User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Outcomes-based measurement of TAVR program quality goes live
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
REPORTING FROM ACC 20
Larger absolute rivaroxaban benefit in diabetes: COMPASS
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
UK TAVI: Similar outcomes to surgery in real world
Transcatheter aortic valve replacement (TAVR) was not inferior to conventional surgery with respect to death from any cause at 1 year in a new real-world study in patients age 70 years or older with severe symptomatic aortic stenosis at increased operative risk due to age or comorbidity.
The UK TAVI study was presented March 29 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The trial involved a broad group of patients who were treated at every medical center that performs the transcatheter procedure across the United Kingdom.
“The importance of this trial is that it confirms the effectiveness of the TAVR strategy in a real-world setting,” said lead author, William D. Toff, MD, professor of cardiology at the University of Leicester, United Kingdom.
Previous clinical trials have found TAVR to be noninferior or superior to open-heart surgery for various patient groups, but most trials have been limited to medical centers that perform a high volume of procedures or focus on the use of specific types of replacement valves, he noted.
“Our results are concordant with those from earlier trials in intermediate- and low-risk patients, but those earlier trials were performed in the best centers and had many exclusion criteria. We have replicated those results in populations more representative of the real world.”
“I think it is a very important message that supports the findings in earlier trials that were focused on showing whether TAVR can work under ideal conditions, while our trial shows that it does work in real-world clinical practice,” he added.
The UK TAVI trial enrolled 913 patients referred for treatment of severe aortic stenosis at 34 UK sites from 2014 to 2018. They were randomly assigned to receive TAVR or open-heart surgery.
Enrollment was limited to participants age 70 years or older (with additional risk factors) or age 80 years or older (with or without additional risk factors). The average age was 81 years.
Overall, participants were at intermediate to low risk from surgery, with a median Society of Thoracic Surgeons (STS) risk score of 2.6%. However, researchers did not specify a particular risk score cutoff for enrollment.
“This allowed the trial to evolve along with changes in guidelines and practice regarding TAVR over the course of the study and to reflect physicians’ nuanced, real-world approach to considering risk in decision-making rather than taking a formulaic approach,” Toff said.
At 1 year, the rate of death from any cause (the primary endpoint) was 4.6% in the TAVR group and 6.6% in the surgery group, a difference that met the trial’s prespecified threshold for noninferiority of TAVR.
Rates of death from cardiovascular disease or stroke were also similar between the two groups.
Patients who received TAVR had a significantly higher rate of vascular complications (4.8%) than those receiving surgery (1.3%).
TAVR patients were also more likely to have a pacemaker implanted. This occurred in 12.2% of TAVR patients and 6.6% of those undergoing surgery.
In addition, patients who underwent TAVR had a higher rate of aortic regurgitation. Mild aortic regurgitation occurred at 1 year in 38.3% of the TAVR group and 11.7% of the surgery group, whereas moderate regurgitation occurred in 2.3% of TAVR patients and 0.6% of surgery patients.
On the other hand, patients undergoing TAVR had a significantly lower rate of major bleeding complications, which occurred in 6.3% of patients having TAVR and 17.1% of those undergoing surgery.
TAVR was also associated with a shorter hospital stay, fewer days in intensive care, and a faster improvement in functional capacity and quality of life. Functional capacity and quality-of-life measures at 6 weeks after the procedure were better in the TAVR group but by 1 year they were similar in the two groups.
“Longer follow-up is required to confirm sustained clinical benefit and valve durability to inform clinical practice, particularly in younger patients,” Toff concluded.
“The results from our trial and others are encouraging, but patients need to be fully informed and know that the long-term durability of the TAVR valves and the long-term implications of the increased risk of aortic regurgitation are still uncertain,” he added.
The researchers plan to continue to track outcomes for a minimum of 5 years.
Discussant of the UK TAVI trial at an ACC press conference, Julia Grapsa, MD, Guys and St Thomas NHS Trust, London, United Kingdom, said it was a well-designed study.
“It was impressive to see so many UK sites and the age range of patients from 70 to 91 years, and the shorter hospital stays and functional recoveries as well as reduced major bleeding in the TAVR group,” Grapsa said.
“But something that was very striking to me was the increase in moderate aortic regurgitation in the TAVR arm, 2.3% versus 0.6% in the surgical arm, so it is very important to keep following these patients long term,” she added.
In answer to a question during the main session about using age alone as an inclusion criterion in those over 80 years old, Toff said, “We were more comfortable taking all comers over 80 years of age because of the uncertainty about TAVR is more in relation to its durability and the clinical significance of the aortic regurgitation, which may have consequences in the longer term. But the longer term for the over 80s is obviously less of a problem than for those in their 70s.”
This study was funded by the UK National Institute for Health Research Health Technology Assessment Programme. Toff reports no disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20410-ACC. Presented March 29, 2020.
This article first appeared on Medscape.com.
Transcatheter aortic valve replacement (TAVR) was not inferior to conventional surgery with respect to death from any cause at 1 year in a new real-world study in patients age 70 years or older with severe symptomatic aortic stenosis at increased operative risk due to age or comorbidity.
The UK TAVI study was presented March 29 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The trial involved a broad group of patients who were treated at every medical center that performs the transcatheter procedure across the United Kingdom.
“The importance of this trial is that it confirms the effectiveness of the TAVR strategy in a real-world setting,” said lead author, William D. Toff, MD, professor of cardiology at the University of Leicester, United Kingdom.
Previous clinical trials have found TAVR to be noninferior or superior to open-heart surgery for various patient groups, but most trials have been limited to medical centers that perform a high volume of procedures or focus on the use of specific types of replacement valves, he noted.
“Our results are concordant with those from earlier trials in intermediate- and low-risk patients, but those earlier trials were performed in the best centers and had many exclusion criteria. We have replicated those results in populations more representative of the real world.”
“I think it is a very important message that supports the findings in earlier trials that were focused on showing whether TAVR can work under ideal conditions, while our trial shows that it does work in real-world clinical practice,” he added.
The UK TAVI trial enrolled 913 patients referred for treatment of severe aortic stenosis at 34 UK sites from 2014 to 2018. They were randomly assigned to receive TAVR or open-heart surgery.
Enrollment was limited to participants age 70 years or older (with additional risk factors) or age 80 years or older (with or without additional risk factors). The average age was 81 years.
Overall, participants were at intermediate to low risk from surgery, with a median Society of Thoracic Surgeons (STS) risk score of 2.6%. However, researchers did not specify a particular risk score cutoff for enrollment.
“This allowed the trial to evolve along with changes in guidelines and practice regarding TAVR over the course of the study and to reflect physicians’ nuanced, real-world approach to considering risk in decision-making rather than taking a formulaic approach,” Toff said.
At 1 year, the rate of death from any cause (the primary endpoint) was 4.6% in the TAVR group and 6.6% in the surgery group, a difference that met the trial’s prespecified threshold for noninferiority of TAVR.
Rates of death from cardiovascular disease or stroke were also similar between the two groups.
Patients who received TAVR had a significantly higher rate of vascular complications (4.8%) than those receiving surgery (1.3%).
TAVR patients were also more likely to have a pacemaker implanted. This occurred in 12.2% of TAVR patients and 6.6% of those undergoing surgery.
In addition, patients who underwent TAVR had a higher rate of aortic regurgitation. Mild aortic regurgitation occurred at 1 year in 38.3% of the TAVR group and 11.7% of the surgery group, whereas moderate regurgitation occurred in 2.3% of TAVR patients and 0.6% of surgery patients.
On the other hand, patients undergoing TAVR had a significantly lower rate of major bleeding complications, which occurred in 6.3% of patients having TAVR and 17.1% of those undergoing surgery.
TAVR was also associated with a shorter hospital stay, fewer days in intensive care, and a faster improvement in functional capacity and quality of life. Functional capacity and quality-of-life measures at 6 weeks after the procedure were better in the TAVR group but by 1 year they were similar in the two groups.
“Longer follow-up is required to confirm sustained clinical benefit and valve durability to inform clinical practice, particularly in younger patients,” Toff concluded.
“The results from our trial and others are encouraging, but patients need to be fully informed and know that the long-term durability of the TAVR valves and the long-term implications of the increased risk of aortic regurgitation are still uncertain,” he added.
The researchers plan to continue to track outcomes for a minimum of 5 years.
Discussant of the UK TAVI trial at an ACC press conference, Julia Grapsa, MD, Guys and St Thomas NHS Trust, London, United Kingdom, said it was a well-designed study.
“It was impressive to see so many UK sites and the age range of patients from 70 to 91 years, and the shorter hospital stays and functional recoveries as well as reduced major bleeding in the TAVR group,” Grapsa said.
“But something that was very striking to me was the increase in moderate aortic regurgitation in the TAVR arm, 2.3% versus 0.6% in the surgical arm, so it is very important to keep following these patients long term,” she added.
In answer to a question during the main session about using age alone as an inclusion criterion in those over 80 years old, Toff said, “We were more comfortable taking all comers over 80 years of age because of the uncertainty about TAVR is more in relation to its durability and the clinical significance of the aortic regurgitation, which may have consequences in the longer term. But the longer term for the over 80s is obviously less of a problem than for those in their 70s.”
This study was funded by the UK National Institute for Health Research Health Technology Assessment Programme. Toff reports no disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20410-ACC. Presented March 29, 2020.
This article first appeared on Medscape.com.
Transcatheter aortic valve replacement (TAVR) was not inferior to conventional surgery with respect to death from any cause at 1 year in a new real-world study in patients age 70 years or older with severe symptomatic aortic stenosis at increased operative risk due to age or comorbidity.
The UK TAVI study was presented March 29 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The trial involved a broad group of patients who were treated at every medical center that performs the transcatheter procedure across the United Kingdom.
“The importance of this trial is that it confirms the effectiveness of the TAVR strategy in a real-world setting,” said lead author, William D. Toff, MD, professor of cardiology at the University of Leicester, United Kingdom.
Previous clinical trials have found TAVR to be noninferior or superior to open-heart surgery for various patient groups, but most trials have been limited to medical centers that perform a high volume of procedures or focus on the use of specific types of replacement valves, he noted.
“Our results are concordant with those from earlier trials in intermediate- and low-risk patients, but those earlier trials were performed in the best centers and had many exclusion criteria. We have replicated those results in populations more representative of the real world.”
“I think it is a very important message that supports the findings in earlier trials that were focused on showing whether TAVR can work under ideal conditions, while our trial shows that it does work in real-world clinical practice,” he added.
The UK TAVI trial enrolled 913 patients referred for treatment of severe aortic stenosis at 34 UK sites from 2014 to 2018. They were randomly assigned to receive TAVR or open-heart surgery.
Enrollment was limited to participants age 70 years or older (with additional risk factors) or age 80 years or older (with or without additional risk factors). The average age was 81 years.
Overall, participants were at intermediate to low risk from surgery, with a median Society of Thoracic Surgeons (STS) risk score of 2.6%. However, researchers did not specify a particular risk score cutoff for enrollment.
“This allowed the trial to evolve along with changes in guidelines and practice regarding TAVR over the course of the study and to reflect physicians’ nuanced, real-world approach to considering risk in decision-making rather than taking a formulaic approach,” Toff said.
At 1 year, the rate of death from any cause (the primary endpoint) was 4.6% in the TAVR group and 6.6% in the surgery group, a difference that met the trial’s prespecified threshold for noninferiority of TAVR.
Rates of death from cardiovascular disease or stroke were also similar between the two groups.
Patients who received TAVR had a significantly higher rate of vascular complications (4.8%) than those receiving surgery (1.3%).
TAVR patients were also more likely to have a pacemaker implanted. This occurred in 12.2% of TAVR patients and 6.6% of those undergoing surgery.
In addition, patients who underwent TAVR had a higher rate of aortic regurgitation. Mild aortic regurgitation occurred at 1 year in 38.3% of the TAVR group and 11.7% of the surgery group, whereas moderate regurgitation occurred in 2.3% of TAVR patients and 0.6% of surgery patients.
On the other hand, patients undergoing TAVR had a significantly lower rate of major bleeding complications, which occurred in 6.3% of patients having TAVR and 17.1% of those undergoing surgery.
TAVR was also associated with a shorter hospital stay, fewer days in intensive care, and a faster improvement in functional capacity and quality of life. Functional capacity and quality-of-life measures at 6 weeks after the procedure were better in the TAVR group but by 1 year they were similar in the two groups.
“Longer follow-up is required to confirm sustained clinical benefit and valve durability to inform clinical practice, particularly in younger patients,” Toff concluded.
“The results from our trial and others are encouraging, but patients need to be fully informed and know that the long-term durability of the TAVR valves and the long-term implications of the increased risk of aortic regurgitation are still uncertain,” he added.
The researchers plan to continue to track outcomes for a minimum of 5 years.
Discussant of the UK TAVI trial at an ACC press conference, Julia Grapsa, MD, Guys and St Thomas NHS Trust, London, United Kingdom, said it was a well-designed study.
“It was impressive to see so many UK sites and the age range of patients from 70 to 91 years, and the shorter hospital stays and functional recoveries as well as reduced major bleeding in the TAVR group,” Grapsa said.
“But something that was very striking to me was the increase in moderate aortic regurgitation in the TAVR arm, 2.3% versus 0.6% in the surgical arm, so it is very important to keep following these patients long term,” she added.
In answer to a question during the main session about using age alone as an inclusion criterion in those over 80 years old, Toff said, “We were more comfortable taking all comers over 80 years of age because of the uncertainty about TAVR is more in relation to its durability and the clinical significance of the aortic regurgitation, which may have consequences in the longer term. But the longer term for the over 80s is obviously less of a problem than for those in their 70s.”
This study was funded by the UK National Institute for Health Research Health Technology Assessment Programme. Toff reports no disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20410-ACC. Presented March 29, 2020.
This article first appeared on Medscape.com.
VICTORIA: Vericiguat seen as novel success in tough-to-treat, high-risk heart failure
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
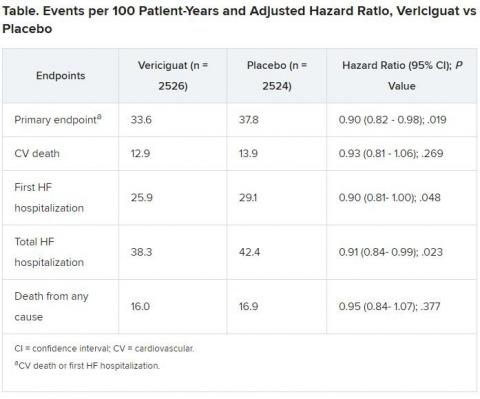
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
TAILOR-PCI: Clopidogrel genotyping trial narrowly misses endpoint
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
More than one in three cardiologists burned out, many ready to bolt
Even before the COVID-19 pandemic, , a new survey shows.
“It is important to recognize the personal and professional repercussions of physician burnout,” lead author Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said during an online session of the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The new ACC 2019 Well Being Survey was sent to 19,348 ACC members in the fall of 2019 and sought to take a deeper dive into the issue of burnout after the ACC’s most recent Professional Life Survey revealed that one in four U.S. cardiologists were burned out in 2015.
While the number of cardiologists who reported feeling stressed fell from 49.5% in 2015 to 43.9% in 2019, the number of cardiologists who reported being burned out increased by 32% from 26.8% to 35.4%, Mehta said.
Among those currently feeling burned out, 23.9% reported having one or more symptoms of burnout, 9.9% had chronic burnout and work frustrations, and 1.6% were “completely burned out” and at the point where they may need to seek help.
Burned-out cardiologists were more likely than those who felt stressed or no burnout to say they may have made a major medical error in the past 3 months (58.3% vs 33.1% and 8.6%; P ≤ .001).
The Usual Suspects
As previously observed, burnout was highest among mid-career cardiologists with 8 to 21 years in practice vs early-career and late-career cardiologists (45.3% vs 35.4% and 31.5%; P ≤ .001) and in women vs men (45.3% vs 33.5%; P ≤ .001). Of the 2025 ACC members who responded, 362 were women.
Several initiatives are underway by the ACC to increase the diversity of cardiology as a specialty, but attention is also needed for mid-career cardiologists, who may not see the “light at the end of the tunnel,” as they take on more clinical demands and more administrative roles, Mehta observed.
Not surprising, clocking 60 or more hours per week increased the risk for burnout, compared with working 40 to 59 hours per week or fewer than 40 hours per week (41.5% vs 29.5% and 17.9%; P ≤ .001).
Burned-out cardiologists were also more likely than those who felt stressed or no burnout to report working in a hectic work environment (59.5% vs 32.3% and 14.6%; P ≤ .001) and to have plans to leave their current practice setting (58.1% vs 27.9% and 14.0%; P ≤ .001).
Factors that played a significant role in those plans were the desire to spend more time with family, on-call time, excessive work or relative value unit (RVU) targets, electronic health records, and the pressure to maintain high patient satisfaction scores, Mehta noted.
“Is any of this relatable to decreasing numbers of cardiologists in the U.S., or is there work to try and relate actual work force availability to burnout?” asked session moderator B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute in Charlotte, North Carolina, and a member of ACC’s Board of Trustees, following the presentation.
“It’s hard to decipher all of those exact details, but we do know that the cardiology work force tends to be older, so the mid-careers are going to be pulling on a lot more weight in the next few years, so that is a concern,” Mehta replied.
A big factor, however, is the excessive work hours put in by all cardiologists, especially the increasing amount of time spent with electronic medical records and administrative tasks, which is “taking away the fun we had in cardiology,” she added.
Limitations of the survey include the potential for bias; burnout was self-reported and may vary over time; and the 14% response rate was less than ideal, although the results are consistent with other national surveys, Mehta said.
In the recent Medscape Cardiologist Lifestyle, Happiness & Burnout Report 2020, 29% of respondents reported feeling burnout, 2% depressed, and 15% both burned out and depressed.
The Elephant in the Room
The new findings are “certainly a call to action, but it’s hard to avoid the elephant in the room, which is COVID-19,” said panelist Sandra Lewis, MD, Legacy Good Samaritan Hospital & Medical Center, Portland, Oregon.
“The implications of burnout are really front-and-center with our colleagues, who are working long hours, have hectic work environments, lack of control, and, more than that, a lack of safety of the work situations that we have worked so hard to achieve, as we run out of protective gear, we don’t have masks, as we see our colleagues falling victim to this.”
During her presentation, Mehta highlighted the ACC Clinician Well Being Portal and its COVID-19 Hub, but also several self-care strategies to employ, such as relinquishing control during these uncharted waters, revisiting personal strengths and abilities leveraged in other times of uncertainty, and giving yourself a “brain break” by challenging yourself to chat with a colleague for 30 minutes on topics unrelated to COVID-19 and other workplace stressors.
Wilson said the global pandemic only heightens concerns about burnout among cardiologists, which he likened to a “runaway train.”
“These are not great signals, I think they’re shocking, quite frankly,” Wilson told theheart.org | Medscape Cardiology.
“ACC is setting up a task force from the board of trustees to get to work right away and see about ways we can turn this around as quickly as possible and be a voice for the clinicians,” he said. “It’s not only cardiologists, it’s everybody on our cardiovascular care team, including nurses, physician assistants, nurse practitioners, and even pharmacists. Everybody’s burning out.”
The authors and Wilson report no relevant conflicts of interest.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 403.08. Presented March 28, 2020.
This article first appeared on Medscape.com.
Even before the COVID-19 pandemic, , a new survey shows.
“It is important to recognize the personal and professional repercussions of physician burnout,” lead author Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said during an online session of the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The new ACC 2019 Well Being Survey was sent to 19,348 ACC members in the fall of 2019 and sought to take a deeper dive into the issue of burnout after the ACC’s most recent Professional Life Survey revealed that one in four U.S. cardiologists were burned out in 2015.
While the number of cardiologists who reported feeling stressed fell from 49.5% in 2015 to 43.9% in 2019, the number of cardiologists who reported being burned out increased by 32% from 26.8% to 35.4%, Mehta said.
Among those currently feeling burned out, 23.9% reported having one or more symptoms of burnout, 9.9% had chronic burnout and work frustrations, and 1.6% were “completely burned out” and at the point where they may need to seek help.
Burned-out cardiologists were more likely than those who felt stressed or no burnout to say they may have made a major medical error in the past 3 months (58.3% vs 33.1% and 8.6%; P ≤ .001).
The Usual Suspects
As previously observed, burnout was highest among mid-career cardiologists with 8 to 21 years in practice vs early-career and late-career cardiologists (45.3% vs 35.4% and 31.5%; P ≤ .001) and in women vs men (45.3% vs 33.5%; P ≤ .001). Of the 2025 ACC members who responded, 362 were women.
Several initiatives are underway by the ACC to increase the diversity of cardiology as a specialty, but attention is also needed for mid-career cardiologists, who may not see the “light at the end of the tunnel,” as they take on more clinical demands and more administrative roles, Mehta observed.
Not surprising, clocking 60 or more hours per week increased the risk for burnout, compared with working 40 to 59 hours per week or fewer than 40 hours per week (41.5% vs 29.5% and 17.9%; P ≤ .001).
Burned-out cardiologists were also more likely than those who felt stressed or no burnout to report working in a hectic work environment (59.5% vs 32.3% and 14.6%; P ≤ .001) and to have plans to leave their current practice setting (58.1% vs 27.9% and 14.0%; P ≤ .001).
Factors that played a significant role in those plans were the desire to spend more time with family, on-call time, excessive work or relative value unit (RVU) targets, electronic health records, and the pressure to maintain high patient satisfaction scores, Mehta noted.
“Is any of this relatable to decreasing numbers of cardiologists in the U.S., or is there work to try and relate actual work force availability to burnout?” asked session moderator B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute in Charlotte, North Carolina, and a member of ACC’s Board of Trustees, following the presentation.
“It’s hard to decipher all of those exact details, but we do know that the cardiology work force tends to be older, so the mid-careers are going to be pulling on a lot more weight in the next few years, so that is a concern,” Mehta replied.
A big factor, however, is the excessive work hours put in by all cardiologists, especially the increasing amount of time spent with electronic medical records and administrative tasks, which is “taking away the fun we had in cardiology,” she added.
Limitations of the survey include the potential for bias; burnout was self-reported and may vary over time; and the 14% response rate was less than ideal, although the results are consistent with other national surveys, Mehta said.
In the recent Medscape Cardiologist Lifestyle, Happiness & Burnout Report 2020, 29% of respondents reported feeling burnout, 2% depressed, and 15% both burned out and depressed.
The Elephant in the Room
The new findings are “certainly a call to action, but it’s hard to avoid the elephant in the room, which is COVID-19,” said panelist Sandra Lewis, MD, Legacy Good Samaritan Hospital & Medical Center, Portland, Oregon.
“The implications of burnout are really front-and-center with our colleagues, who are working long hours, have hectic work environments, lack of control, and, more than that, a lack of safety of the work situations that we have worked so hard to achieve, as we run out of protective gear, we don’t have masks, as we see our colleagues falling victim to this.”
During her presentation, Mehta highlighted the ACC Clinician Well Being Portal and its COVID-19 Hub, but also several self-care strategies to employ, such as relinquishing control during these uncharted waters, revisiting personal strengths and abilities leveraged in other times of uncertainty, and giving yourself a “brain break” by challenging yourself to chat with a colleague for 30 minutes on topics unrelated to COVID-19 and other workplace stressors.
Wilson said the global pandemic only heightens concerns about burnout among cardiologists, which he likened to a “runaway train.”
“These are not great signals, I think they’re shocking, quite frankly,” Wilson told theheart.org | Medscape Cardiology.
“ACC is setting up a task force from the board of trustees to get to work right away and see about ways we can turn this around as quickly as possible and be a voice for the clinicians,” he said. “It’s not only cardiologists, it’s everybody on our cardiovascular care team, including nurses, physician assistants, nurse practitioners, and even pharmacists. Everybody’s burning out.”
The authors and Wilson report no relevant conflicts of interest.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 403.08. Presented March 28, 2020.
This article first appeared on Medscape.com.
Even before the COVID-19 pandemic, , a new survey shows.
“It is important to recognize the personal and professional repercussions of physician burnout,” lead author Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said during an online session of the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC).
The new ACC 2019 Well Being Survey was sent to 19,348 ACC members in the fall of 2019 and sought to take a deeper dive into the issue of burnout after the ACC’s most recent Professional Life Survey revealed that one in four U.S. cardiologists were burned out in 2015.
While the number of cardiologists who reported feeling stressed fell from 49.5% in 2015 to 43.9% in 2019, the number of cardiologists who reported being burned out increased by 32% from 26.8% to 35.4%, Mehta said.
Among those currently feeling burned out, 23.9% reported having one or more symptoms of burnout, 9.9% had chronic burnout and work frustrations, and 1.6% were “completely burned out” and at the point where they may need to seek help.
Burned-out cardiologists were more likely than those who felt stressed or no burnout to say they may have made a major medical error in the past 3 months (58.3% vs 33.1% and 8.6%; P ≤ .001).
The Usual Suspects
As previously observed, burnout was highest among mid-career cardiologists with 8 to 21 years in practice vs early-career and late-career cardiologists (45.3% vs 35.4% and 31.5%; P ≤ .001) and in women vs men (45.3% vs 33.5%; P ≤ .001). Of the 2025 ACC members who responded, 362 were women.
Several initiatives are underway by the ACC to increase the diversity of cardiology as a specialty, but attention is also needed for mid-career cardiologists, who may not see the “light at the end of the tunnel,” as they take on more clinical demands and more administrative roles, Mehta observed.
Not surprising, clocking 60 or more hours per week increased the risk for burnout, compared with working 40 to 59 hours per week or fewer than 40 hours per week (41.5% vs 29.5% and 17.9%; P ≤ .001).
Burned-out cardiologists were also more likely than those who felt stressed or no burnout to report working in a hectic work environment (59.5% vs 32.3% and 14.6%; P ≤ .001) and to have plans to leave their current practice setting (58.1% vs 27.9% and 14.0%; P ≤ .001).
Factors that played a significant role in those plans were the desire to spend more time with family, on-call time, excessive work or relative value unit (RVU) targets, electronic health records, and the pressure to maintain high patient satisfaction scores, Mehta noted.
“Is any of this relatable to decreasing numbers of cardiologists in the U.S., or is there work to try and relate actual work force availability to burnout?” asked session moderator B. Hadley Wilson, MD, executive vice chair of the Sanger Heart & Vascular Institute in Charlotte, North Carolina, and a member of ACC’s Board of Trustees, following the presentation.
“It’s hard to decipher all of those exact details, but we do know that the cardiology work force tends to be older, so the mid-careers are going to be pulling on a lot more weight in the next few years, so that is a concern,” Mehta replied.
A big factor, however, is the excessive work hours put in by all cardiologists, especially the increasing amount of time spent with electronic medical records and administrative tasks, which is “taking away the fun we had in cardiology,” she added.
Limitations of the survey include the potential for bias; burnout was self-reported and may vary over time; and the 14% response rate was less than ideal, although the results are consistent with other national surveys, Mehta said.
In the recent Medscape Cardiologist Lifestyle, Happiness & Burnout Report 2020, 29% of respondents reported feeling burnout, 2% depressed, and 15% both burned out and depressed.
The Elephant in the Room
The new findings are “certainly a call to action, but it’s hard to avoid the elephant in the room, which is COVID-19,” said panelist Sandra Lewis, MD, Legacy Good Samaritan Hospital & Medical Center, Portland, Oregon.
“The implications of burnout are really front-and-center with our colleagues, who are working long hours, have hectic work environments, lack of control, and, more than that, a lack of safety of the work situations that we have worked so hard to achieve, as we run out of protective gear, we don’t have masks, as we see our colleagues falling victim to this.”
During her presentation, Mehta highlighted the ACC Clinician Well Being Portal and its COVID-19 Hub, but also several self-care strategies to employ, such as relinquishing control during these uncharted waters, revisiting personal strengths and abilities leveraged in other times of uncertainty, and giving yourself a “brain break” by challenging yourself to chat with a colleague for 30 minutes on topics unrelated to COVID-19 and other workplace stressors.
Wilson said the global pandemic only heightens concerns about burnout among cardiologists, which he likened to a “runaway train.”
“These are not great signals, I think they’re shocking, quite frankly,” Wilson told theheart.org | Medscape Cardiology.
“ACC is setting up a task force from the board of trustees to get to work right away and see about ways we can turn this around as quickly as possible and be a voice for the clinicians,” he said. “It’s not only cardiologists, it’s everybody on our cardiovascular care team, including nurses, physician assistants, nurse practitioners, and even pharmacists. Everybody’s burning out.”
The authors and Wilson report no relevant conflicts of interest.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 403.08. Presented March 28, 2020.
This article first appeared on Medscape.com.
Primordial cardiovascular prevention draws closer
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
REPORTING FROM ACC 20
Rivaroxaban plus aspirin safely benefits PAD patients after limb revascularization
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
REPORTING FROM ACC 20
Key clinical point: Combined treatment with rivaroxaban plus aspirin safely reduced a composite measure of adverse ischemic events in PAD patients following lower-limb revascularization.
Major finding: The primary event outcome occurred in 17.3% of patients on rivaroxaban plus aspirin, and in 19.9% on aspirin alone.
Study details: VOYAGER PAD, a multicenter, international randomized trial with 6,564 patients.
Disclosures: VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
Source: Bonaca MP. ACC 20, Abstract 402-10.
Before the COVID-19 surge hits your facility, take steps to boost capacity
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
REPORTING FROM AN ALLIANCE FOR HEALTH POLICY WEBINAR
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”