User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
VEXAS syndrome: More common, variable, and severe than expected
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
FROM JAMA
Mastocytosis: Rare, underdiagnosed, potentially fatal
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Long-pulsed 1,064 nm Nd:YAG for nonaggressive BCC ‘effective and easy’
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
AT MOAS 2022
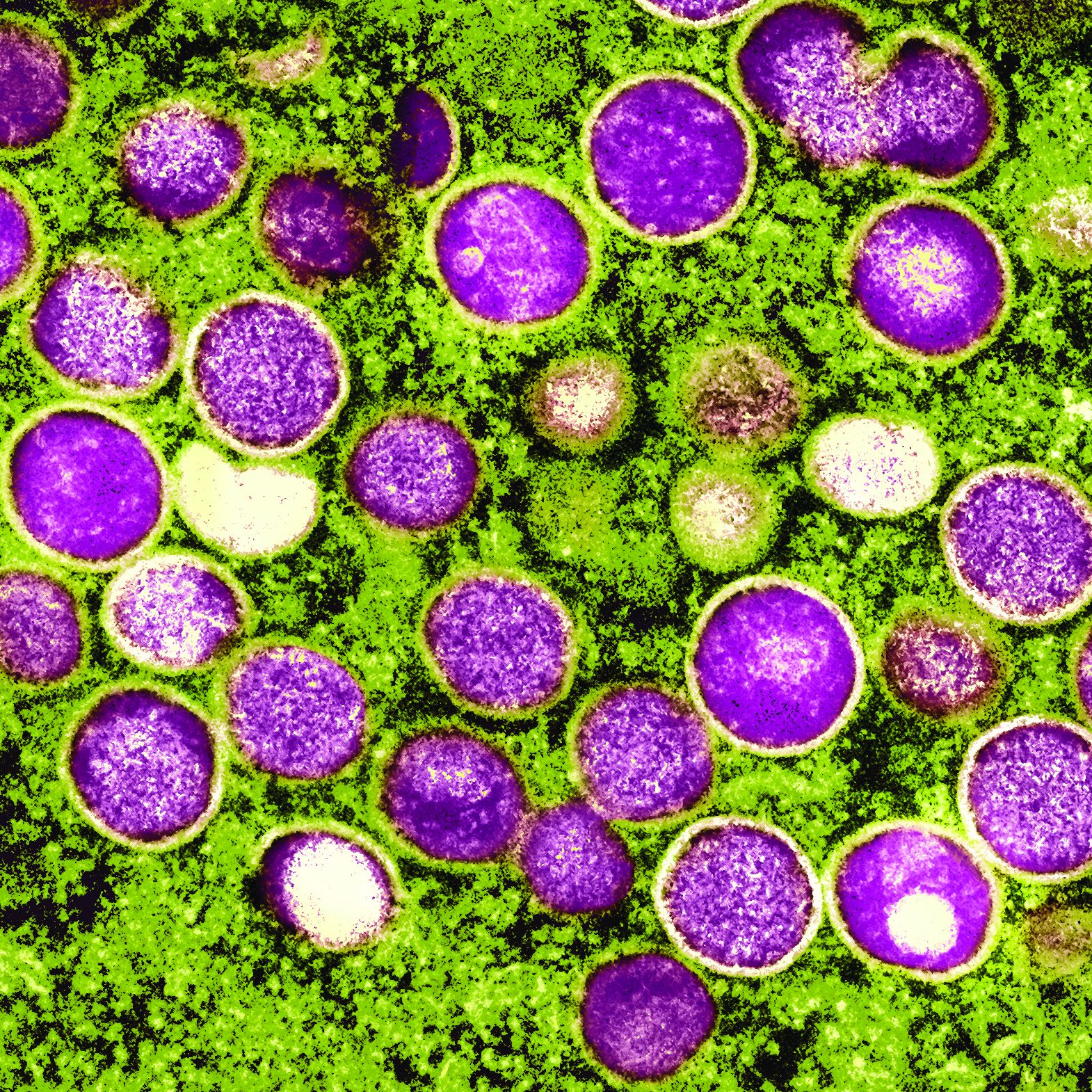
Mpox: Dermatology registry data pinpoints unique signs
that frequently appeared before systemic illness and a much lower overall numbers of lesions.
“Just these two findings alone show how important it is to remain clinically vigilant as dermatologists,” Esther Freeman, MD, PhD, director of global health dermatology at Massachusetts General Hospital, Boston, said in an interview. She is the corresponding author of the study, which analyzed 101 mpox cases from 13 countries and was published online on in the Journal of the American Academy of Dermatology.
“Mpox appeared to manifest differently than in previous outbreaks with morphologic and clinical evolutions much different than previously reported in endemic and prior outbreaks,” added Dr. Freeman. “Dermatologists should continue to keep mpox on the differential as it continues to circulate at low levels in the population and is a mimicker of many other common skin diseases.”
According to the Centers for Disease Control and Prevention, as of Jan. 20, 2023, there have been 30,061 cases of mpox in the United States during the outbreak that began in 2022; 23 people died. Worldwide, the number of cases neared 85,000.
Most of the affected cases were among gay, bisexual, and other men who have sex with men. A vaccination effort began last summer, and the number of cases soon plummeted. The national daily case count in January has been in the single digits.
For the new report, dermatologists tracked cases via the American Academy of Dermatology/International League of Dermatologic Societies (AAD/ILDS) Dermatology COVID-19, Monkeypox (mpox), and Emerging Infections Registry. The new report includes data about cases entered from Aug. 4 to Nov. 13. Of these cases, 97% were male, median age was 35 years, 62% were White, 20% were Hispanic, and 11% were Black.
Just over half (54%) of patients reported skin lesions as the first sign of disease, while others had signs such as fever (16%) and malaise (9%). “This is a sharp contrast to endemic or prior outbreaks in which a ‘flu-like’ prodrome preceded lesions,” Dr. Freeman said. “Dermatologists should be aware that patients may come in with mpox skin lesions as their only initial symptoms.”
In contrast to past outbreaks where patients may have had dozens or hundreds of lesions, 20% had only 1 lesion, while 52% had 2-5 lesions, and 20% had 6-20 lesions. “There may be only a few lesions, so index of suspicion needs to be high,” Dr. Freeman said.
According to the study, “the most common skin lesion morphologies and secondary characteristics reported included papules, vesicles/blisters, pustules, erosions/ulcers and crust/scabs.” Dr. Freeman cautioned that “lesions may not go through the ‘typical’ progression from papule to pustule. The initial lesion could even be an ulceration or a crust. For dermatologists, this means you need to have a high index of suspicion, especially if you see a new onset lesion in the groin or perianal area, though they can also start elsewhere.”
She added that “the lesion you see on exam could be a classic pustule/pseudopustule, but it might not be – it could be a small perianal erosion or ulceration. If you have any concern it could be mpox, it’s a good idea to test by PCR.”
Morbilliform rash, scarring reported
The study also highlighted 10 cases of morbilliform rash. “A morbilliform exanthem is pretty nonspecific, and usually cases of mpox have more specific features,” dermatologist and study coauthor Misha Rosenbach, MD, of the University of Pennsylvania, Philadelphia, said in an interview.
“Given the current low rates of mpox, I do not think most dermatologists need to worry about mpox when evaluating morbilliform exanthems. However, in high-risk patients or patients with other morphologies, it is worth noting that there’s a chance that this may be related.”
Emory University dermatologist Howa Yeung, MD, MSc, who wasn’t involved with the study, said in an interview that morbilliform rashes in the mouth/tongue area, mostly on days 1-5, should be considered a possible sign of mpox. “While I didn’t typically think of monkeypox virus as a cause of viral exanthems, I will now add it to my differential diagnoses.”
In the report, 13% of patients had scarring, “an outcome underemphasized in the current literature” that could have long-term emotional and mental effects, the authors noted. “Some patients, particularly immunosuppressed patients, have had very large and/or ulceronecrotic lesions,” Dr. Rosenbach said. “Their scarring can be quite significant. There is, to date, very little guidance for clinicians or patients on how to mitigate this risk and, if scarring is developing, how best to manage it.”
As for lessons from the findings, Dr. Yeung said, “dermatologists need to be aware that patients with mpox can have multiple morphologies at the same time and lesions can skip stages.” And, he pointed out, it’s clear that wound care is important to prevent scarring.
The AAD has a resource page on skin care in patients with mpox that includes information about preventing scarring. Examples of mpox rashes are available on the CDC website.
The study was supported by a grant from the International League of Dermatologic Societies and in-kind support from the American Academy of Dermatology. Dr. Freeman is a coauthor for UpToDate. Dr. Freeman and Dr. Rosenbach are members of the AAD Ad Hoc Task Force to Create Monkeypox Content. Study authors reported no other disclosures, and Dr. Yeung has no disclosures.
that frequently appeared before systemic illness and a much lower overall numbers of lesions.
“Just these two findings alone show how important it is to remain clinically vigilant as dermatologists,” Esther Freeman, MD, PhD, director of global health dermatology at Massachusetts General Hospital, Boston, said in an interview. She is the corresponding author of the study, which analyzed 101 mpox cases from 13 countries and was published online on in the Journal of the American Academy of Dermatology.
“Mpox appeared to manifest differently than in previous outbreaks with morphologic and clinical evolutions much different than previously reported in endemic and prior outbreaks,” added Dr. Freeman. “Dermatologists should continue to keep mpox on the differential as it continues to circulate at low levels in the population and is a mimicker of many other common skin diseases.”
According to the Centers for Disease Control and Prevention, as of Jan. 20, 2023, there have been 30,061 cases of mpox in the United States during the outbreak that began in 2022; 23 people died. Worldwide, the number of cases neared 85,000.
Most of the affected cases were among gay, bisexual, and other men who have sex with men. A vaccination effort began last summer, and the number of cases soon plummeted. The national daily case count in January has been in the single digits.
For the new report, dermatologists tracked cases via the American Academy of Dermatology/International League of Dermatologic Societies (AAD/ILDS) Dermatology COVID-19, Monkeypox (mpox), and Emerging Infections Registry. The new report includes data about cases entered from Aug. 4 to Nov. 13. Of these cases, 97% were male, median age was 35 years, 62% were White, 20% were Hispanic, and 11% were Black.
Just over half (54%) of patients reported skin lesions as the first sign of disease, while others had signs such as fever (16%) and malaise (9%). “This is a sharp contrast to endemic or prior outbreaks in which a ‘flu-like’ prodrome preceded lesions,” Dr. Freeman said. “Dermatologists should be aware that patients may come in with mpox skin lesions as their only initial symptoms.”
In contrast to past outbreaks where patients may have had dozens or hundreds of lesions, 20% had only 1 lesion, while 52% had 2-5 lesions, and 20% had 6-20 lesions. “There may be only a few lesions, so index of suspicion needs to be high,” Dr. Freeman said.
According to the study, “the most common skin lesion morphologies and secondary characteristics reported included papules, vesicles/blisters, pustules, erosions/ulcers and crust/scabs.” Dr. Freeman cautioned that “lesions may not go through the ‘typical’ progression from papule to pustule. The initial lesion could even be an ulceration or a crust. For dermatologists, this means you need to have a high index of suspicion, especially if you see a new onset lesion in the groin or perianal area, though they can also start elsewhere.”
She added that “the lesion you see on exam could be a classic pustule/pseudopustule, but it might not be – it could be a small perianal erosion or ulceration. If you have any concern it could be mpox, it’s a good idea to test by PCR.”
Morbilliform rash, scarring reported
The study also highlighted 10 cases of morbilliform rash. “A morbilliform exanthem is pretty nonspecific, and usually cases of mpox have more specific features,” dermatologist and study coauthor Misha Rosenbach, MD, of the University of Pennsylvania, Philadelphia, said in an interview.
“Given the current low rates of mpox, I do not think most dermatologists need to worry about mpox when evaluating morbilliform exanthems. However, in high-risk patients or patients with other morphologies, it is worth noting that there’s a chance that this may be related.”
Emory University dermatologist Howa Yeung, MD, MSc, who wasn’t involved with the study, said in an interview that morbilliform rashes in the mouth/tongue area, mostly on days 1-5, should be considered a possible sign of mpox. “While I didn’t typically think of monkeypox virus as a cause of viral exanthems, I will now add it to my differential diagnoses.”
In the report, 13% of patients had scarring, “an outcome underemphasized in the current literature” that could have long-term emotional and mental effects, the authors noted. “Some patients, particularly immunosuppressed patients, have had very large and/or ulceronecrotic lesions,” Dr. Rosenbach said. “Their scarring can be quite significant. There is, to date, very little guidance for clinicians or patients on how to mitigate this risk and, if scarring is developing, how best to manage it.”
As for lessons from the findings, Dr. Yeung said, “dermatologists need to be aware that patients with mpox can have multiple morphologies at the same time and lesions can skip stages.” And, he pointed out, it’s clear that wound care is important to prevent scarring.
The AAD has a resource page on skin care in patients with mpox that includes information about preventing scarring. Examples of mpox rashes are available on the CDC website.
The study was supported by a grant from the International League of Dermatologic Societies and in-kind support from the American Academy of Dermatology. Dr. Freeman is a coauthor for UpToDate. Dr. Freeman and Dr. Rosenbach are members of the AAD Ad Hoc Task Force to Create Monkeypox Content. Study authors reported no other disclosures, and Dr. Yeung has no disclosures.
that frequently appeared before systemic illness and a much lower overall numbers of lesions.
“Just these two findings alone show how important it is to remain clinically vigilant as dermatologists,” Esther Freeman, MD, PhD, director of global health dermatology at Massachusetts General Hospital, Boston, said in an interview. She is the corresponding author of the study, which analyzed 101 mpox cases from 13 countries and was published online on in the Journal of the American Academy of Dermatology.
“Mpox appeared to manifest differently than in previous outbreaks with morphologic and clinical evolutions much different than previously reported in endemic and prior outbreaks,” added Dr. Freeman. “Dermatologists should continue to keep mpox on the differential as it continues to circulate at low levels in the population and is a mimicker of many other common skin diseases.”
According to the Centers for Disease Control and Prevention, as of Jan. 20, 2023, there have been 30,061 cases of mpox in the United States during the outbreak that began in 2022; 23 people died. Worldwide, the number of cases neared 85,000.
Most of the affected cases were among gay, bisexual, and other men who have sex with men. A vaccination effort began last summer, and the number of cases soon plummeted. The national daily case count in January has been in the single digits.
For the new report, dermatologists tracked cases via the American Academy of Dermatology/International League of Dermatologic Societies (AAD/ILDS) Dermatology COVID-19, Monkeypox (mpox), and Emerging Infections Registry. The new report includes data about cases entered from Aug. 4 to Nov. 13. Of these cases, 97% were male, median age was 35 years, 62% were White, 20% were Hispanic, and 11% were Black.
Just over half (54%) of patients reported skin lesions as the first sign of disease, while others had signs such as fever (16%) and malaise (9%). “This is a sharp contrast to endemic or prior outbreaks in which a ‘flu-like’ prodrome preceded lesions,” Dr. Freeman said. “Dermatologists should be aware that patients may come in with mpox skin lesions as their only initial symptoms.”
In contrast to past outbreaks where patients may have had dozens or hundreds of lesions, 20% had only 1 lesion, while 52% had 2-5 lesions, and 20% had 6-20 lesions. “There may be only a few lesions, so index of suspicion needs to be high,” Dr. Freeman said.
According to the study, “the most common skin lesion morphologies and secondary characteristics reported included papules, vesicles/blisters, pustules, erosions/ulcers and crust/scabs.” Dr. Freeman cautioned that “lesions may not go through the ‘typical’ progression from papule to pustule. The initial lesion could even be an ulceration or a crust. For dermatologists, this means you need to have a high index of suspicion, especially if you see a new onset lesion in the groin or perianal area, though they can also start elsewhere.”
She added that “the lesion you see on exam could be a classic pustule/pseudopustule, but it might not be – it could be a small perianal erosion or ulceration. If you have any concern it could be mpox, it’s a good idea to test by PCR.”
Morbilliform rash, scarring reported
The study also highlighted 10 cases of morbilliform rash. “A morbilliform exanthem is pretty nonspecific, and usually cases of mpox have more specific features,” dermatologist and study coauthor Misha Rosenbach, MD, of the University of Pennsylvania, Philadelphia, said in an interview.
“Given the current low rates of mpox, I do not think most dermatologists need to worry about mpox when evaluating morbilliform exanthems. However, in high-risk patients or patients with other morphologies, it is worth noting that there’s a chance that this may be related.”
Emory University dermatologist Howa Yeung, MD, MSc, who wasn’t involved with the study, said in an interview that morbilliform rashes in the mouth/tongue area, mostly on days 1-5, should be considered a possible sign of mpox. “While I didn’t typically think of monkeypox virus as a cause of viral exanthems, I will now add it to my differential diagnoses.”
In the report, 13% of patients had scarring, “an outcome underemphasized in the current literature” that could have long-term emotional and mental effects, the authors noted. “Some patients, particularly immunosuppressed patients, have had very large and/or ulceronecrotic lesions,” Dr. Rosenbach said. “Their scarring can be quite significant. There is, to date, very little guidance for clinicians or patients on how to mitigate this risk and, if scarring is developing, how best to manage it.”
As for lessons from the findings, Dr. Yeung said, “dermatologists need to be aware that patients with mpox can have multiple morphologies at the same time and lesions can skip stages.” And, he pointed out, it’s clear that wound care is important to prevent scarring.
The AAD has a resource page on skin care in patients with mpox that includes information about preventing scarring. Examples of mpox rashes are available on the CDC website.
The study was supported by a grant from the International League of Dermatologic Societies and in-kind support from the American Academy of Dermatology. Dr. Freeman is a coauthor for UpToDate. Dr. Freeman and Dr. Rosenbach are members of the AAD Ad Hoc Task Force to Create Monkeypox Content. Study authors reported no other disclosures, and Dr. Yeung has no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Not all white coats are doctors: Why titles are important at the doctor’s office
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
Age-related atopic dermatitis phenotypes evaluated in study
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
FROM JAAD INTERNATIONAL
Physician sues AMA for defamation over 2022 election controversy
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
If Willarda Edwards, MD, MBA, had won her 2022 campaign for president-elect of the American Medical Association (AMA), she would have been the second Black woman to head the group.
The lawsuit sheds light on the power dynamics of a politically potent organization that has more than 271,000 members and holds assets of $1.2 billion. The AMA president is one of the most visible figures in American medicine.
“The AMA impugned Dr. Edwards with these false charges, which destroyed her candidacy and irreparably damaged her reputation,” according to the complaint, which was filed Nov. 9, 2022, in Baltimore County Circuit Court. The case was later moved to federal court.
The AMA “previously rejected our attempt to resolve this matter without litigation,” Dr. Edwards’ attorney, Timothy Maloney, told this news organization. An AMA spokesman said the organization had no comment on Dr. Edwards’ suit.
Dr. Edwards is a past president of the National Medical Association, MedChi, the Baltimore City Medical Society, the Monumental City Medical Society, and the Sickle Cell Disease Association of America. She joined the AMA in 1994 and has served as a trustee since 2016.
As chair of the AMA Task Force on Health Equity, “she helped lead the way in consensus building and driving action that in 2019 resulted in the AMA House of Delegates establishing the AMA Center on Health Equity,” according to her AMA bio page.
‘Quid pro quo’ alleged
In June 2022, Dr. Edwards was one of three individuals running to be AMA president-elect.
According to Dr. Edwards’ complaint, she was “incorrectly advised by colleagues” that Virginia urologist William Reha, MD, had decided not to seek the AMA vice-speakership in 2023. This was important because both Dr. Edwards and Dr. Reha were in the Southeastern delegation. It could be in Dr. Edwards’ favor if Dr. Reha was not running, as it would mean one less leadership candidate from the same region.
Dr. Edwards called Dr. Reha on June 6 to discuss the matter. When they talked, Dr. Reha allegedly recorded the call without Dr. Edwards’ knowledge or permission – a felony in Maryland – and also steered her toward discussions about how his decision could benefit her campaign, according to the complaint.
The suit alleges that Dr. Reha’s questions were “clearly calculated to draw some statements by Dr. Edwards that he could use later to thwart her candidacy and to benefit her opponent.”
On June 10, at the AMA’s House of Delegates meeting in Chicago, Dr. Edwards was taken aside and questioned by members of the AMA’s Election Campaign Committee, according to the complaint. They accused her of “vote trading” but did not provide any evidence or a copy of a complaint they said had been filed against her, the suit said.
Dr. Edwards was given no opportunity to produce her own evidence or rebut the accusations, the suit alleges.
Just before the delegates started formal business on June 13, House Speaker Bruce Scott, MD, read a statement to the assembly saying that a complaint of a possible campaign violation had been filed against Dr. Edwards.
Dr. Scott told the delegates that “committee members interviewed the complainant and multiple other individuals said to have knowledge of the circumstances. In addition to conducting multiple interviews, the committee reviewed evidence that was deemed credible and corroborated that a campaign violation did in fact occur,” according to the complaint.
The supposed violation: A “quid pro quo” in which an unnamed delegation would support Dr. Edwards’ current candidacy, and the Southeastern delegation would support a future candidate from that other unnamed delegation.
Dr. Edwards was given a short opportunity to speak, in which she denied any violations.
According to a news report, Dr. Edwards said, “I’ve been in the House of Delegates for 30 years, and you know me as a process person – a person who truly believes in the process and trying to follow the complexities of our election campaign.”
The lawsuit alleges that “this defamatory conduct was repeated the next day to more than 600 delegates just minutes prior to the casting of votes, when Dr Scott repeated these allegations.”
Dr. Edwards lost the election.
AMA: Nothing more to add
The suit alleges that neither the Election Campaign Committee nor the AMA itself has made any accusers or complaints available to Dr. Edwards and that it has not provided any audio or written evidence of her alleged violation.
In July, the AMA’s Southeastern delegation told its membership, “We continue to maintain that Willarda was ‘set up’ ... The whole affair lacked any reasonable semblance of due process.”
The delegation has filed a counter claim against the AMA seeking “to address this lack of due process as well as the reputational harm” to the delegation.
The AMA said that it has nothing it can produce. “The Speaker of the House presented a verbal report to the attending delegates,” said a spokesman. “The Speaker’s report remains the only remarks from an AMA officer, and no additional remarks can be expected at this time.”
He added that there “is no official transcript of the Speaker’s report.”
A version of this article first appeared on Medscape.com.
Will your smartphone be the next doctor’s office?
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The loss of letters
My desk looks nothing like my grandfather’s. It stands about mid-abdomen high and has a small surface, perhaps just enough for the monitor and a mug. Yes, I can move it up and down (thank you 21st century), but it has no drawers. It is lean and immaculate, but it has no soul.
My grandfather sat at a large oak desk with three drawers on each side. Each was so heavy you had to be at least 6 years old to pull one open for exploring the contents inside. The desk surface was vast and although immobile, it had a greenish leather blotter for writing. Alongside his pile of correspondences was a treasure for those of us tall enough to get it: A heavy brass letter opener. It came, I believe, with a secretary who would open his letters and stack them neatly before placing this sometimes-pirate’s-sword far enough away from the edge for us to not reach it.
Upon my skinny, adaptable desk the other day sat a white envelope that was hand addressed to me. It was postmarked more than 2 weeks before as it had been waylaid in Endocrinology before being couriered to the rightful recipient. It had not been opened. Nor did I have any way to do so gracefully. I tore it apart with a fat finger while clicking through path reports that just arrived in my inbox.
Dear Dr. Benabio,
Pat
. She carefully looped her “y’s” and crossed her “t’s.” Not one cross out. She thought about each sentence before transcribing it. The paper once sat on her desk, touched her fingers and the envelope sealed with her saliva. It was not filled with trifling requests or complaints. It was not efficient, but it was more than just communication. She took the time to choose the words to capture her emotion and express her gratitude. It was respectful, dignified, decidedly nondigital. For a brief moment I thought I might write back, but quickly realized that was impractical. I knew I wouldn’t make the time to do so. I wish I had.
Having no drawers to save it, I held it up with just a corner of the page resting on my desk and scribbled in black ink “Reviewed. Please scan to media file. 12/8/22. JAB”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
My desk looks nothing like my grandfather’s. It stands about mid-abdomen high and has a small surface, perhaps just enough for the monitor and a mug. Yes, I can move it up and down (thank you 21st century), but it has no drawers. It is lean and immaculate, but it has no soul.
My grandfather sat at a large oak desk with three drawers on each side. Each was so heavy you had to be at least 6 years old to pull one open for exploring the contents inside. The desk surface was vast and although immobile, it had a greenish leather blotter for writing. Alongside his pile of correspondences was a treasure for those of us tall enough to get it: A heavy brass letter opener. It came, I believe, with a secretary who would open his letters and stack them neatly before placing this sometimes-pirate’s-sword far enough away from the edge for us to not reach it.
Upon my skinny, adaptable desk the other day sat a white envelope that was hand addressed to me. It was postmarked more than 2 weeks before as it had been waylaid in Endocrinology before being couriered to the rightful recipient. It had not been opened. Nor did I have any way to do so gracefully. I tore it apart with a fat finger while clicking through path reports that just arrived in my inbox.
Dear Dr. Benabio,
Pat
. She carefully looped her “y’s” and crossed her “t’s.” Not one cross out. She thought about each sentence before transcribing it. The paper once sat on her desk, touched her fingers and the envelope sealed with her saliva. It was not filled with trifling requests or complaints. It was not efficient, but it was more than just communication. She took the time to choose the words to capture her emotion and express her gratitude. It was respectful, dignified, decidedly nondigital. For a brief moment I thought I might write back, but quickly realized that was impractical. I knew I wouldn’t make the time to do so. I wish I had.
Having no drawers to save it, I held it up with just a corner of the page resting on my desk and scribbled in black ink “Reviewed. Please scan to media file. 12/8/22. JAB”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
My desk looks nothing like my grandfather’s. It stands about mid-abdomen high and has a small surface, perhaps just enough for the monitor and a mug. Yes, I can move it up and down (thank you 21st century), but it has no drawers. It is lean and immaculate, but it has no soul.
My grandfather sat at a large oak desk with three drawers on each side. Each was so heavy you had to be at least 6 years old to pull one open for exploring the contents inside. The desk surface was vast and although immobile, it had a greenish leather blotter for writing. Alongside his pile of correspondences was a treasure for those of us tall enough to get it: A heavy brass letter opener. It came, I believe, with a secretary who would open his letters and stack them neatly before placing this sometimes-pirate’s-sword far enough away from the edge for us to not reach it.
Upon my skinny, adaptable desk the other day sat a white envelope that was hand addressed to me. It was postmarked more than 2 weeks before as it had been waylaid in Endocrinology before being couriered to the rightful recipient. It had not been opened. Nor did I have any way to do so gracefully. I tore it apart with a fat finger while clicking through path reports that just arrived in my inbox.
Dear Dr. Benabio,
Pat
. She carefully looped her “y’s” and crossed her “t’s.” Not one cross out. She thought about each sentence before transcribing it. The paper once sat on her desk, touched her fingers and the envelope sealed with her saliva. It was not filled with trifling requests or complaints. It was not efficient, but it was more than just communication. She took the time to choose the words to capture her emotion and express her gratitude. It was respectful, dignified, decidedly nondigital. For a brief moment I thought I might write back, but quickly realized that was impractical. I knew I wouldn’t make the time to do so. I wish I had.
Having no drawers to save it, I held it up with just a corner of the page resting on my desk and scribbled in black ink “Reviewed. Please scan to media file. 12/8/22. JAB”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].