User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
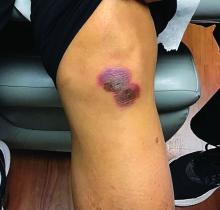
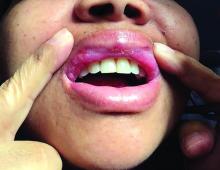
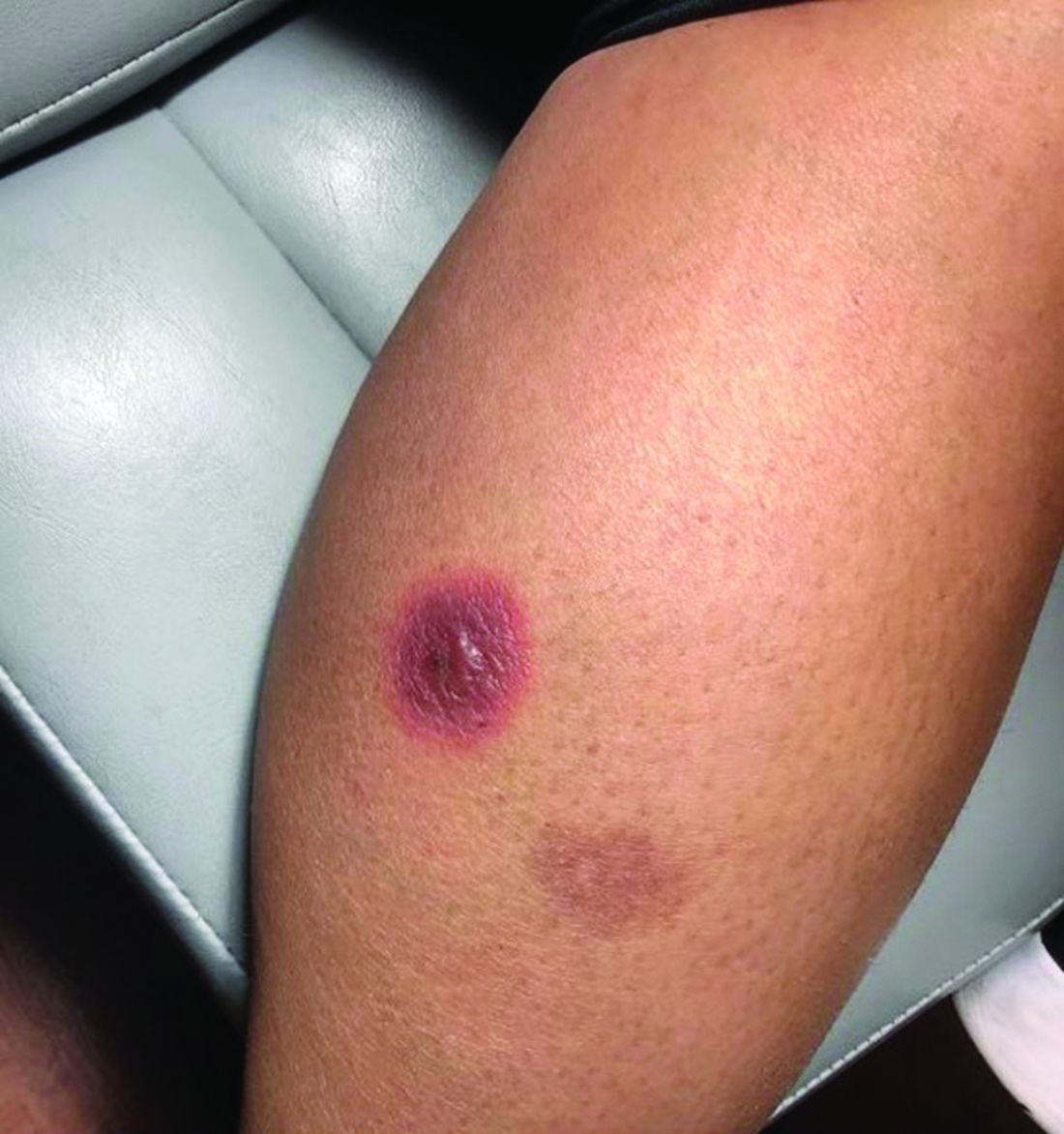
A 42-year-old woman presented with a few days of erosions on her buccal mucosa, tongue, and soft palate
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
in which lesions present in the same location upon repeated intake of the offending drug. The lesions typically present within 30 minutes to 8 hours of administration of the drug. These reactions can be considered allergic or pseudo-allergic, in which case, there is no notable adaptive immune response. CD8+ T cells appear to play a role in the epidermal injury via release of interferons and interactions with other inflammatory cells.
There are numerous drugs that can precipitate these findings. NSAIDs; antibiotics, such as tetracyclines, sulfonamides; and phenytoin are common offenders. In the case of our patient, naproxen was the offending medication.
The classic presentation of FDE features annular, erythematous to violaceous macules on the skin or mucosa that can be asymptomatic or can produce burning, pain, or pruritus. The most common locations include the trunk and extremities, but the palms, soles, face, scalp, and mucosa can also be impacted. The oral mucosa seems to be the most common mucosal location. Intravenous administration of a drug is associated with more severe symptoms. Systemic symptoms are typically absent, and the eruption may initially be in one location, but may appear elsewhere upon repeated exposure to the offending medication.
The differential diagnosis includes arthropod bite reactions, urticaria, and erythema multiforme. Although FDEs are typically a clinical diagnosis, the histopathology will commonly show a vacuolar interface dermatitis. Furthermore, a variety of immune cells can be found, including neutrophilic, eosinophilic, and lymphocytic infiltrate. A combination of two or more histological patterns often favors the diagnosis of FDE.
Steroid creams can be prescribed to decrease the inflammatory reaction and improve symptoms; however, the definitive treatment of this condition is cessation of the offending agent. Postinflammatory hyperpigmentation is a common symptom after resolution of the condition, and it may take months to fade away. Further darkening can be prevented by practicing sun safety measures such as wearing sunblock, covering the affected areas, and avoiding prolonged sun exposure.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Igor Chaplik, DO, Aesthetix Dermatology, Fort Lauderdale. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Shaker G et al. Cureus. 2022 Aug 23;14(8):e28299.
Srivastava R et al. Indian J Dent. 2015 Apr-Jun;6(2):103-6.
Weyers W, Metze D. Dermatol Pract Concept. 2011 Jan 31;1(1):33-47.
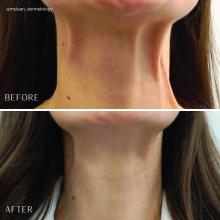
Treatment of the neck and lower face with botulinum toxin
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
Patch testing finds higher prevalence of ACD among children with AD
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Anti-acid meds lower strength of systemic sclerosis drug
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Anti-acid drugs used by patients with systemic sclerosis reduce the bioavailability of mycophenolate mofetil (MMF).
METHODOLOGY:
- Researchers conducted an open-label, pragmatic crossover study of 20 patients (all female) with systemic sclerosis at a single center who were on a stable MMF dose (1.5-2 g/day) for the last 3 months or more.
- Participants sequentially took MMF alone for 1 month, then with the H2 receptor blocker (HRB) ranitidine 300 mg/day in the second month, then with the proton pump inhibitor (PPI) esomeprazole 40 mg/day in the third month.
- Researchers measured the bioavailability of MMF in the patients during treatment with ranitidine or esomeprazole and the impact of the drugs on the total GI score of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 instrument.
- Patients were excluded if they were receiving co-prescription of cholestyramine, magnesium- or aluminum-containing antacids, and rifampicin; taking prednisolone-equivalent dose > 5 mg/day; taking MMF plus a PPI or an HRB at baseline; living with chronic kidney disease with a glomerular filtration rate < 30 mL/min; positive for HIV, HCV, or HBV; or living with end-stage lung disease or gastroduodenal ulcers.
TAKEAWAY:
- Mean estimated 12-hour area under curve levels of mycophenolic acid dropped by 32.7% (mean difference = 22.28 mcg h mL–1) when patients added esomeprazole, and they dipped by 21.97% (mean difference = 14.93 mcg h mL–1) when they added ranitidine vs. MMF alone.
- The pharmacokinetic parameter T-max did not differ significantly between MMF alone vs. MMF plus ranitidine but was significantly different with esomeprazole. C-max significantly declined with administration of ranitidine or esomeprazole vs. MMF alone.
- Total GI scores dipped when patients added esomeprazole or ranitidine.
IN PRACTICE:
In patients with significant gastroesophageal reflux disease symptoms who need to take MMF, management options may include monitoring MMF drug levels, switching to enteric-coated mycophenolate sodium, and spacing doses with anti-acid drugs.
SOURCE:
Glaxon Alex, MD, and colleagues from the Center for Arthritis and Rheumatism Excellence in Kochi, India, conducted the study, which was published online in Seminars in Arthritis & Rheumatism.
LIMITATIONS:
The sample size is small, and the optimum dose of MMF is unknown.
DISCLOSURES:
The study had no outside funding. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Every click you make, the EHR is watching you
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.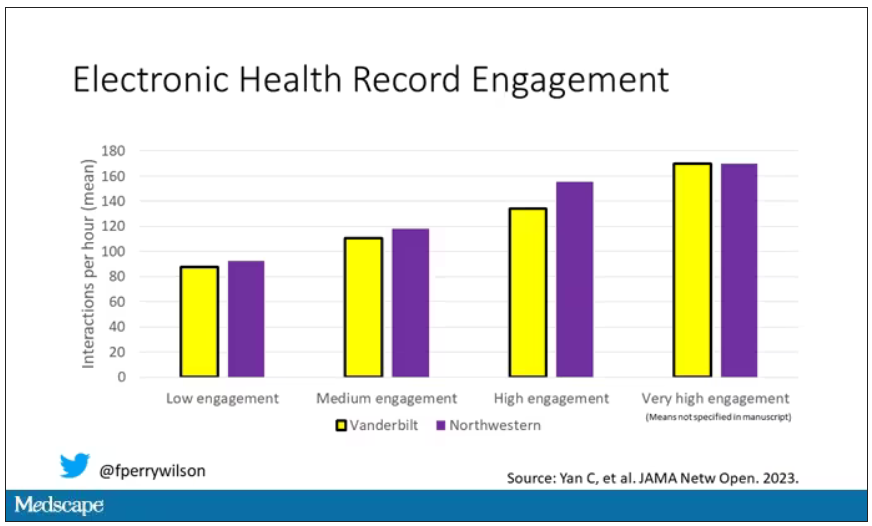
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.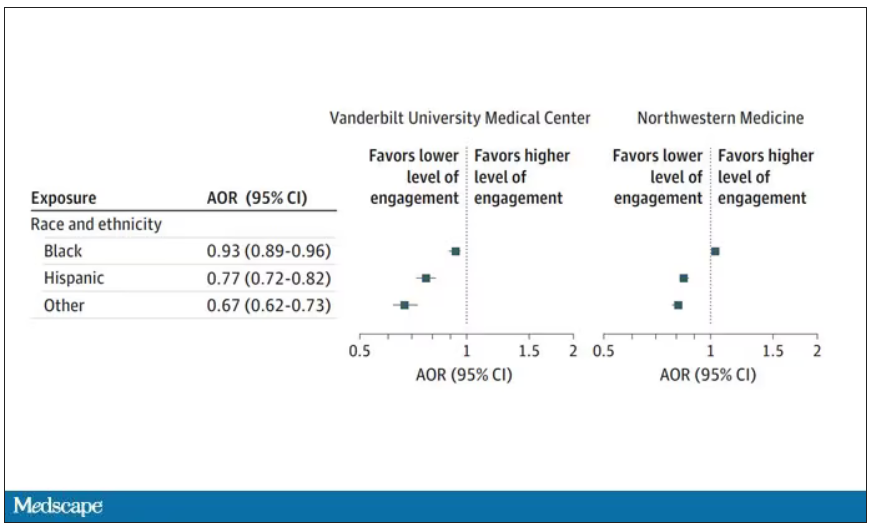
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.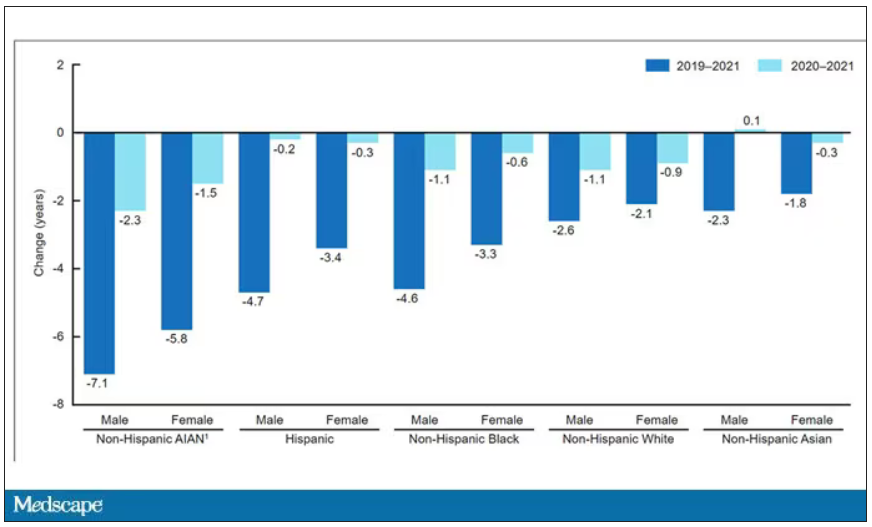
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I close my eyes and imagine what it is I do for a living, I see a computer screen.
I’m primarily a clinical researcher, so much of what I do is looking at statistical software, or, more recently, writing grant applications. But even when I think of my clinical duties, I see that computer screen.
The reason? The electronic health record (EHR) – the hot, beating heart of medical care in the modern era. Our most powerful tool and our greatest enemy.
The EHR records everything – not just the vital signs and lab values of our patients, not just our notes and billing codes. Everything. Every interaction we have is tracked and can be analyzed. The EHR is basically Sting in the song “Every Breath You Take.” Every click you make, it is watching you.
Researchers are leveraging that panopticon to give insight into something we don’t talk about frequently: the issue of racial bias in medicine. Is our true nature revealed by our interactions with the EHR?
We’re talking about this study in JAMA Network Open.
Researchers leveraged huge amounts of EHR data from two big academic medical centers, Vanderbilt University Medical Center and Northwestern University Medical Center. All told, there are data from nearly 250,000 hospitalizations here.
The researchers created a metric for EHR engagement. Basically, they summed the amount of clicks and other EHR interactions that occurred during the hospitalization, divided by the length of stay in days, to create a sort of average “engagement per day” metric. This number was categorized into four groups: low engagement, medium engagement, high engagement, and very high engagement.
What factors would predict higher engagement? Well, , except among Black patients who actually got a bit more engagement.
So, right away we need to be concerned about the obvious implications. Less engagement with the EHR may mean lower-quality care, right? Less attention to medical issues. And if that differs systematically by race, that’s a problem.
But we need to be careful here, because engagement in the health record is not random. Many factors would lead you to spend more time in one patient’s chart vs. another. Medical complexity is the most obvious one. The authors did their best to account for this, adjusting for patients’ age, sex, insurance status, comorbidity score, and social deprivation index based on their ZIP code. But notably, they did not account for the acuity of illness during the hospitalization. If individuals identifying as a minority were, all else being equal, less likely to be severely ill by the time they were hospitalized, you might see results like this.
The authors also restrict their analysis to individuals who were discharged alive. I’m not entirely clear why they made this choice. Most people don’t die in the hospital; the inpatient mortality rate at most centers is 1%-1.5%. But excluding those patients could potentially bias these results, especially if race is, all else being equal, a predictor of inpatient mortality, as some studies have shown.
But the truth is, these data aren’t coming out of nowhere; they don’t exist in a vacuum. Numerous studies demonstrate different intensity of care among minority vs. nonminority individuals. There is this study, which shows that minority populations are less likely to be placed on the liver transplant waitlist.
There is this study, which found that minority kids with type 1 diabetes were less likely to get insulin pumps than were their White counterparts. And this one, which showed that kids with acute appendicitis were less likely to get pain-control medications if they were Black.
This study shows that although life expectancy decreased across all races during the pandemic, it decreased the most among minority populations.
This list goes on. It’s why the CDC has called racism a “fundamental cause of ... disease.”
So, yes, it is clear that there are racial disparities in health care outcomes. It is clear that there are racial disparities in treatments. It is also clear that virtually every physician believes they deliver equitable care. Somewhere, this disconnect arises. Could the actions we take in the EHR reveal the unconscious biases we have? Does the all-seeing eye of the EHR see not only into our brains but into our hearts? And if it can, are we ready to confront what it sees?
F. Perry Wilson, MD, MSCE, is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Intravenous formulation of secukinumab gets FDA approval
The Food and Drug Administration has approved an intravenous (IV) formulation of secukinumab (Cosentyx) for the treatment of adults with psoriatic arthritis (PsA), ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis (nr-axSpA).
Secukinumab is the only treatment approved in an IV formulation that specifically targets and blocks interleukin-17A and the only non–tumor necrosis factor alpha IV option available to treat the three indications of PsA, AS, and nr-axSpA, according to a press release from the drug’s manufacturer, Novartis.
The approval marks the first new IV treatment in 6 years for these three conditions. The drug was first approved in 2015 and up to now has been available only as a subcutaneous injection.
The new formulation is also approved for secukinumab’s other indications of plaque psoriasis in people aged 6 years or older, children aged 2 years or older with PsA, and enthesitis-related arthritis in patients aged 4 years or older.
“A significant portion of the millions of PsA, AS, and nr-axSpA patients in the United States require treatment through IV infusions for a variety of reasons, including not being comfortable with self-injections or simply preferring to have treatments administered in their health care provider’s office,” Philip J. Mease, MD, clinical professor at the University of Washington, Seattle, and director of rheumatology research at the Swedish Medical Center, Seattle, said in the press release. “The approval of Cosentyx as an IV formulation is an important milestone for patients because it expands the treatment options available to them with a different mechanism of action than existing biologic IV therapies, along with the comfort and familiarity of an established treatment.”
This IV formulation is administered monthly in a 30-minute, weight-based dosing regimen. This new option will become available before the end of the year, Novartis said.
“With this approval of Cosentyx as an IV formulation, along with the subcutaneous formulation, we can broaden the use of Cosentyx to help more patients manage their condition with a medicine backed by more than a decade of clinical research and 8 years of real-world experience,” said Christy Siegel, vice president and head of immunology, Novartis U.S.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an intravenous (IV) formulation of secukinumab (Cosentyx) for the treatment of adults with psoriatic arthritis (PsA), ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis (nr-axSpA).
Secukinumab is the only treatment approved in an IV formulation that specifically targets and blocks interleukin-17A and the only non–tumor necrosis factor alpha IV option available to treat the three indications of PsA, AS, and nr-axSpA, according to a press release from the drug’s manufacturer, Novartis.
The approval marks the first new IV treatment in 6 years for these three conditions. The drug was first approved in 2015 and up to now has been available only as a subcutaneous injection.
The new formulation is also approved for secukinumab’s other indications of plaque psoriasis in people aged 6 years or older, children aged 2 years or older with PsA, and enthesitis-related arthritis in patients aged 4 years or older.
“A significant portion of the millions of PsA, AS, and nr-axSpA patients in the United States require treatment through IV infusions for a variety of reasons, including not being comfortable with self-injections or simply preferring to have treatments administered in their health care provider’s office,” Philip J. Mease, MD, clinical professor at the University of Washington, Seattle, and director of rheumatology research at the Swedish Medical Center, Seattle, said in the press release. “The approval of Cosentyx as an IV formulation is an important milestone for patients because it expands the treatment options available to them with a different mechanism of action than existing biologic IV therapies, along with the comfort and familiarity of an established treatment.”
This IV formulation is administered monthly in a 30-minute, weight-based dosing regimen. This new option will become available before the end of the year, Novartis said.
“With this approval of Cosentyx as an IV formulation, along with the subcutaneous formulation, we can broaden the use of Cosentyx to help more patients manage their condition with a medicine backed by more than a decade of clinical research and 8 years of real-world experience,” said Christy Siegel, vice president and head of immunology, Novartis U.S.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an intravenous (IV) formulation of secukinumab (Cosentyx) for the treatment of adults with psoriatic arthritis (PsA), ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis (nr-axSpA).
Secukinumab is the only treatment approved in an IV formulation that specifically targets and blocks interleukin-17A and the only non–tumor necrosis factor alpha IV option available to treat the three indications of PsA, AS, and nr-axSpA, according to a press release from the drug’s manufacturer, Novartis.
The approval marks the first new IV treatment in 6 years for these three conditions. The drug was first approved in 2015 and up to now has been available only as a subcutaneous injection.
The new formulation is also approved for secukinumab’s other indications of plaque psoriasis in people aged 6 years or older, children aged 2 years or older with PsA, and enthesitis-related arthritis in patients aged 4 years or older.
“A significant portion of the millions of PsA, AS, and nr-axSpA patients in the United States require treatment through IV infusions for a variety of reasons, including not being comfortable with self-injections or simply preferring to have treatments administered in their health care provider’s office,” Philip J. Mease, MD, clinical professor at the University of Washington, Seattle, and director of rheumatology research at the Swedish Medical Center, Seattle, said in the press release. “The approval of Cosentyx as an IV formulation is an important milestone for patients because it expands the treatment options available to them with a different mechanism of action than existing biologic IV therapies, along with the comfort and familiarity of an established treatment.”
This IV formulation is administered monthly in a 30-minute, weight-based dosing regimen. This new option will become available before the end of the year, Novartis said.
“With this approval of Cosentyx as an IV formulation, along with the subcutaneous formulation, we can broaden the use of Cosentyx to help more patients manage their condition with a medicine backed by more than a decade of clinical research and 8 years of real-world experience,” said Christy Siegel, vice president and head of immunology, Novartis U.S.
A version of this article first appeared on Medscape.com.
More evidence shows COVID-19’s link to risk for autoimmune disease
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Choosing which biologic to prescribe for psoriasis
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
CARLSBAD, CALIF. –
“When you look at the list of options it can be confusing to many clinicians in deciding which one to choose,” April W. Armstrong, MD, MPH, professor and chief of dermatology at the University of California, Los Angeles, said at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
One approach is to consider how the biologics compare in short- and long-term efficacy. “Several different meta-analyses of biologics have been conducted,” which include some head-to head studies, Dr. Armstrong said. “In terms of efficacy, [biologics] are similar at the population level,” she said.
In a meta-analysis of 71 randomized, controlled trials through July 2020, Dr. Armstrong and colleagues found that in the short-term, Psoriasis Area and Severity Index (PASI) 90 response rates at 10-16 weeks from baseline were highest for ixekizumab (72.9%), risankizumab (72.5%), and brodalumab (72%). These PASI 90 responses were significantly higher than among patients on guselkumab (65%), secukinumab (65%), infliximab (56.8%), certolizumab (400 mg: 49.6%; 200 mg: 42.2%), ustekinumab (90 mg: 47.9%; weight-based: 45.7%; 45 mg: 44.6%), adalimumab (43%), tildrakizumab (200 mg: 39.7%; 100 mg: 37.2%), etanercept (18.0%), apremilast (12.4%), and dimethyl fumarate (12.2%).
In a more recent meta-analysis, Dr. Armstrong and coauthors used area under the curve (AUC) analyses to compare the cumulative clinical benefits of biologics over 1 year. They found that the placebo-adjusted normalized maximum AUC for a PASI 100 response was greatest for ixekizumab (0.436), risankizumab (0.423), and brodalumab (0.378), followed by guselkumab (0.358), secukinumab (0.324), ustekinumab (0.201), adalimumab (0.183), and etanercept (0.087).
In Dr. Armstrong’s opinion, the tumor necrosis factor (TNF) inhibitors etanercept, infliximab, adalimumab, and certolizumab “have served their purpose for plaque psoriasis over time, but these days I would probably choose either an IL [interleukin]-17 inhibitor or an IL-23 inhibitor first,” she said. Still, TNF inhibitors “are certainly good for psoriatic arthritis, and certolizumab is appropriate for patients who are pregnant or breastfeeding,” she said. “Avoid them in patients with demyelinating disease and in those with hepatitis B. They are not preferred in patients with latent TB or advanced CHF.”
Dr. Armstrong said that there are robust efficacy data for the IL-17 inhibitors ixekizumab, secukinumab, and brodalumab in psoriasis and in the peripheral and axial forms of psoriatic arthritis (PsA). “Avoid using them in patients with a personal history of inflammatory bowel disease,” she advised.
Low rates of oral candidiasis have been reported in the literature, “but this has not been issue with our approved IL-17 inhibitors so far,” she said.
The IL-23 inhibitors guselkumab, risankizumab, tildrakizumab, and ustekinumab have robust data for psoriasis efficacy, she said, and three – guselkumab, risankizumab, and ustekinumab – are also approved for PsA. “These agents have the advantage of fewer injections, and the evidence [of efficacy] for IL-23 inhibitors continues to evolve, such as in patients with psoriatic arthritis involving the spine,” Dr. Armstrong said.
She also shared how she deals with patients who fail to respond to biologics. “Do you switch drugs, or do you dose escalate?” she asked. “In most cases, the strategy for dose escalation is to shorten the interval between the injections so the dosing is delivered more frequently.” In a case of primary failure, which Dr. Armstrong defined as a patient who has never responded optimally to a biologic, consider revisiting the diagnosis. “Maybe it’s cutaneous T-cell lymphoma or some other condition, because our current IL-17 and IL-23 medications work extremely well,” she said. “So, if you have a patient who is not responding at all, I would question the diagnosis and consider a biopsy.”
She generally waits about 6 months before switching a patient to another biologic, “to see if they’re one of the late bloomers who may catch up in efficacy,” she explained. “Switching the class of biologic is another consideration.”
If a patient had responded to the biologic for a long time and then lost response – known as secondary failure – Dr. Armstrong considers dose escalation or a switch to another agent within the same class “if it helps to address comorbidities such as PsA,” she said. “You can also try across-class switching.”
Dr. Armstrong disclosed ties with AbbVie, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dermavant, EPI, Galderma, InCyte, Janssen, Leo, Lilly, Meiji, Modmed, Nimbus, Novartis, Ortho Dermatologics, Parexel, Pfizer, Regeneron, Sanofi, Suna, UCB, and Ventyx.
AT CALDERM 2023
FDA approves topical roflumilast for psoriasis in children aged 6-11
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
FDA approves ninth Humira biosimilar, with interchangeability
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.