User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
MIS-C cardiac evaluation requires more than EF
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
More U.S. states cap insulin cost, but activists will ‘fight harder’
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.
Twelve U.S. states have now passed laws aimed at making insulin more affordable – and more than 30 are considering such legislation – but they all have gaps that still put the cost of this basic and essential medication out of reach for many with diabetes.
The laws only apply to health insurance through state-regulated plans, and not to the majority of health plans that cover most Americans: Medicare, Medicaid, the Veterans Affairs health system, or self-funded employer-sponsored plans.
Overall, Hannah Crabtree, an activist who writes the blog Data for Insulin, estimates state laws that limit copays, deductibles, or other out-of-pocket costs for insulin cover an average of 27% of people with diabetes across the United States.
And while diabetes activists have applauded state actions, most want more help for the under- and uninsured.
“Our chapter will be fighting harder next legislative session for the uninsured,” said Mindie Hooley, the leader of the Utah #insulin4all chapter, which successfully lobbied legislators to pass a bill signed by the state’s governor on March 30.
“With so many losing their jobs because of the pandemic, there’s no better time than now to fight for these patients who don’t have insurance,” Ms. Hooley said in an interview.
The American Diabetes Association has also been lobbying for state caps as one of many avenues for making insulin more affordable, said Stephen Habbe, the ADA’s director for state government affairs.
One in four insulin users report rationing the medication, Mr. Habbe said.
The state laws “can really provide important relief in terms of affordability for their insulin costs, which we know can be critical in terms of preserving their life and helping to prevent complications that can potentially be disabling or even deadly,” he said in an interview.
Activists with T1 International, which created the #insulin4all campaign, are working nationwide to convince state legislators to back measures that limit out-of-pocket costs for insulin, or for other diabetes medications and supplies.
Colorado, Connecticut, Delaware, Illinois, Maine, New Hampshire, New Mexico, New York, Utah, Virginia, Washington, and West Virginia have enacted such limits, with caps ranging from $25 to $100.
Insulin makers unfazed, blame insurers, PBMs for high prices
The three insulin manufacturers in the United States – Eli Lilly, Novo Nordisk, and Sanofi– have not overtly fought against the laws, although in July, the Pharmaceutical Research and Manufacturers of America did sue to block a related Minnesota law that provides a free emergency supply of insulin.
And the nonprofit news organization FairWarning reported in August that a lobbyist from Eli Lilly had attempted to push a Tennessee legislator to keep the uninsured from being eligible for any out-of-pocket limits.
The insulin makers have also not lowered prices in response to the mounting number of state laws.
They see no need, said Tara O’Neill Hayes, director of human welfare policy at the American Action Forum, a center right–leaning Washington, D.C., think tank.
“You’re going to do what you can get away with,” Ms. O’Neill Hayes said in an interview. “To the extent that they can keep their prices high and people are still buying, they have limited incentives to lower those costs.”
The insulin market is dysfunctional, she added. “The increasing cost of insulin seems primarily to be the result of a lack of competition in the market and convoluted drug pricing and insurance practices,” Ms. O’Neill Hayes and colleagues wrote in a report in April on federal and state attempts to address insulin affordability.
Novo Nordisk, however, maintains that drugmakers are not solely to blame.
“Everyone in the health care system has a role to play in affordability,” said Ken Inchausti, Novo Nordisk’s senior director for corporate communications. State legislation “attempts to address a systemic issue in [U.S.] health care: How benefit design can make medicines unaffordable for many, especially for those in high-deductible health plans,” he said in an interview.
“Efforts to place copay caps on insurance plans covering insulin can certainly help lower out-of-pocket costs,” said Mr. Inchausti.
Sanofi spokesperson Jon Florio said the company supports actions that increase affordable access to insulin. However, “while we support capped copays, we feel this should not be limited to just one class of medicines,” he said. Mr. Florio also noted that Sanofi provides out-of-pocket caps to anyone with commercial insurance and that anyone without insurance can buy one or multiple Sanofi insulins for a fixed price of $99 per month, up to 10 boxes of pens and/or 10-mL vials.
And Sanofi will take part in the Centers for Medicare & Medicaid Services’ new insulin demonstration program. Starting in 2021, CMS will cap insulin copays at $35 for people in Part D plans that participate.
Eli Lilly spokesperson Brad Jacklin said the company “believes in the common goal of ensuring affordable access to insulin and other life-saving medicines because nobody should have to forgo or ration because of cost.”
Lilly supports efforts “that more directly affect patients’ cost-sharing based on their health care coverage,” he said. Insurers and pharmacy benefit managers (PBMs) should pass savings on to patients, Mr. Jacklin urged. Lilly caps some insulins at $35 for the uninsured or commercially insured. The company will also participate in the CMS program.
Meanwhile, a PhRMA-sponsored website www.letstalkaboutcost.org said that, because they do not share savings, insurers and PBMs are responsible for high insulin costs.
Manufacturer assistance programs for patients with diabetes and other chronic diseases, on the other hand, can save individuals $300-$500 a year, PhRMA said in August.
PBMs point back at insulin manufacturers
PBMs, however, point back at drug companies. “PBMs have been able to moderate insulin costs for most consumers with insurance,” said J.C. Scott, president and CEO of the Pharmaceutical Care Management Association, the PBM trade group, in a statement.
The rising cost of insulin is caused by a lack of competition and overuse of patent extensions, PCMA maintains.
Health insurers, which, in tandem with PBMs, give insulins formulary preference based on a discounted price, are most likely to feel the impact of laws limiting out-of-pocket costs.
If they have to make up the shortfall from a patient’s reduced payment for a prescription, they will likely raise premiums, said Ms. O’Neill Hayes.
And if patients pay the same price for insulin – regardless of who makes it – drugmakers won’t have much incentive to offer discounts or rebates for formulary placement, she said. Again, that would likely lead to higher premiums.
David Allen, a spokesperson for America’s Health Insurance Plans, said in an interview that AHIP believes lack of competition has driven up insulin prices.
“High prices for insulin correspond with high health insurance costs for insulin,” he said. When CMS starts requiring drugmakers to discount their insulins for Medicare that will allow “health plans to use those savings to reduce out-of-pocket [costs] for seniors.”
He did not respond to a question as to why health insurers were not already passing savings on to commercially insured patients, especially in states with out-of-pocket limits.
Mr. Allen did say that AHIP’s plans “stand ready to work with state policymakers to remove barriers to lower insulin prices for Americans.”
Utah savings hopefully saving lives already
In Utah, legislators tuned out the blame game, and instead were keen to listen to patients, who had many stories about how the high cost of insulin had hurt them, said Ms. Hooley.
She noted an estimated 50,000 Utahans rely on insulin to stay alive.
Ms. Hooley and her chapter convinced legislators to pass a bill that gives insurers the option to cap patient copays at $30 per month, or to put insulin on its lowest formulary tier and waive any patient deductible. That aspect of the law does not go into effect until January 2021, but insurers are already starting to move insulin to the lowest formulary tier.
That has helped some people immediately. One state resident said her most recent insulin prescription cost $7 – instead of the usual $200.
The uninsured are not left totally high and dry either. Starting June 1, anyone in the state could buy through a state bulk-purchasing program, which guaranteed a 60% discount.
Ms. Hooley said she’d recently heard about a patient who usually spent $300 per prescription but was able to buy insulin for $100 through the program.
“Although $100 is still too much, it is nice knowing the Utah Insulin Savings Program is saving lives,” Ms. Hooley concluded.
A version of this article originally appeared on Medscape.com.
Early evolocumab quickly lowers LDL cholesterol after primary PCI
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
FROM ESC CONGRESS 2020
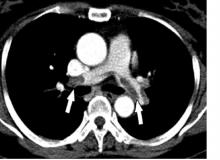
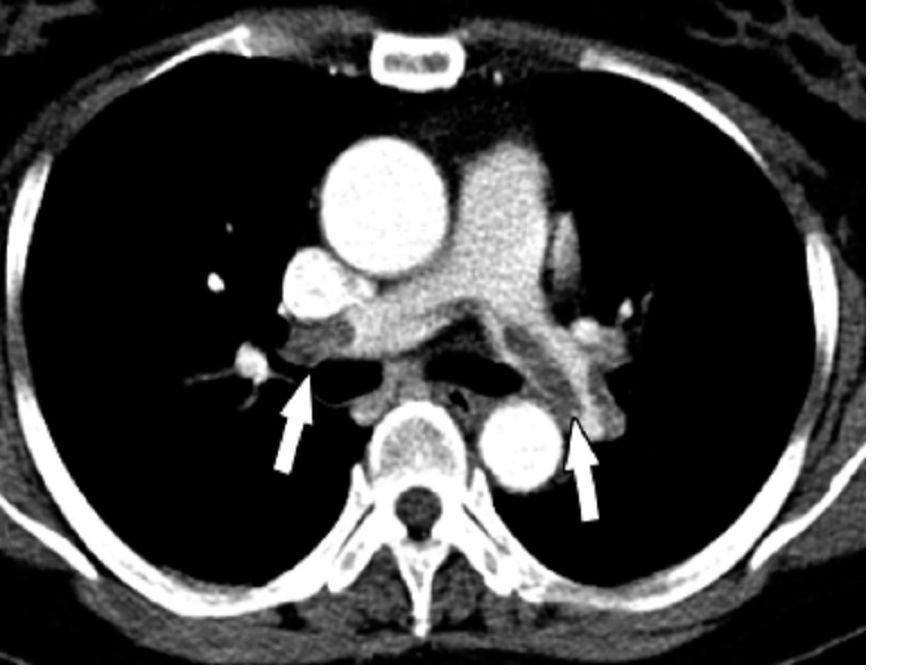
LoDoCo2: Added steam for colchicine as secondary prevention
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
Statins linked to reduced mortality in COVID-19
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
REALITY trial supports restrictive transfusion in anemic MI
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM ESC CONGRESS 2020
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Obesity boosts risks in COVID-19 from diagnosis to death
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
FROM OBESITY REVIEWS
HOME-PE trial clarifies which pulmonary embolism patients to treat at home
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
REPORTING FROM ESC CONGRESS 2020
Evolocumab safe and effective in pediatric FH
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.
The PCSK9 monoclonal antibody evolocumab (Repatha) was well tolerated and effectively lowered LDL cholesterol by 38% compared with placebo in a randomized controlled trial in pediatric patients with heterozygous familial hypercholesterolemia (FH) already taking statins with or without ezetimibe.
“HAUSER-RCT is the largest study and the first placebo-controlled randomized trial of a PCSK9 inhibitor in pediatric FH,” senior author Daniel Gaudet, MD, PhD, Universite de Montreal, said in an interview.
“The study showed good safety and efficacy of the drug in this population, with an excellent 44% reduction in LDL cholesterol compared with 6% in the placebo group.”
The trial also found evolocumab to be well tolerated in this group, with adverse effects similar in the active and placebo groups.
“Some people have wondered about using a drug with a monthly injection in a pediatric population, but this was not an issue in our study,” Dr. Gaudet said. “The idea of a monthly injection was well received, and no patient withdrew because of this.”
The HAUSER-RCT trial was presented on Aug. 29 at the virtual annual congress of the European Society of Cardiology (ESC Congress 2020) and simultaneously published online in the New England Journal of Medicine.
“With patients recruited from 23 countries in five continents, the study provides an accurate picture of the safety and efficacy of evolocumab in pediatric FH patients worldwide,” Dr. Gaudet said.
The 24-week, randomized, double-blind, placebo-controlled trial involved 157 patients aged 10-17 years with heterozygous FH already taking statins with or without ezetimibe and who had an LDL cholesterol level of 130 mg/dL or more and a triglyceride level of 400 mg/dL or less.
They were randomly assigned in a 2:1 ratio to receive monthly subcutaneous injections of evolocumab (420 mg) or placebo.
Results showed that at week 24, the mean percentage change from baseline in LDL cholesterol level was −44.5% in the evolocumab group and −6.2% in the placebo group, giving a difference of −38.3 percentage points (P < .001).
The absolute change in the LDL cholesterol level was −77.5 mg/dL in the evolocumab group and −9.0 mg/dL in the placebo group, giving a difference of −68.6 mg/dL (P < .001).
Results for all secondary lipid variables were significantly better with evolocumab than with placebo. The incidence of adverse events that occurred during the treatment period was similar in the evolocumab and placebo groups. Laboratory abnormalities did not differ between groups.
Dr. Gaudet noted that FH is the most common genetic disease worldwide, affecting 1 in 250 people. “It is very treatable, so it is important to identify these patients, but it is massively underdiagnosed, with only around 15%-20% of patients with the condition having been identified,” he said.
“The vast majority of pediatric FH patients can reach target LDL levels with statins and ezetimibe, but there are 5%-10% of patients who may need additional therapy. We have now shown that evolocumab is safe and effective for these patients and can be used to fill this gap,” Dr. Gaudet said. “We can now say that we can cover all situations in treating FH whatever the severity of the disease.”
However, the challenge remains to improve the diagnosis of FH. “If there is one person with FH in a family, then it is essential that the whole extended family is tested. Our toolbox for treating this condition is now sufficiently effective, so there is no reason not to diagnose this disease,” Dr. Gaudet stressed.
The HAUSER-RCT study was supported by Amgen. Gaudet reports grants and personal fees from Amgen during the conduct of the study.
A version of this article originally appeared on Medscape.com.