User login
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
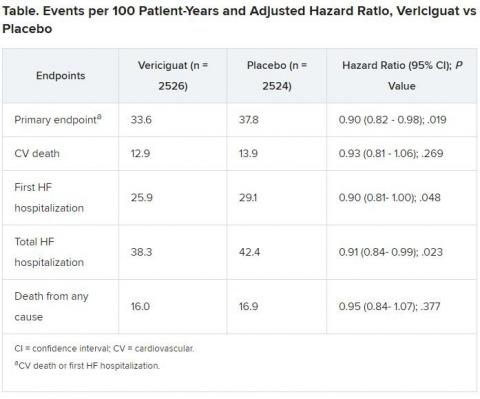
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Not too many years ago, clinicians who treat patients with heart failure, especially those at high risk for decompensation, lamented what seemed a dearth of new drug therapy options.
Now, with the toolbox brimming with new guideline-supported alternatives, .
Importantly, it entered an especially high-risk population with heart failure and reduced ejection fraction (HFrEF); everyone in the trial had experienced a prior, usually quite recent, heart failure exacerbation.
In such patients, the addition of vericiguat (Merck/Bayer) to standard drug and device therapies was followed by a moderately but significantly reduced relative risk for the trial’s primary clinical endpoint over about 11 months.
Recipients benefited with a 10% drop in adjusted risk (P = .019) for cardiovascular (CV) death or first heart failure hospitalization compared to a placebo control group.
But researchers leading the 5050-patient Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA), as well as unaffiliated experts who have studied the trial, say that in this case, risk reduction in absolute numbers is a more telling outcome.
“Remember who we’re talking about here in terms of the patients who have this degree of morbidity and mortality,” VICTORIA study chair Paul W. Armstrong, MD, University of Alberta, Edmonton, Canada, told theheart.org | Medscape Cardiology, pointing to the “incredible placebo-group event rate and relatively modest follow-up of 10.8 months.”
The control group’s primary-endpoint event rate was 37.8 per 100 patient-years, 4.2 points higher than the rate for patients who received vericiguat. “And from there you get a number needed to treat of 24 to prevent one event, which is low,” Armstrong said.
“Think about the hundreds of thousands of people with this disease and what that means at the public health level.” About one in four patients with heart failure experience such exacerbations each year, he said.
Armstrong is lead author on the 42-country trial’s publication today in the New England Journal of Medicine, timed to coincide with his online presentation for the American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). The annual session was conducted virtually this year following the traditional live meeting’s cancelation due to the COVID-19 pandemic.
The VICTORIA presentation and publication flesh out the cursory top-line primary results that Merck unveiled in November 2019, which had not included the magnitude of the vericiguat relative benefit for the primary endpoint.
The trial represents “another win” for the treatment of heart failure, Clyde W. Yancy, MD, Northwestern University, Chicago, Illinois, said as an invited discussant following Armstrong’s presentation.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
“Hospitalization for heart failure generates a major inflection point in the natural history of this condition, with a marked change in the risk for re-hospitalization and death. Up until now, no prior therapies have attenuated this risk, except for more intensive processes and care improvement strategies,” he said.
“Now we have a therapy that may be the first one to change that natural history after a person with heart failure has had a worsening event.”
Interestingly, the primary-endpoint reduction was driven by a significant drop in heart failure hospitalizations, even within a fairly short follow-up time.
“What was fascinating is that the requisite number of events were accrued in less than 12 months — meaning that inexplicably, this is one of the few times we’ve had a trial where the event rate realized was higher than the event rate predicted,” Yancy observed for theheart.org | Medscape Cardiology.
Although the effect size was similar to what was observed for dapagliflozin (Farxiga, AstraZeneca) in DAPA-HF and sacubitril/valsartan (Entresto, Novartis) in PARADIGM-HF, he said, VICTORIA’s population was much sicker and had an “astonishingly high” event rate even while receiving aggressive background heart failure therapy.
It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.