make a plan to kill themselves, and more than 46,000 succeed, the investigators note.
The findings were published online in the Journal of Clinical Psychiatry.
Molecule mixture
Primarily used as an anesthetic in hospitals, ketamine is also taken illegally as a recreational drug. Users may aim for an intense high or feeling of dissociation, or an out-of-body–type experience.
Ketamine is a mixture of two mirror-image molecules. An intranasal version of one of these molecules (esketamine) is approved by the U.S. Food and Drug Administration for TRD. Both esketamine and ketamine are believed to increase neurotrophic signaling that affects synaptic function.
The study included 424 patients (mean age, 41.7 years) with major depressive disorder or another mood disorder and who received at least one ketamine infusion at a specialty clinic. Most participants had failed prior medication trials.
Patients in the study were typically started on 0.5 mg/kg of ketamine, with the dose titrated to achieve symptoms of partial dissociation. The median dose administered after titration was 0.93 mg/kg over 40 minutes.
The main treatment course of at least six infusions within 21 days was completed by 70% of the patients.
At each clinic visit, all participants completed the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7).
The primary outcome was PHQ-9 total scores, for which researchers looked at seven time periods: 1 week, 2-3 weeks, 4-6 weeks, 7-12 weeks, 13-24 weeks, 25-51 weeks, and 52+ weeks.
‘Blows it out of the water’
Results showed PHQ-9 total scores declined by 50% throughout the course of treatment, with much of the improvement gained within 4-6 weeks. There was a significant difference between week 1 and all later time periods (all P values < .001) and between weeks 2 and 3 and all later periods (all P values < .001).
Other measures included treatment response, defined as at least a 50% improvement on the PHQ-9, and depression remission, defined as a PHQ-9 score of less than 5. After three infusions, 14% of the patients responded and 7% were in remission. After 10 infusions, 72% responded and 38% were in remission.
These results compare favorably to other depression treatments, said Dr. Oliver. “Truthfully, with the exception of ECT [electroconvulsive therapy], this blows it all out of the water,” he added.
Dr. Oliver noted that the success rate for repetitive transcranial magnetic stimulation is 40%-60% depending on the modality; and for selective serotonin reuptake inhibitors, the success rate “is somewhere between the mid-20s and low-30s percent range.”
Another outcome measure was the self-harm/suicidal ideation item of the PHQ-9 questionnaire, which asks about “thoughts that you would be better off dead, or of hurting yourself in some way.” About 22% of the study participants no longer reported suicidal ideation after 3 infusions, 50% by 6 infusions, and 75% by 10 infusions.
By 15 infusions, 85% no longer reported these thoughts. “Nothing else has shown that, ever,” said Dr. Oliver.
Symptoms of generalized anxiety were also substantially improved. There was about a 30% reduction in the GAD-7 score during treatment and, again, most of the response occurred by 4-6 weeks.
Study limitations
Sex, age, and other demographic characteristics did not predict response or remission, but suicide planning trended toward higher response rates (P = .083). This suggests that a more depressed subgroup can achieve greater benefit from the treatment than can less symptomatic patients, the investigators note.
A history of psychosis also trended toward better response to treatment (P = .086) but not remission.
The researchers note that study limitations include that it was retrospective, lacked a control group, and did not require patients to be hospitalized – so the study sample may have been less severely ill than in other studies.
In addition, most patients paid out of pocket for the treatment at $495 per infusion, and they self-reported their symptoms.
As well, the researchers did not assess adverse events, although nurses made follow-up calls to patients. Dr. Oliver noted the most common side effects of ketamine are nausea, vomiting, and anxiety.
Previous research has suggested that ketamine therapy is not linked to long-term side effects, such as sexual dysfunction, weight gain, lethargy, or cognitive issues, said Dr. Oliver.
The investigators point out another study limitation was lack of detailed demographic information, such as race, income, and education, which might affect its generalizability.
Concerns and questions
Pouya Movahed Rad, MD, PhD, senior consultant and researcher in psychiatry, Lund (Sweden) University, noted several concerns, including that the clinics treating the study participants with ketamine profited from it.
He also speculated about who can afford the treatment because only a few patients in the study were reimbursed through insurance.
Dr. Movahed Rad was not involved with the current research but was principal investigator for a recent study that compared intravenous ketamine to ECT.
He questioned whether the patient population in the new study really was “real world.” Well-designed randomized controlled trials have been carried out in a “naturalistic setting, [which] get closer to real-life patients,” he said.
He also noted that the median dose after clinician titration (0.93 mg/kg over 40 minutes) “may be considered very high.”
With regard to doses being titrated to achieve symptoms of partial dissociation, “there is no obvious evidence to my knowledge that patients need to develop dissociative symptoms in order to have antidepressant effect,” said Dr. Movahed Rad.
Finally, he noted that the finding that 28% of the participants were using illegal drugs “is worrying” and wondered what drugs they were taking; he also questioned why 81% of the study population needed to take antidepressants.
The study did not receive outside funding. Dr. Oliver is the founder of MindPeace Clinics, which specialize in ketamine therapeutics. Dr. Movahed Rad has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL PSYCHIATRY
Is acetaminophen really safer than NSAIDs in heart disease?
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
‘Game changer’ semaglutide halves diabetes risk from obesity
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Treatment of people with obesity but without diabetes with the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy) – hailed at its approval in 2021 as a “game changer” for the treatment of obesity – led to beneficial changes in body mass index (BMI), glycemic control, and other clinical measures.
This collectively cut the calculated risk for possible future development of type 2 diabetes in study participants by more than half, based on post-hoc analysis of data from two pivotal trials that compared semaglutide with placebo.
The findings “suggest that semaglutide could help prevent type 2 diabetes in people with overweight or obesity,” said W. Timothy Garvey, MD, in a presentation at the annual meeting of the European Association for the Study of Diabetes.
Asked to comment, Rodolfo J. Galindo, MD, said: “We devote a significant amount of effort to treating people with diabetes but very little effort for diabetes prevention. We hope that further scientific findings showing the benefits of weight loss, as illustrated by [Dr.] Garvey [and colleagues], for diabetes prevention will change the pandemic of adiposity-based chronic disease.”
GLP-1 agonists as complication-reducing agents
Finding a link between treatment with semaglutide and a reduced future risk of developing type 2 diabetes is important because it shows that this regimen is not just a BMI-centric approach to treating people with obesity but is also a way to potentially reduce complications of obesity such as diabetes onset, explained Dr. Garvey, a professor and director of the Diabetes Research Center at the University of Alabama at Birmingham.
Recent obesity-management recommendations have focused on interventions aimed at avoiding complications, as in 2016 guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology, he noted.
Having evidence that treatment with a GLP-1 agonist such as semaglutide can reduce the incidence of diabetes in people with obesity might also help convince payers to more uniformly reimburse for this type of obesity intervention, which up to now has commonly faced coverage limitations, especially in the United States, he said in an interview.
Dr. Garvey added that evidence for a reduction in the incidence of cardiovascular disease complications such as myocardial infarction and stroke may need to join diabetes prevention as proven effects from obesity intervention before coverage decisions change.
He cited the SELECT trial, which is testing the hypothesis that semaglutide treatment of people with overweight or obesity can reduce the incidence of cardiovascular events in about 17,500 participants and with expected completion toward the end of 2023.
“A complication-centric approach to management of people with obesity needs prediction tools that allow a focus on prevention strategies for people with obesity who are at increased risk of developing diabetes,” commented Dr. Galindo, an endocrinologist at Emory University, Atlanta, in an interview.
Combined analysis of STEP 1 and STEP 4 data
The analysis conducted by Dr. Garvey and colleagues used data from the STEP 1 trial, which compared semaglutide 2.4 mg subcutaneous once weekly with placebo for weight loss in more than 1,500 people predominantly with obesity (about 6% were overweight) and showed that after 68 weeks semaglutide cut the calculated risk of developing type 2 diabetes over the subsequent 10 years from 18% at baseline to 7%, compared with a drop from 18% at baseline to 16% among those who received placebo.
A second, similar analysis of data from people predominantly with obesity in the STEP 4 trial – which treated around 800 people with semaglutide 2.4 mg for 20 weeks and then randomized them to placebo or continued semaglutide treatment – showed that semaglutide treatment cut their calculated 10-year risk for incident type 2 diabetes from 20% at baseline to about 11% after 20 weeks. The risk rebounded in the study participants who then switched from semaglutide to placebo. Among those randomized to remain on semaglutide for a total of 68 weeks, the 10-year risk fell further to 8%.
Dr. Garvey and associates used a validated prognostic formula, the cardiometabolic disease staging (CMDS) tool, they had previously developed and reported to calculate 10-year risk for development of type 2 diabetes based on three unmodifiable factors (age, sex, and race) and five modifiable factors (BMI, blood pressure, glucose level, HDL cholesterol, and triglycerides). They applied the analysis to data from 1,561 of the STEP 1 participants and 766 participants in the STEP 4 study.
“There is no better tool I know of to predict diabetes incidence,” commented Michael A. Nauck, MD, professor and chief of clinical research, diabetes division, St. Josef Hospital, Bochum, Germany.
In his opinion, the CMDS tool is appropriate for estimating the risk of developing incident type 2 diabetes in populations but not in specific individuals.
The new analyses also showed that, in STEP 1, the impact of semaglutide on reducing future risk of developing type 2 diabetes was roughly the same regardless of whether participants entered the study with prediabetes or were normoglycemic at entry.
Blood glucose changes confer the biggest effect
The biggest contributor among the five modifiable components of the CMDS tool for altering the predicted risk for incident diabetes was the reduction in blood glucose produced by semaglutide treatment, which influenced just under half of the change in predicted risk, Dr. Garvey said. The four other modifiable components had roughly similar individual effects on predicted risk, with change in BMI influencing about 15% of the observed effect.
“Our analysis shows that semaglutide treatment is preventing diabetes via several mechanisms. It’s not just a reduction in glucose,” Dr. Garvey said.
Dr. Nauck cautioned, however, that it is hard to judge the efficacy of an intervention like semaglutide for preventing incident diabetes when one of its effects is to dampen down hyperglycemia, the signal indicator of diabetes onset.
Indeed, semaglutide was first approved as a treatment for type 2 diabetes (known as Ozempic, Novo Nordisk) at slightly lower doses than it is approved for obesity. It is also available as an oral agent to treat diabetes (Rybelsus).
Dr. Nauck also noted that the results from at least one previously reported study had already shown the same relationship between treatment with the GLP-1 agonist liraglutide as an anti-obesity agent (3.0 mg dose daily, known as Saxenda) and a reduced subsequent incidence of type 2 diabetes but using actual clinical outcomes during 3 years of follow-up rather than a calculated projection of diabetes likelihood.
The SCALE Obesity and Prediabetes trial randomized 2,254 people with prediabetes and overweight or obesity to weekly treatment with 3.0 mg of liraglutide or placebo. After 160 weeks on treatment, the cumulative incidence of type 2 diabetes was 2% in those who received liraglutide and 6% among those on placebo, with a significant hazard ratio reduction of 79% in the incidence of diabetes on liraglutide treatment.
The STEP 1 and STEP 4 trials were sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Garvey has reported serving as an advisor without compensation to Novo Nordisk as well as Boehringer Ingelheim, Eli Lilly, Jazz, and Pfizer. He is also a site principal investigator for multicentered clinical trials sponsored by the University of Alabama at Birmingham and funded by Novo Nordisk, Eli Lilly, Epitomee, and Pfizer. Dr .Galindo has reported being a consultant or advisor for Boehringer Ingelheim, Eli Lilly, Pfizer, Sanofi, and Weight Watchers and receiving research funding from Dexcom, Eli Lilly, and Novo Nordisk. Dr. Nauck has reported being an advisor or consultant to Novo Nordisk as well as to Boehringer Ingelheim, Eli Lilly, Menarini/Berlin Chemie, MSD, Regor, and ShouTi/Gasherbrum, receiving research funding from MSD, being a member of a data monitoring and safety board for Inventiva, and being a speaker on behalf of Novo Nordisk as well as for Eli Lilly, Menarini/Berlin Chemie, MSD, and Sun Pharmaceuticals.
A version of this article first appeared on Medscape.com.
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
AAP guidance helps distinguish bleeding disorders from abuse
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
FROM PEDIATRICS