User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
No-implant interatrial shunt remains patent at a year
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
The first in-human trials of a no-implant approach to interatrial shunting to alleviate heart failure symptoms have shown a signal that the procedure reduces peak exercise wedge pressure in recipients a month afterward, according to early trial results.
Colin M. Barker, MD, reported 30-day results of 31 patients who had no-implant interatrial shunting for heart failure across three studies, at the Society for Cardiovascular Angiography & Interventions scientific sessions. The studies included patients with HF with preserved and reduced ejection fraction (HFpEF and HFrEF).
“At 30 days, there was a response with a decrease in the wedge pressures both at rest and at peak exercise, and that was consistent through all three of these initial trials,” Dr. Barker said. In all 33 patients who have been treated to date, there were no major adverse cardiac and cerebrovascular or thromboembolic events through 1 month. (Two of the patients weren’t included in the results Dr. Barker presented.)
The three studies he reported on were the Alleviate-HF-1 (n = 15), Alleviate-HF-2 (n = 11) for patients with HFpEF, and Alleviate-HFrEF (n = 5). The average patient age was 67 years, and all were New York Heart Association class II, III, or IV with elevated peak pulmonary capillary wedge pressure (PCWP).
The device that creates the no-implant shunt as “not very exotic, but it is very effective, and what it does is create a very predictable, reproducible atrial septostomy” between the left and right atria. The device obtains “almost a biopsy” that’s 7 mm in diameter. “There’s no hardware or foreign bodies left inside the patient,” said Dr. Barker, director of interventional cardiology at Vanderbilt University in Nashville, Tenn. “There’s a natural healing process at the rims after the radiofrequency ablation has been done.” Femoral access was used.
Study participants were also asked to complete the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and at 1 and 3 months across all three studies, and at 6 months in the Alleviate-HF-1 study. “Just as important is how patients feel,” Dr. Barker said. KCCQ overall summary scores increased at each time interval across all three studies.
“Durability has been proven with multiple different imaging modalities,” Dr. Barker added, explaining that CT scans in 10 of 10 shunts demonstrated patency through 12 months, and 15 of 15 at 6 months. He noted that none of the created shunts have closed yet. At 6 months, the average shunt measured 7.5 mm (± 1.1 mm, n = 22), left atrial diameter decreased 2.4 mm (P = .031) in HFpEF patients, and no significant changes were observed in right ventricular fractional area change or right atrial volume index.
None of the septostomies have had to be closed or enlarged to date, Dr. Barker said. “We are creating an atrial septal defect that we have a lot of comfort and experience with closing with other devices if need be, but that hasn’t been an issue,” he said. “As of now, it’s one size, but as you can imagine, one-size-fits-all is not the way this will go, and this does allow for variations in size ultimately.”
Kirk N. Garratt, MD, director of the Center for Heart and Vascular Health at Christiana Care in Newark, Del., noted that the approach to unload the left atrium “is novel, but I think is becoming well accepted in the advanced HF population. There remain questions about long-term consequences of an intentional interatrial shunt – what happens to pulmonary flow dynamics and the like – but to date the impact of this approach has been favorable.
“The liabilities that come with an implanted device in the septal space, both in terms of the durability of the shunt and the impact that it would have on the ability to perform other transseptal procedures, is overcome with this approach,” he added.
Dr. Barker disclosed he is an advisory board member and consultant to Alleviant Medical. Dr. Garratt is an advisory board member for Abbott.
FROM SCAI 2022
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Thrombolysis is safe in stroke patients on oral anticoagulants
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new observational study suggests, prompting researchers to ask whether guidelines that restrict its use should be updated.
Researchers found that DOAC users were significantly less likely to develop symptomatic intracerebral hemorrhage (sICH) after IVT, and there was no difference in functional independence at 3 months, compared with patients who received IVT but who did not receive DOAC.
“At the moment, the guidelines really pose a barrier and stop sign in front of the most important medical reperfusion therapy, which is thrombolysis,” said principal investigator Jan Purrucker, MD, professor of neurology at Heidelberg University Hospital.
“The main question we have to answer is, is IVT safe in patients with acute ischemic stroke who were pretreated with direct oral anticoagulants or not?”
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022, Lyon, France.
A ‘daily clinical problem’
As many as 20% of patients with atrial fibrillation experience ischemic stroke while receiving DOAC therapy. Reperfusion therapy with intravenous alteplase is considered standard of care for acute ischemic stroke, but current guidelines recommend against the use of IVT for patients who have recently received a DOAC, owing to safety concerns that researchers say are not backed by strong clinical evidence.
A recent study found no significant difference in sICH among patients who received IV alteplase for acute ischemic stroke within 7 days of receiving therapy with non–vitamin K antagonist oral anticoagulants.
“In our daily clinical practice, we face a lot of patients who have received oral anticoagulation, many with atrial fibrillation, but a lot of other indicators as well, and they suffer from ischemic stroke,” Dr. Purrucker said. “They usually are ineligible for medical reperfusion therapy because of quite strict guideline recommendations at the moment. This is a daily clinical problem.”
Dr. Purrucker and colleagues in New Zealand and Switzerland launched an international, observational, multicenter cohort study to examine the issue.
Researchers collected data on patients with ischemic stroke who had last received DOAC therapy 48 hours or less before the event or whose last intake was unknown and who had received IVT. They included 20,448 patients, 830 of whom were receiving DOAC therapy at the time of stroke onset.
Among the DOAC users, 30% received DOAC reversal prior to IVT, 27% had their DOAC level measured, and 42% received IVT without reversal treatment or knowledge of DOAC levels.
Overall, 4.5% of patients developed sICH. Compared with the control group, DOAC users were half as likely to develop sICH (adjusted odds ratio, 0.47; P = .003).
There was no significant difference between groups in independent outcome at 3 months, defined as a Modified Rankin Scale score of 1 to 3 (aOR, 1.21; 95% confidence interval, 0.99-1.49).
This finding held across patient subgroups, including patients for whom selection methods differed and patients with very recent intake of less than 12 hours.
“The question is whether we are so confident in these data that we would change our clinical practice now,” Dr. Purrucker said.
Infrastructure needed
While the findings are promising, more data are needed to strengthen the argument for revising current IVT guidelines, said Ho-Yan Yvonne Chun, PhD, honorary senior clinical lecturer with the Centre for Clinical Brain Sciences at the University of Edinburgh and a consultant in stroke medicine for NHS Lothian and Borders General Hospital, who commented on the findings.
“The study sample are a highly selected group of patients from selected centers that have the infrastructure to offer DOAC level checking and DOAC reversal,” Dr. Chun said. “The selected centers are not representative of the majority of hospitals that offer IVT to stroke patients with acute stroke.”
Most hospitals lack the equipment necessary to test DOAC levels and don’t have immediate access to DOAC reversal agents, Dr. Chun said. In those centers, she added, the administration of IVT could be delayed, which might affect clinical outcomes.
“Infrastructure needs to be in place to ensure timely delivery of IVT to these patients,” Dr. Chun added. “This means that in real-world practice, hospitals need to have right logistical pathway in place in order to provide timely DOAC level checking and DOAC reversal agents.”
Dr. Chun added that “large pragmatic clinical trials, preferably multicentered, are needed to provide the definitive evidence on the safety and effectiveness of using these approaches to select patients with prior DOAC use for IVT.”
But such a study may not be feasible, Dr. Purrucker said. Among the hurdles he noted are the large sample size needed for such a trial, uncertainty regarding funding, and patient selection bias, resulting from the fact that such studies would likely exclude patients eligible for mechanical thrombectomy or those eligible for reversal treatment.
In light of earlier studies, including preclinical data that support the safety of DOACs in IVT, and these new data, Dr. Purrucker said he hopes a change in guidelines might be taken up in the future.
“But it should be good academic practice to first let the results be externally evaluated, for example, during the manuscript submission process,” he said. “But once published, guideline working groups will have to evaluate the recent and new evidence and might reconsider previous recommendations.”
The study received no commercial funding. Dr. Purrucker and Dr. Chun reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESOC 2022
PA convicted of distributing more than 1.2 million opioid pills
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
A federal sting operation led to the recent conviction of a Texas physician assistant on charges of illegally prescribing a total of $3 million in drugs to patients at two “pill mill” clinics in Houston and helping others do the same.
The May 20 conviction of Charles Thompson, 76, of Houston, was based on charges of distributing more than 1.2 million opioid pills to thousands of individuals posing as patients at two pain management clinics, according to the U.S. Department of Justice.
Thompson’s conviction was the latest legal action in a string of cases involving the operation, including a doctor convicted in March who worked with Thompson at the West Parker Medical Clinic. Internist James Pierre, MD, 52, faces charges of unlawfully prescribing more than $1 million worth of opioid hydrocodone, according to federal officials.
Thompson also worked at Priority Wellness Clinic. Six people have pled guilty in connection with their conduct at West Parker or Priority Wellness, the justice department reported.
From June 2015 through July 2016, while Thompson was at West Parker, he helped Dr. Pierre unlawfully prescribe hydrocodone and the muscle relaxant carisoprodol, a combination of controlled substances for pain management known as the “Las Vegas cocktail,” to people in the sting operations pretending to be patients, authorities stated.
Thompson also distributed unlawful prescriptions for carisoprodol. So-called “runners” brought numerous people to pose as patients at West Parker and paid the clinic about $220 to $500 in cash for each visit that resulted in prescriptions for dangerous drugs. Throughout the scheme, West Parker pocketed about $1.75 million from prescriptions; Thompson was paid more than $208,000.
According to authorities, Thompson also helped others illegally prescribe controlled substances, including hydrocodone and oxycodone, from May to July 2017 at Priority Wellness, which opened in December 2016 after West Parker closed.
Priority Wellness reportedly operated as a pill mill similar to West Parker’s. Runners brought people posing as patients to Priority Wellness and paid the clinic between $300 and $600. The cost depended on whether the purported patient received a prescription for hydrocodone or oxycodone, almost always prescribed in combination with carisoprodol, authorities said. Throughout the scheme, Priority Wellness made about $1.1 million, and Thompson made between $700 and $900 a day.
He was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and seven counts of unlawfully distributing and dispensing controlled substances in connection with his conduct at West Parker. For his conduct at Priority Wellness, he was convicted of one count of conspiracy to unlawfully distribute and dispense controlled substances and one count of unlawfully distributing and dispensing controlled substances.
He faces up to 20 years in prison for each count of conviction with sentencing scheduled for Oct. 3.
A version of this article first appeared on Medscape.com.
Newly approved tirzepatide’s retail price announced
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Tirzepatide (Mounjaro) – the new twincretin approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes – was priced by Lilly, the company that will market the drug, at a list price of $974.33 for four weekly doses regardless of dose size, a cost that adds up to about $12,666 per year, according to a statement made on May 20 by a Lilly spokesperson.
This price puts tirzepatide, which combines the activity of two of the primary human incretins in one molecule, roughly in the same ballpark as what might be its main competitor, semaglutide (Ozempic) for type 2 diabetes, which retails at many U.S. pharmacies for about $925 for four weekly doses, or about $12,025 per year, although Ozempic’s posted retail price is about $100 higher for four doses.
According to the Lilly spokesperson, discount programs could reduce the monthly out-of-pocket cost for patients to as little as $25.
Tirzepatide, which received approval from the FDA on May 13, is a dual glucagonlike peptide–1 (GLP-1) receptor agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, which is indicated as Wegovy for weight loss in patients with obesity regardless of diabetes status.
A version of this article first appeared on Medscape.com.
Adsorbent offers promise for irritable bowel syndrome diarrhea
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
AT DDW 2022
Monkeypox quarantines not needed in U.S., Biden says
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
A psychiatric patient confesses to murder: Now what?
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
AT APA 2022
Fever after a tropical trip: A guide to differential diagnosis
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.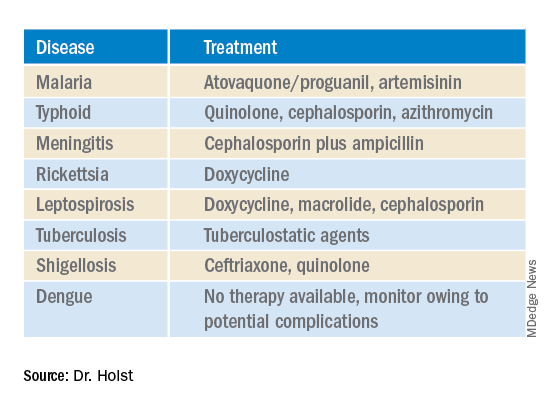
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.
After 2 years of a pandemic in which traveling was barely possible, tropical diseases are becoming important once more. At a 2022 conference for internal medicine specialists, tropical medicine specialist Fritz Holst, MD, of the Center for Tropical and Travel Medicine in Marburg, Germany, explained what questions you should be asking travelers with a fever at your practice and how to proceed with a suspected case.
The following article is based on the lecture: “Differential Diagnosis of Fever After a Trip to the Tropics,” which Dr. Holst gave at the 128th conference of the German Society of Internal Medicine.
A meta-analysis of studies concerning the topic, “returnee travelers from the tropics with fever,” was published in 2020. According to the analysis, purely tropical infections make up a third (33%) of fever diagnoses worldwide following an exotic trip. Malaria accounts for a fifth (22%), 5% are dengue fever, and 2.2% are typhoid (enteric fever).
In 26% of the returnee travelers investigated, nontropical infections were the cause of the fever. Acute gastroenteritis was responsible for 14%, and respiratory infections were responsible for 13%. In 18% of the cases, the cause of the fever remained unclear.
In Germany, the number of malaria cases has increased, said Dr. Holst. In Hessen, for example, there was recently a malaria fatality. “What we should do has been forgotten again,” he warned. More attention should also be paid once more to prophylaxis.
How to proceed
Dr. Holst described the following steps for treating recently returned travelers who are sick:
- Severely ill or not: If there are signs of a severe disease, such as dyspnea, signs of bleeding, hypotension, or central nervous system symptoms, the patient should be referred to a clinic. A diagnosis should be made within 1 day and treatment should be started.
- Transmissible or dangerous disease: This question should be quickly clarified to protect health care personnel, especially those treating patients. By using a thorough medical history (discussed below), a range of diseases may be clarified.
- Disease outbreak in destination country: Find out about possible disease outbreaks in the country that the traveler visited.
- Malaria? Immediate diagnostics: Malaria should always be excluded in patients at the practice on the same day by using a thick blood smear, even if no fever is present. If this is not possible because of time constraints, the affected person should be transferred directly to the clinic.
- Fever independent of the travel? Exclude other causes of the fever (for example, endocarditis).
- Involve tropical medicine specialists in a timely manner.
Nine mandatory questions
Dr. Holst also listed nine questions that clinicians should ask this patient population.
Where were you exactly?
Depending on the regional prevalence of tropical diseases, certain pathogens can be excluded quickly. Approximately 35% of travelers returning from Africa have malaria, whereas typhoid is much rarer. In contrast, typhoid and dengue fever are much more widespread in Southeast Asia. In Latin America, this is the case for both dengue fever and leptospirosis.
When did you travel?
By using the incubation time of the pathogen in question, as well as the time of return journey, you can determine which diseases are possible and which are not. In one patient who visited the practice 4 weeks after his return, dengue or typhoid were excluded.
Where did you stay overnight?
Whether in an unhygienic bed or under the stars, the question regarding how and where travelers stayed overnight provides important evidence of the following nocturnal vectors:
- Sandflies: Leishmaniasis
- Kissing bugs: Chagas disease
- Fleas: Spotted fever, bubonic plague
- Mosquitoes: Malaria, dengue, filariasis
What did you eat?
Many infections can be attributed to careless eating. For example, when eating fish, crabs, crawfish, or frogs, especially if raw, liver fluke, lung fluke, or ciguatera should be considered. Mussel toxins have been found on the coast of Kenya and even in the south of France. In North African countries, you should be cautious when eating nonpasteurized milk products (for example, camel milk). They can transmit the pathogens for brucellosis and tuberculosis. In beef or pork that has not been cooked thoroughly, there is the risk of trichinosis or of a tapeworm. Even vegetarians need to be careful. Infections with the common liver fluke are possible after eating watercress.
What have you been doing?
You can only get some diseases through certain activities, said Dr. Holst. If long-distance travelers tell you about the following excursions, prick up your ears:
- Freshwater contact: Schistosomiasis, leptospirosis
- Caving: Histoplasmosis, rabies
- Excavations: Anthrax, coccidioidomycosis
- Camel tour: MERS coronavirus (Do not mount a sniffling camel!)
- Walking around barefoot: Strongyloides, hookworm
Was there contact with animals?
Because of the risk of rabies following contact with cats or biting apes, Dr. Holst advised long-distance travelers to get vaccinated.
Were there new sexual partners?
In the event of new sexual contacts, tests for hepatitis A, B, C, and HIV should be performed.
Are you undergoing medical treatment?
The patient may already be under medical supervision because of having a disease.
What prophylactic measures did you take before traveling?
To progress in the differential diagnosis, questions should also be asked regarding prophylactic measures. Vaccination against hepatitis A provides very efficient infection protection, whereas vaccines against typhoid offer a much lower level of protection.
Diagnostic tests
As long as there are no abnormalities, such as meningism or heart murmurs, further diagnostics include routine infectiologic laboratory investigations (C-reactive protein, blood count, etc), blood culture (aerobic, anaerobic), a urine dipstick test, and rapid tests for malaria and dengue.
To exclude malaria, a thick blood smear should always be performed on the same day, said Dr. Holst. “The rapid test is occasionally negative. But you often only detect tertian malaria in the thick blood smear. And you have to repeat the diagnostics the following day.” For this, it is important to know that a single test result does not exclude malaria right away. In contrast, detecting malaria antibodies is obsolete. Depending on the result, further tests include serologies, antigen investigations, and polymerase chain reaction.
Treat early
A complete set of results is not always available promptly. Experts recommend that, “if you already have a hunch, then start the therapy, even without a definite diagnosis.” This applies in particular for the suspected diagnoses in the following table.
This article was translated from Coliquio. A version of this article appeared on Medscape.com.





