User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
COVID-19: What are the major cardiovascular issues?
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Acute viral myocarditis often confounds with ischemic injury
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
What will pediatrics look like in 2022?
In 1966 I was struggling with the decision of whether to become an art historian or go to medical school. I decided corporate ladder climbs and tenure chases were not for me. I wanted to be my own boss. I reckoned that medicine would offer me rock-solid job security and a comfortable income that I could adjust to my needs simply by working harder. In my Norman Rockwell–influenced view of the world, there would always be sick children. There would never be a quiet week or even a day when I would have to worry about not having an income.
So it was an idyllic existence for decades, tarnished only slightly when corporate entities began gobbling up owner-operator practices. But I never envisioned a pandemic that would turn the world – including its pediatricians – upside down. For the last several weeks as I pedal past my old office, I am dumbstruck by the empty parking lot. For the present I appear to be buffered by my retirement, but know that many of you are under serious financial pressure as a result of the pandemic.
We are all yearning to return to business as usual, but we know that it isn’t going to happen because everything has changed. The usual has yet to be defined. When you finally reopen your offices, you will be walking into a strange and eerie new normal. Initially you may struggle to make it feel like nothing has changed, but very quickly the full force of the postpandemic tsunami will hit us all broadside. In 2 years, the ship may still be rocking but what will clinical pediatrics look like in the late spring of 2022?
Will the patient mix have shifted even more toward behavioral and mental health complaints as a ripple effect of the pandemic’s emotional turmoil? Will your waiting room have become a maze of plexiglass barriers to separate the sick from the well? Has the hospital invested hundreds of thousands of dollars in a ventilation system in hopes of minimizing contagion in your exam rooms? Maybe you will have instituted an appointment schedule with sick visits in the morning and well checks in the afternoon. Or you may no longer have a waiting room because patients are queuing in their cars in the parking lot. Your support staff may be rollerskating around like carhops at a drive-in recording histories and taking vital signs.
Telemedicine will hopefully have gone mainstream with more robust guidelines for billing and quality control. Medical schools may be devoting more attention to teaching student how to assess remotely. Parents may now be equipped with a tool kit of remote sensors so that you can assess their child’s tympanic membranes, pulse rate, oxygen saturation, and blood pressure on your office computer screen.
Will the EHR finally have begun to emerge from its awkward and at times painful adolescence into an easily accessible and transportable nationwide data bank that includes immunization records for all ages? Patients may have been asked or ordered to allow their cell phones to be used as tracking devices for serious communicable diseases. How many vaccine-resistant people will have responded to the pandemic by deciding that immunizations are worth the minimal risks? I fear not many.
How many of your colleagues will have left pediatrics and heeded the call for more epidemiologists? Will you be required to take a CME course in ventilation management? The good news may be that to keep the pediatric workforce robust the government has decided to forgive your student loans.
None of these changes may have come to pass because we have notoriously short memories. But I am sure that we will all still bear the deep scars of this world changing event.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In 1966 I was struggling with the decision of whether to become an art historian or go to medical school. I decided corporate ladder climbs and tenure chases were not for me. I wanted to be my own boss. I reckoned that medicine would offer me rock-solid job security and a comfortable income that I could adjust to my needs simply by working harder. In my Norman Rockwell–influenced view of the world, there would always be sick children. There would never be a quiet week or even a day when I would have to worry about not having an income.
So it was an idyllic existence for decades, tarnished only slightly when corporate entities began gobbling up owner-operator practices. But I never envisioned a pandemic that would turn the world – including its pediatricians – upside down. For the last several weeks as I pedal past my old office, I am dumbstruck by the empty parking lot. For the present I appear to be buffered by my retirement, but know that many of you are under serious financial pressure as a result of the pandemic.
We are all yearning to return to business as usual, but we know that it isn’t going to happen because everything has changed. The usual has yet to be defined. When you finally reopen your offices, you will be walking into a strange and eerie new normal. Initially you may struggle to make it feel like nothing has changed, but very quickly the full force of the postpandemic tsunami will hit us all broadside. In 2 years, the ship may still be rocking but what will clinical pediatrics look like in the late spring of 2022?
Will the patient mix have shifted even more toward behavioral and mental health complaints as a ripple effect of the pandemic’s emotional turmoil? Will your waiting room have become a maze of plexiglass barriers to separate the sick from the well? Has the hospital invested hundreds of thousands of dollars in a ventilation system in hopes of minimizing contagion in your exam rooms? Maybe you will have instituted an appointment schedule with sick visits in the morning and well checks in the afternoon. Or you may no longer have a waiting room because patients are queuing in their cars in the parking lot. Your support staff may be rollerskating around like carhops at a drive-in recording histories and taking vital signs.
Telemedicine will hopefully have gone mainstream with more robust guidelines for billing and quality control. Medical schools may be devoting more attention to teaching student how to assess remotely. Parents may now be equipped with a tool kit of remote sensors so that you can assess their child’s tympanic membranes, pulse rate, oxygen saturation, and blood pressure on your office computer screen.
Will the EHR finally have begun to emerge from its awkward and at times painful adolescence into an easily accessible and transportable nationwide data bank that includes immunization records for all ages? Patients may have been asked or ordered to allow their cell phones to be used as tracking devices for serious communicable diseases. How many vaccine-resistant people will have responded to the pandemic by deciding that immunizations are worth the minimal risks? I fear not many.
How many of your colleagues will have left pediatrics and heeded the call for more epidemiologists? Will you be required to take a CME course in ventilation management? The good news may be that to keep the pediatric workforce robust the government has decided to forgive your student loans.
None of these changes may have come to pass because we have notoriously short memories. But I am sure that we will all still bear the deep scars of this world changing event.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In 1966 I was struggling with the decision of whether to become an art historian or go to medical school. I decided corporate ladder climbs and tenure chases were not for me. I wanted to be my own boss. I reckoned that medicine would offer me rock-solid job security and a comfortable income that I could adjust to my needs simply by working harder. In my Norman Rockwell–influenced view of the world, there would always be sick children. There would never be a quiet week or even a day when I would have to worry about not having an income.
So it was an idyllic existence for decades, tarnished only slightly when corporate entities began gobbling up owner-operator practices. But I never envisioned a pandemic that would turn the world – including its pediatricians – upside down. For the last several weeks as I pedal past my old office, I am dumbstruck by the empty parking lot. For the present I appear to be buffered by my retirement, but know that many of you are under serious financial pressure as a result of the pandemic.
We are all yearning to return to business as usual, but we know that it isn’t going to happen because everything has changed. The usual has yet to be defined. When you finally reopen your offices, you will be walking into a strange and eerie new normal. Initially you may struggle to make it feel like nothing has changed, but very quickly the full force of the postpandemic tsunami will hit us all broadside. In 2 years, the ship may still be rocking but what will clinical pediatrics look like in the late spring of 2022?
Will the patient mix have shifted even more toward behavioral and mental health complaints as a ripple effect of the pandemic’s emotional turmoil? Will your waiting room have become a maze of plexiglass barriers to separate the sick from the well? Has the hospital invested hundreds of thousands of dollars in a ventilation system in hopes of minimizing contagion in your exam rooms? Maybe you will have instituted an appointment schedule with sick visits in the morning and well checks in the afternoon. Or you may no longer have a waiting room because patients are queuing in their cars in the parking lot. Your support staff may be rollerskating around like carhops at a drive-in recording histories and taking vital signs.
Telemedicine will hopefully have gone mainstream with more robust guidelines for billing and quality control. Medical schools may be devoting more attention to teaching student how to assess remotely. Parents may now be equipped with a tool kit of remote sensors so that you can assess their child’s tympanic membranes, pulse rate, oxygen saturation, and blood pressure on your office computer screen.
Will the EHR finally have begun to emerge from its awkward and at times painful adolescence into an easily accessible and transportable nationwide data bank that includes immunization records for all ages? Patients may have been asked or ordered to allow their cell phones to be used as tracking devices for serious communicable diseases. How many vaccine-resistant people will have responded to the pandemic by deciding that immunizations are worth the minimal risks? I fear not many.
How many of your colleagues will have left pediatrics and heeded the call for more epidemiologists? Will you be required to take a CME course in ventilation management? The good news may be that to keep the pediatric workforce robust the government has decided to forgive your student loans.
None of these changes may have come to pass because we have notoriously short memories. But I am sure that we will all still bear the deep scars of this world changing event.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Angiotensin drugs and COVID-19: More reassuring data
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AUGUSTUS: After ACS or PCI, aspirin gives AFib patients scant benefit
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
FROM ACC 2020
Hydroxychloroquine ineffective for COVID-19, VA study suggests
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).
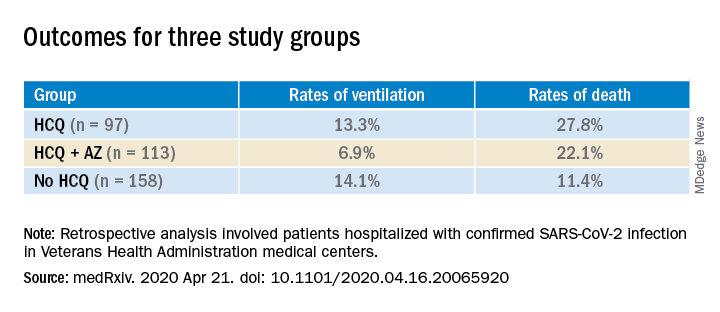
Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prioritizing ambulatory gynecology care during COVID-19: The latest guidance
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
European COVID-19 insights: Try helmet CPAP
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Are patients with epilepsy at increased risk of COVID-19 infection?
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
COVID-19 antibody tests proliferate, but what do they show?
Noopur Raje, MD, has been sitting at home for 5 weeks waiting for her COVID-19 test to turn negative so she can get back to work. She’s a cancer specialist – head of the Massachusetts General Hospital’s Center for Multiple Myeloma – but Raje says as soon as she’s allowed back to the hospital, she’ll head straight to the front line of COVID-19 caregivers.
“It’s people like us who have to get back in the trenches and do the work now,” she told Medscape Medical News.
“I still will be at risk,” she said. But, having nursed her physician husband through COVID-19 at home until he was admitted to an intensive care unit, she is determined to help in the COVID-19 wards.
“I will be the first one to volunteer to take care of these patients,” she said. “I can’t wait, as I want to give these folks hope. They are so scared.”
Around the world, it’s assumed that she and others like her who’ve recovered from COVID-19 will be immune to the infection.
Some have suggested that with antibodies to the virus coursing through their veins, these survivors might be given immunity passports. They could be the ones to jump-start people’s lives again ― the first to be let out from lockdown, and in healthcare, the ones to head the ongoing battle against this pandemic.
So, there has been a race to develop COVID-19 antibody tests to identify these people.
Circumventing the Usual Clearance Process
To speed up the process, the US Food and Drug Administration (FDA) made a much-criticized move to allow a free-for-all for developers to begin marketing antibody tests that had not gone through the agency’s usual evaluation process. The result was a flood of more than 90 unapproved tests “that have, frankly, dubious quality,” said Scott Becker, CEO of the Association of Public Health Laboratories (APHL), which represents local and state public laboratories.
The APHL spoke out in dismay – its chief program officer, Eric Blank, decried the “Wild West” of tests unleashed on the public.
“These tests create more uncertainty than before,” said Kelly Wroblewski, APHL’s director of infectious diseases, in a news conference on April 14. “Having many inaccurate tests is worse than having no tests at all.”
The APHL and the FDA, working with the Centers for Disease Control and Prevention and the National Institutes of Health (NIH), have moved quickly into damage control, conducting evaluations of the tests in an effort to distinguish the potentially useful from the useless.
So far, they have succeeded in issuing emergency use authorizations (EUAs) to only four tests, those marketed by Cellex, Ortho Clinical Diagnostics, Chembio Diagnostic Systems, and the Mount Sinai Laboratory.
For all the other antibody tests on the market that do not have an EUA, “They’re trusting that the test developer has done a good job in validation,” Becker said. But there are worrying anecdotes. “Our members have reported that they’ve seen fraudulent marketing.... We’ve seen the FDA clamp down on some companies... [and] a number of cities and health departments have issued warnings because of what they’ve seen,” he added.
In particular, Wroblewski said, some companies are marketing tests for use in physicians’ offices or pharmacies. “Today, there are no serology tests approved for point-of-care settings,” she warned. “We don’t know how to interpret the test results, if the presence of antibodies indicates immunity, how long it will last, or what titer might be sufficient.”
Uncertainty Emphasized
The FDA emphasized the uncertainty about antibody tests in a statement released on April 18.
Although the tests can identify people who have been exposed and who developed an immune response to the virus, the agency noted, “we don’t yet know that just because someone has developed antibodies, that they are fully protected from reinfection, or how long any immunity lasts.”
The FDA says that the role of these antibody tests, at present, lies in providing information to “help us track the spread of the virus nationwide and assess the impact of our public health efforts now, while also informing our COVID-19 response as we continue to move forward.”
The World Health Organization (WHO) also emphasized the current uncertainty over antibody tests at a press briefing on April 17. “Nobody is sure about the length of protection that antibodies may give and whether they fully protect against ... the disease,” said Mike Ryan, MD, executive director of the WHO’s emergencies program. There is also a concern that such tests may give false assurance or be misused. “There is still a lot of work that needs to be done to validate these antibody tests,” he added.
“The WHO are right to highlight that any antibody test, if we get one, won’t be able to definitely say whether someone is immune to the infection, because we just don’t know enough yet about how immunity works with COVID-19,” commented Prof. Chris Dye, Oxford Martin School, University of Oxford, in reaction on the UK Science Media Center.
Expanding on this point on the same site, Andrew Easton PhD, professor of virology at the University of Warwick, noted that “a serology test does not discriminate between neutralising and non-neutralising antibodies; a discriminatory test is much more complex and slow.”
Only the neutralizing antibodies have the ability to inactivate the invading virus, he noted.
“When people are infected, the proportions of neutralising and non-neutralising antibodies can differ. It is not always understood what makes an antibody neutralising and another non-neutralising, or why an infection leads to production of more of one of these types of antibodies,” he explained. “The initial immune response immediately following infection sets the memory of the immune system, so if the person had generated mostly non-neutralising antibodies, the next time that person encounters the same virus, they may not be able to prevent an infection.”
So at present, the information from antibody testing is largely unhelpful to individuals, but it could be valuable to epidemiologists and policy makers.
“States are looking at ways they can integrate reliable serologic tests for surveillance,” explained APHL’s Blank.
Knowing how widespread the infection has been within a community could guide research and possibly public health decisions, Wroblewski said at the APHL press conference. But she’s hesitant here, too. “I know there has been a lot of talk about using this testing to ease restrictions, but I do think we need to be cautious on how quickly we move in that direction.” If people don’t have antibodies, it means they haven’t been exposed and that they’re still vulnerable, she noted. “If nothing else, that still informs policy decisions, even if they’re not the policy decisions we want.”
Trials Recruiting, Medical Centers Develop Own Tests
Despite the uncertainties over antibody testing, many efforts are still being guided by this strategy.
The NIH is recruiting volunteers to its antibody testing study and suggests that immunity is “likely” for those who test positive.
In addition, several large medical centers have developed their own antibody tests, including Stanford, the Yale New Haven Hospital, and the Mayo Clinic.
The Stanford test detects two types of antibodies: IgM, which is made early in an immune response and usually wanes quickly, and IgG, which rises more slowly after infection but usually persists longer.
“There’s limited data out of China and Europe showing that this appears to be the response pattern followed with this virus,” commented Thomas Montine, MD, PhD, professor and chair of pathology at Stanford University. “But no one has had this long enough to know how long after infection the antibodies persist,” he added.
“There is enormous demand for serologic testing,” said William Morice, MD, PhD, president of Mayo Clinic Laboratories. “At this time, serology testing needs to be prioritized for efforts to identify individuals in areas where potential immunity is key ― supporting healthcare workers, screening for potential plasma donors, and helping advance the most promising vaccine candidates.”
During a recent webinar with the Association for Value-Based Cancer Care, the largest physician-owned oncology-hematology practice in the country, the president, Lucio Gordan, MD, said his organization was looking into antibody testing for staff. “They wanted to see how many have been exposed,” he said, although “what it means is uncertain.”
When Medscape Medical News checked back with him a few weeks later, Gordan, president of Florida Cancer Specialists and Research Institute, reported that no progress had been made.
“We unfortunately have not been able to test yet, due to concerns with reliability of kits. We are waiting for a better solution so we can reassess our strategy,” he said.
This article first appeared on Medscape.com.
Noopur Raje, MD, has been sitting at home for 5 weeks waiting for her COVID-19 test to turn negative so she can get back to work. She’s a cancer specialist – head of the Massachusetts General Hospital’s Center for Multiple Myeloma – but Raje says as soon as she’s allowed back to the hospital, she’ll head straight to the front line of COVID-19 caregivers.
“It’s people like us who have to get back in the trenches and do the work now,” she told Medscape Medical News.
“I still will be at risk,” she said. But, having nursed her physician husband through COVID-19 at home until he was admitted to an intensive care unit, she is determined to help in the COVID-19 wards.
“I will be the first one to volunteer to take care of these patients,” she said. “I can’t wait, as I want to give these folks hope. They are so scared.”
Around the world, it’s assumed that she and others like her who’ve recovered from COVID-19 will be immune to the infection.
Some have suggested that with antibodies to the virus coursing through their veins, these survivors might be given immunity passports. They could be the ones to jump-start people’s lives again ― the first to be let out from lockdown, and in healthcare, the ones to head the ongoing battle against this pandemic.
So, there has been a race to develop COVID-19 antibody tests to identify these people.
Circumventing the Usual Clearance Process
To speed up the process, the US Food and Drug Administration (FDA) made a much-criticized move to allow a free-for-all for developers to begin marketing antibody tests that had not gone through the agency’s usual evaluation process. The result was a flood of more than 90 unapproved tests “that have, frankly, dubious quality,” said Scott Becker, CEO of the Association of Public Health Laboratories (APHL), which represents local and state public laboratories.
The APHL spoke out in dismay – its chief program officer, Eric Blank, decried the “Wild West” of tests unleashed on the public.
“These tests create more uncertainty than before,” said Kelly Wroblewski, APHL’s director of infectious diseases, in a news conference on April 14. “Having many inaccurate tests is worse than having no tests at all.”
The APHL and the FDA, working with the Centers for Disease Control and Prevention and the National Institutes of Health (NIH), have moved quickly into damage control, conducting evaluations of the tests in an effort to distinguish the potentially useful from the useless.
So far, they have succeeded in issuing emergency use authorizations (EUAs) to only four tests, those marketed by Cellex, Ortho Clinical Diagnostics, Chembio Diagnostic Systems, and the Mount Sinai Laboratory.
For all the other antibody tests on the market that do not have an EUA, “They’re trusting that the test developer has done a good job in validation,” Becker said. But there are worrying anecdotes. “Our members have reported that they’ve seen fraudulent marketing.... We’ve seen the FDA clamp down on some companies... [and] a number of cities and health departments have issued warnings because of what they’ve seen,” he added.
In particular, Wroblewski said, some companies are marketing tests for use in physicians’ offices or pharmacies. “Today, there are no serology tests approved for point-of-care settings,” she warned. “We don’t know how to interpret the test results, if the presence of antibodies indicates immunity, how long it will last, or what titer might be sufficient.”
Uncertainty Emphasized
The FDA emphasized the uncertainty about antibody tests in a statement released on April 18.
Although the tests can identify people who have been exposed and who developed an immune response to the virus, the agency noted, “we don’t yet know that just because someone has developed antibodies, that they are fully protected from reinfection, or how long any immunity lasts.”
The FDA says that the role of these antibody tests, at present, lies in providing information to “help us track the spread of the virus nationwide and assess the impact of our public health efforts now, while also informing our COVID-19 response as we continue to move forward.”
The World Health Organization (WHO) also emphasized the current uncertainty over antibody tests at a press briefing on April 17. “Nobody is sure about the length of protection that antibodies may give and whether they fully protect against ... the disease,” said Mike Ryan, MD, executive director of the WHO’s emergencies program. There is also a concern that such tests may give false assurance or be misused. “There is still a lot of work that needs to be done to validate these antibody tests,” he added.
“The WHO are right to highlight that any antibody test, if we get one, won’t be able to definitely say whether someone is immune to the infection, because we just don’t know enough yet about how immunity works with COVID-19,” commented Prof. Chris Dye, Oxford Martin School, University of Oxford, in reaction on the UK Science Media Center.
Expanding on this point on the same site, Andrew Easton PhD, professor of virology at the University of Warwick, noted that “a serology test does not discriminate between neutralising and non-neutralising antibodies; a discriminatory test is much more complex and slow.”
Only the neutralizing antibodies have the ability to inactivate the invading virus, he noted.
“When people are infected, the proportions of neutralising and non-neutralising antibodies can differ. It is not always understood what makes an antibody neutralising and another non-neutralising, or why an infection leads to production of more of one of these types of antibodies,” he explained. “The initial immune response immediately following infection sets the memory of the immune system, so if the person had generated mostly non-neutralising antibodies, the next time that person encounters the same virus, they may not be able to prevent an infection.”
So at present, the information from antibody testing is largely unhelpful to individuals, but it could be valuable to epidemiologists and policy makers.
“States are looking at ways they can integrate reliable serologic tests for surveillance,” explained APHL’s Blank.
Knowing how widespread the infection has been within a community could guide research and possibly public health decisions, Wroblewski said at the APHL press conference. But she’s hesitant here, too. “I know there has been a lot of talk about using this testing to ease restrictions, but I do think we need to be cautious on how quickly we move in that direction.” If people don’t have antibodies, it means they haven’t been exposed and that they’re still vulnerable, she noted. “If nothing else, that still informs policy decisions, even if they’re not the policy decisions we want.”
Trials Recruiting, Medical Centers Develop Own Tests
Despite the uncertainties over antibody testing, many efforts are still being guided by this strategy.
The NIH is recruiting volunteers to its antibody testing study and suggests that immunity is “likely” for those who test positive.
In addition, several large medical centers have developed their own antibody tests, including Stanford, the Yale New Haven Hospital, and the Mayo Clinic.
The Stanford test detects two types of antibodies: IgM, which is made early in an immune response and usually wanes quickly, and IgG, which rises more slowly after infection but usually persists longer.
“There’s limited data out of China and Europe showing that this appears to be the response pattern followed with this virus,” commented Thomas Montine, MD, PhD, professor and chair of pathology at Stanford University. “But no one has had this long enough to know how long after infection the antibodies persist,” he added.
“There is enormous demand for serologic testing,” said William Morice, MD, PhD, president of Mayo Clinic Laboratories. “At this time, serology testing needs to be prioritized for efforts to identify individuals in areas where potential immunity is key ― supporting healthcare workers, screening for potential plasma donors, and helping advance the most promising vaccine candidates.”
During a recent webinar with the Association for Value-Based Cancer Care, the largest physician-owned oncology-hematology practice in the country, the president, Lucio Gordan, MD, said his organization was looking into antibody testing for staff. “They wanted to see how many have been exposed,” he said, although “what it means is uncertain.”
When Medscape Medical News checked back with him a few weeks later, Gordan, president of Florida Cancer Specialists and Research Institute, reported that no progress had been made.
“We unfortunately have not been able to test yet, due to concerns with reliability of kits. We are waiting for a better solution so we can reassess our strategy,” he said.
This article first appeared on Medscape.com.
Noopur Raje, MD, has been sitting at home for 5 weeks waiting for her COVID-19 test to turn negative so she can get back to work. She’s a cancer specialist – head of the Massachusetts General Hospital’s Center for Multiple Myeloma – but Raje says as soon as she’s allowed back to the hospital, she’ll head straight to the front line of COVID-19 caregivers.
“It’s people like us who have to get back in the trenches and do the work now,” she told Medscape Medical News.
“I still will be at risk,” she said. But, having nursed her physician husband through COVID-19 at home until he was admitted to an intensive care unit, she is determined to help in the COVID-19 wards.
“I will be the first one to volunteer to take care of these patients,” she said. “I can’t wait, as I want to give these folks hope. They are so scared.”
Around the world, it’s assumed that she and others like her who’ve recovered from COVID-19 will be immune to the infection.
Some have suggested that with antibodies to the virus coursing through their veins, these survivors might be given immunity passports. They could be the ones to jump-start people’s lives again ― the first to be let out from lockdown, and in healthcare, the ones to head the ongoing battle against this pandemic.
So, there has been a race to develop COVID-19 antibody tests to identify these people.
Circumventing the Usual Clearance Process
To speed up the process, the US Food and Drug Administration (FDA) made a much-criticized move to allow a free-for-all for developers to begin marketing antibody tests that had not gone through the agency’s usual evaluation process. The result was a flood of more than 90 unapproved tests “that have, frankly, dubious quality,” said Scott Becker, CEO of the Association of Public Health Laboratories (APHL), which represents local and state public laboratories.
The APHL spoke out in dismay – its chief program officer, Eric Blank, decried the “Wild West” of tests unleashed on the public.
“These tests create more uncertainty than before,” said Kelly Wroblewski, APHL’s director of infectious diseases, in a news conference on April 14. “Having many inaccurate tests is worse than having no tests at all.”
The APHL and the FDA, working with the Centers for Disease Control and Prevention and the National Institutes of Health (NIH), have moved quickly into damage control, conducting evaluations of the tests in an effort to distinguish the potentially useful from the useless.
So far, they have succeeded in issuing emergency use authorizations (EUAs) to only four tests, those marketed by Cellex, Ortho Clinical Diagnostics, Chembio Diagnostic Systems, and the Mount Sinai Laboratory.
For all the other antibody tests on the market that do not have an EUA, “They’re trusting that the test developer has done a good job in validation,” Becker said. But there are worrying anecdotes. “Our members have reported that they’ve seen fraudulent marketing.... We’ve seen the FDA clamp down on some companies... [and] a number of cities and health departments have issued warnings because of what they’ve seen,” he added.
In particular, Wroblewski said, some companies are marketing tests for use in physicians’ offices or pharmacies. “Today, there are no serology tests approved for point-of-care settings,” she warned. “We don’t know how to interpret the test results, if the presence of antibodies indicates immunity, how long it will last, or what titer might be sufficient.”
Uncertainty Emphasized
The FDA emphasized the uncertainty about antibody tests in a statement released on April 18.
Although the tests can identify people who have been exposed and who developed an immune response to the virus, the agency noted, “we don’t yet know that just because someone has developed antibodies, that they are fully protected from reinfection, or how long any immunity lasts.”
The FDA says that the role of these antibody tests, at present, lies in providing information to “help us track the spread of the virus nationwide and assess the impact of our public health efforts now, while also informing our COVID-19 response as we continue to move forward.”
The World Health Organization (WHO) also emphasized the current uncertainty over antibody tests at a press briefing on April 17. “Nobody is sure about the length of protection that antibodies may give and whether they fully protect against ... the disease,” said Mike Ryan, MD, executive director of the WHO’s emergencies program. There is also a concern that such tests may give false assurance or be misused. “There is still a lot of work that needs to be done to validate these antibody tests,” he added.
“The WHO are right to highlight that any antibody test, if we get one, won’t be able to definitely say whether someone is immune to the infection, because we just don’t know enough yet about how immunity works with COVID-19,” commented Prof. Chris Dye, Oxford Martin School, University of Oxford, in reaction on the UK Science Media Center.
Expanding on this point on the same site, Andrew Easton PhD, professor of virology at the University of Warwick, noted that “a serology test does not discriminate between neutralising and non-neutralising antibodies; a discriminatory test is much more complex and slow.”
Only the neutralizing antibodies have the ability to inactivate the invading virus, he noted.
“When people are infected, the proportions of neutralising and non-neutralising antibodies can differ. It is not always understood what makes an antibody neutralising and another non-neutralising, or why an infection leads to production of more of one of these types of antibodies,” he explained. “The initial immune response immediately following infection sets the memory of the immune system, so if the person had generated mostly non-neutralising antibodies, the next time that person encounters the same virus, they may not be able to prevent an infection.”
So at present, the information from antibody testing is largely unhelpful to individuals, but it could be valuable to epidemiologists and policy makers.
“States are looking at ways they can integrate reliable serologic tests for surveillance,” explained APHL’s Blank.
Knowing how widespread the infection has been within a community could guide research and possibly public health decisions, Wroblewski said at the APHL press conference. But she’s hesitant here, too. “I know there has been a lot of talk about using this testing to ease restrictions, but I do think we need to be cautious on how quickly we move in that direction.” If people don’t have antibodies, it means they haven’t been exposed and that they’re still vulnerable, she noted. “If nothing else, that still informs policy decisions, even if they’re not the policy decisions we want.”
Trials Recruiting, Medical Centers Develop Own Tests
Despite the uncertainties over antibody testing, many efforts are still being guided by this strategy.
The NIH is recruiting volunteers to its antibody testing study and suggests that immunity is “likely” for those who test positive.
In addition, several large medical centers have developed their own antibody tests, including Stanford, the Yale New Haven Hospital, and the Mayo Clinic.
The Stanford test detects two types of antibodies: IgM, which is made early in an immune response and usually wanes quickly, and IgG, which rises more slowly after infection but usually persists longer.
“There’s limited data out of China and Europe showing that this appears to be the response pattern followed with this virus,” commented Thomas Montine, MD, PhD, professor and chair of pathology at Stanford University. “But no one has had this long enough to know how long after infection the antibodies persist,” he added.
“There is enormous demand for serologic testing,” said William Morice, MD, PhD, president of Mayo Clinic Laboratories. “At this time, serology testing needs to be prioritized for efforts to identify individuals in areas where potential immunity is key ― supporting healthcare workers, screening for potential plasma donors, and helping advance the most promising vaccine candidates.”
During a recent webinar with the Association for Value-Based Cancer Care, the largest physician-owned oncology-hematology practice in the country, the president, Lucio Gordan, MD, said his organization was looking into antibody testing for staff. “They wanted to see how many have been exposed,” he said, although “what it means is uncertain.”
When Medscape Medical News checked back with him a few weeks later, Gordan, president of Florida Cancer Specialists and Research Institute, reported that no progress had been made.
“We unfortunately have not been able to test yet, due to concerns with reliability of kits. We are waiting for a better solution so we can reassess our strategy,” he said.
This article first appeared on Medscape.com.
Sudden loss of taste and smell should be part of COVID-19 screen
A number of new publications show that a high proportion of people infected with COVID-19 report loss of smell and/or taste, with their authors adding to the clamor to recognize these symptoms as potentially indicative of the infection.
In particular, there is a belief that these signs may be present in many with asymptomatic COVID-19, and therefore asking about them could be a way to prioritize people for initial testing for the SARS-CoV-2 virus in the absence of other symptoms.
Anyone testing positive could then quarantine, and their contacts could be traced.
Despite this, the World Health Organization (WHO) has not listed loss of smell or taste as potential symptoms of SARS-CoV-2 infection.
But the US Centers for Disease Control and Prevention (CDC) has now added “new loss of taste or smell” as a symptom on its COVID-19 information page.
American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) executive vice president and CEO James C. Denneny III, MD, believes the symptoms may be an early warning signal.
And there’s no downside to checking for these, Denneny told Medscape Medical News.
“Given the fact that this doesn’t require any surgical procedure, biopsy, or specific treatment, I think the upside of getting it early is great,” he said. “The downside of using it as a symptom, and if someone doesn’t turn out to have it, is virtually zero.”
Claire Hopkins, MD, president of the British Rhinological Society, and colleagues, writing in Lancet Infectious Diseases, agree.
“Physicians evaluating patients with acute-onset loss of smell or taste, particularly in the context of a patent nasal airway, should have a high index of suspicion for concomitant SARS-CoV-2 infection.”
They also observe that this appears to occur, in contrast to other respiratory infections, “in the absence of nasal congestion or rhinorrhea.”
Newest Publications Find Smell and Taste Loss Is Common
Author of one of the newly published studies, Carol H. Yan, MD, an otolaryngologist and head and neck surgeon at the University of California, San Diego, also thinks that sudden smell and taste loss seem to be fairly specific markers of COVID-19.
In her survey of patients who presented to UC San Diego Health for SARS-CoV-2 testing, Yan and colleagues reported that 68% (40 of 59) of COVID-19–positive patients reported olfactory impairment and 71% (42 of 59) reported taste impairment.
Among the 203 people in the “control” group who were polymerase chain reaction–negative (PCR–) for SARS-CoV-2, just 16% had smell loss and 17% had taste loss, according to their results published in the International Forum of Allergy & Rhinology.
“Based on our study, if you have smell and taste loss, you are more than 10 times more likely to have COVID-19 infection than other causes of infection. The most common first sign of a COVID-19 infection remains fever, but fatigue and loss of smell and taste follow as other very common initial symptoms,” said Yan.
“We know COVID-19 is an extremely contagious virus. This study supports the need to be aware of smell and taste loss as early signs of COVID-19.”
Yan told Medscape Medical News that another not-yet-published analysis indicates that sudden loss of smell or taste “may be more representative of a mild form of disease.”
Getting these people tested and isolated could therefore help prevent spread of COVID-19, she urged.
Based on Yan’s report and other case reports, the UC San Diego Health system is now asking all callers to its COVID-19 hotlines, and all visitors and staff, if they’ve had a sudden loss of taste or smell in the last few weeks, she explained.
And Ahmad R. Sedaghat, MD, PhD, at the University of Cincinnati, Ohio, takes a similar view.
In a new systematic review of the topic published April 14 in Laryngoscope Investigative Otolaryngology, Sedaghat and colleagues write: “Anosmia (total loss of smell) without nasal obstruction, in particular, appears to be a highly specific indicator of COVID-19.”
Sedaghat said a sudden loss of sense of smell wouldn’t necessarily lead people to think they have COVID-19, particularly if they remain asymptomatic, so “these individuals could continue business as usual and spread the disease as a carrier.”
“If someone experiences anosmia without nasal obstruction, aside from quarantining, it would not be unreasonable to reach out to one’s primary care physician about getting tested,” he said in a statement from his institution.
Symptom Checkers Add Weight
Several organizations around the world have begun collecting symptom reports from patients and clinicians, which has shone more light on the sudden loss of taste and smell as potential flags for COVID-19.
In an April 14 Morbidity and Mortality Weekly report from the CDC on COVID-19 infections in healthcare workers, of the 5000 who reported symptoms, 750 (16%) wrote “loss of smell or taste” as an “other” symptom.
Meanwhile, the COVID Symptom Tracker smartphone app, a joint effort by Massachusetts General Hospital, Boston, Stanford (Calif.) University, and King’s College, London, which as of press time, was monitoring some 2.5 million people, has had similar findings.
In a preprint publication on 400,000 people reporting one or more symptoms between March 24 and 29 on the tracker, 18% had lost their sense of smell or taste — more than the 10% who reported fever, but far less than the 53% who reported fatigue.
Only 1702 of the 400,000 had received a COVID-19 test.
Of those, 579 had tested positive and 1123 were negative.
The organizers estimated that of those who were positive, 59% reported losing smell or taste, compared with just 18% who tested negative.
“When combined with other symptoms, people with loss of smell and taste appear to be three times more likely to have contracted COVID-19 according to our data,” said Tim Spector, MD, a genetic epidemiologist at King’s College and the app’s lead researcher, on the symptom tracker’s website.
These people “should therefore self-isolate for 7 days to reduce the spread of the disease,” he urged.
Anosmia Is the Initial Symptom in Many Patients With COVID-19
The AAO-HNS also began collecting data from physicians and patients on March 25 through its Web-based 16-question symptom tracking tool.
It has received more than 500 reports of sudden taste or smell loss, said Denneny.
In a report on the first 237 responses, published in Otolaryngology-Head and Neck Surgery, anosmia (profound loss of smell) was found in 73% of subjects before a COVID-19 diagnosis and was the initial symptom in 27% of those subjects.
That latter determination “was the single most important finding,” said Denneny, noting it shows that smell and taste loss are “a sentinel symptom.”
Anosmia led to testing in only 40% of the cases.
Half of the reports came from otolaryngologists, but a large number came from other medical specialties, especially from family medicine.
Just 2% of reports came from patients in that first group, which was based on responses through April 3.
Denneny said that more reports are now coming in from patients, which he attributes to widespread media coverage about the loss of taste and smell.
It’s still not entirely clear why SARS-CoV-2 might inhibit taste or smell. More common viruses like influenza and other coronaviruses can also cause smell and taste loss.
So far, it seems like the sensory recovery is faster for SARS-CoV-2 than the other viruses, which suggests a potentially different mechanism of action, said Yan. Patients she surveyed at UC San Diego recovered the senses within a few weeks to a month, compared to months or a year with the more common viruses.
Yan’s study was partially supported by the National Institutes of Health. Sedaghat has reported no relevant financial relationships. The COVID Symptom Tracker is supported by Zoe Global Limited and has received grants from the Wellcome Trust, Medical Research Council/British Heart Foundation, and Biological Informative Markers for Stratification of Hypertension.
This article first appeared on Medscape.com.
A number of new publications show that a high proportion of people infected with COVID-19 report loss of smell and/or taste, with their authors adding to the clamor to recognize these symptoms as potentially indicative of the infection.
In particular, there is a belief that these signs may be present in many with asymptomatic COVID-19, and therefore asking about them could be a way to prioritize people for initial testing for the SARS-CoV-2 virus in the absence of other symptoms.
Anyone testing positive could then quarantine, and their contacts could be traced.
Despite this, the World Health Organization (WHO) has not listed loss of smell or taste as potential symptoms of SARS-CoV-2 infection.
But the US Centers for Disease Control and Prevention (CDC) has now added “new loss of taste or smell” as a symptom on its COVID-19 information page.
American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) executive vice president and CEO James C. Denneny III, MD, believes the symptoms may be an early warning signal.
And there’s no downside to checking for these, Denneny told Medscape Medical News.
“Given the fact that this doesn’t require any surgical procedure, biopsy, or specific treatment, I think the upside of getting it early is great,” he said. “The downside of using it as a symptom, and if someone doesn’t turn out to have it, is virtually zero.”
Claire Hopkins, MD, president of the British Rhinological Society, and colleagues, writing in Lancet Infectious Diseases, agree.
“Physicians evaluating patients with acute-onset loss of smell or taste, particularly in the context of a patent nasal airway, should have a high index of suspicion for concomitant SARS-CoV-2 infection.”
They also observe that this appears to occur, in contrast to other respiratory infections, “in the absence of nasal congestion or rhinorrhea.”
Newest Publications Find Smell and Taste Loss Is Common
Author of one of the newly published studies, Carol H. Yan, MD, an otolaryngologist and head and neck surgeon at the University of California, San Diego, also thinks that sudden smell and taste loss seem to be fairly specific markers of COVID-19.
In her survey of patients who presented to UC San Diego Health for SARS-CoV-2 testing, Yan and colleagues reported that 68% (40 of 59) of COVID-19–positive patients reported olfactory impairment and 71% (42 of 59) reported taste impairment.
Among the 203 people in the “control” group who were polymerase chain reaction–negative (PCR–) for SARS-CoV-2, just 16% had smell loss and 17% had taste loss, according to their results published in the International Forum of Allergy & Rhinology.
“Based on our study, if you have smell and taste loss, you are more than 10 times more likely to have COVID-19 infection than other causes of infection. The most common first sign of a COVID-19 infection remains fever, but fatigue and loss of smell and taste follow as other very common initial symptoms,” said Yan.
“We know COVID-19 is an extremely contagious virus. This study supports the need to be aware of smell and taste loss as early signs of COVID-19.”
Yan told Medscape Medical News that another not-yet-published analysis indicates that sudden loss of smell or taste “may be more representative of a mild form of disease.”
Getting these people tested and isolated could therefore help prevent spread of COVID-19, she urged.
Based on Yan’s report and other case reports, the UC San Diego Health system is now asking all callers to its COVID-19 hotlines, and all visitors and staff, if they’ve had a sudden loss of taste or smell in the last few weeks, she explained.
And Ahmad R. Sedaghat, MD, PhD, at the University of Cincinnati, Ohio, takes a similar view.
In a new systematic review of the topic published April 14 in Laryngoscope Investigative Otolaryngology, Sedaghat and colleagues write: “Anosmia (total loss of smell) without nasal obstruction, in particular, appears to be a highly specific indicator of COVID-19.”
Sedaghat said a sudden loss of sense of smell wouldn’t necessarily lead people to think they have COVID-19, particularly if they remain asymptomatic, so “these individuals could continue business as usual and spread the disease as a carrier.”
“If someone experiences anosmia without nasal obstruction, aside from quarantining, it would not be unreasonable to reach out to one’s primary care physician about getting tested,” he said in a statement from his institution.
Symptom Checkers Add Weight
Several organizations around the world have begun collecting symptom reports from patients and clinicians, which has shone more light on the sudden loss of taste and smell as potential flags for COVID-19.
In an April 14 Morbidity and Mortality Weekly report from the CDC on COVID-19 infections in healthcare workers, of the 5000 who reported symptoms, 750 (16%) wrote “loss of smell or taste” as an “other” symptom.
Meanwhile, the COVID Symptom Tracker smartphone app, a joint effort by Massachusetts General Hospital, Boston, Stanford (Calif.) University, and King’s College, London, which as of press time, was monitoring some 2.5 million people, has had similar findings.
In a preprint publication on 400,000 people reporting one or more symptoms between March 24 and 29 on the tracker, 18% had lost their sense of smell or taste — more than the 10% who reported fever, but far less than the 53% who reported fatigue.
Only 1702 of the 400,000 had received a COVID-19 test.
Of those, 579 had tested positive and 1123 were negative.
The organizers estimated that of those who were positive, 59% reported losing smell or taste, compared with just 18% who tested negative.
“When combined with other symptoms, people with loss of smell and taste appear to be three times more likely to have contracted COVID-19 according to our data,” said Tim Spector, MD, a genetic epidemiologist at King’s College and the app’s lead researcher, on the symptom tracker’s website.
These people “should therefore self-isolate for 7 days to reduce the spread of the disease,” he urged.
Anosmia Is the Initial Symptom in Many Patients With COVID-19
The AAO-HNS also began collecting data from physicians and patients on March 25 through its Web-based 16-question symptom tracking tool.
It has received more than 500 reports of sudden taste or smell loss, said Denneny.
In a report on the first 237 responses, published in Otolaryngology-Head and Neck Surgery, anosmia (profound loss of smell) was found in 73% of subjects before a COVID-19 diagnosis and was the initial symptom in 27% of those subjects.
That latter determination “was the single most important finding,” said Denneny, noting it shows that smell and taste loss are “a sentinel symptom.”
Anosmia led to testing in only 40% of the cases.
Half of the reports came from otolaryngologists, but a large number came from other medical specialties, especially from family medicine.
Just 2% of reports came from patients in that first group, which was based on responses through April 3.
Denneny said that more reports are now coming in from patients, which he attributes to widespread media coverage about the loss of taste and smell.
It’s still not entirely clear why SARS-CoV-2 might inhibit taste or smell. More common viruses like influenza and other coronaviruses can also cause smell and taste loss.
So far, it seems like the sensory recovery is faster for SARS-CoV-2 than the other viruses, which suggests a potentially different mechanism of action, said Yan. Patients she surveyed at UC San Diego recovered the senses within a few weeks to a month, compared to months or a year with the more common viruses.
Yan’s study was partially supported by the National Institutes of Health. Sedaghat has reported no relevant financial relationships. The COVID Symptom Tracker is supported by Zoe Global Limited and has received grants from the Wellcome Trust, Medical Research Council/British Heart Foundation, and Biological Informative Markers for Stratification of Hypertension.
This article first appeared on Medscape.com.
A number of new publications show that a high proportion of people infected with COVID-19 report loss of smell and/or taste, with their authors adding to the clamor to recognize these symptoms as potentially indicative of the infection.
In particular, there is a belief that these signs may be present in many with asymptomatic COVID-19, and therefore asking about them could be a way to prioritize people for initial testing for the SARS-CoV-2 virus in the absence of other symptoms.
Anyone testing positive could then quarantine, and their contacts could be traced.
Despite this, the World Health Organization (WHO) has not listed loss of smell or taste as potential symptoms of SARS-CoV-2 infection.
But the US Centers for Disease Control and Prevention (CDC) has now added “new loss of taste or smell” as a symptom on its COVID-19 information page.
American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) executive vice president and CEO James C. Denneny III, MD, believes the symptoms may be an early warning signal.
And there’s no downside to checking for these, Denneny told Medscape Medical News.
“Given the fact that this doesn’t require any surgical procedure, biopsy, or specific treatment, I think the upside of getting it early is great,” he said. “The downside of using it as a symptom, and if someone doesn’t turn out to have it, is virtually zero.”
Claire Hopkins, MD, president of the British Rhinological Society, and colleagues, writing in Lancet Infectious Diseases, agree.
“Physicians evaluating patients with acute-onset loss of smell or taste, particularly in the context of a patent nasal airway, should have a high index of suspicion for concomitant SARS-CoV-2 infection.”
They also observe that this appears to occur, in contrast to other respiratory infections, “in the absence of nasal congestion or rhinorrhea.”
Newest Publications Find Smell and Taste Loss Is Common
Author of one of the newly published studies, Carol H. Yan, MD, an otolaryngologist and head and neck surgeon at the University of California, San Diego, also thinks that sudden smell and taste loss seem to be fairly specific markers of COVID-19.
In her survey of patients who presented to UC San Diego Health for SARS-CoV-2 testing, Yan and colleagues reported that 68% (40 of 59) of COVID-19–positive patients reported olfactory impairment and 71% (42 of 59) reported taste impairment.
Among the 203 people in the “control” group who were polymerase chain reaction–negative (PCR–) for SARS-CoV-2, just 16% had smell loss and 17% had taste loss, according to their results published in the International Forum of Allergy & Rhinology.
“Based on our study, if you have smell and taste loss, you are more than 10 times more likely to have COVID-19 infection than other causes of infection. The most common first sign of a COVID-19 infection remains fever, but fatigue and loss of smell and taste follow as other very common initial symptoms,” said Yan.
“We know COVID-19 is an extremely contagious virus. This study supports the need to be aware of smell and taste loss as early signs of COVID-19.”
Yan told Medscape Medical News that another not-yet-published analysis indicates that sudden loss of smell or taste “may be more representative of a mild form of disease.”
Getting these people tested and isolated could therefore help prevent spread of COVID-19, she urged.
Based on Yan’s report and other case reports, the UC San Diego Health system is now asking all callers to its COVID-19 hotlines, and all visitors and staff, if they’ve had a sudden loss of taste or smell in the last few weeks, she explained.
And Ahmad R. Sedaghat, MD, PhD, at the University of Cincinnati, Ohio, takes a similar view.
In a new systematic review of the topic published April 14 in Laryngoscope Investigative Otolaryngology, Sedaghat and colleagues write: “Anosmia (total loss of smell) without nasal obstruction, in particular, appears to be a highly specific indicator of COVID-19.”
Sedaghat said a sudden loss of sense of smell wouldn’t necessarily lead people to think they have COVID-19, particularly if they remain asymptomatic, so “these individuals could continue business as usual and spread the disease as a carrier.”
“If someone experiences anosmia without nasal obstruction, aside from quarantining, it would not be unreasonable to reach out to one’s primary care physician about getting tested,” he said in a statement from his institution.
Symptom Checkers Add Weight
Several organizations around the world have begun collecting symptom reports from patients and clinicians, which has shone more light on the sudden loss of taste and smell as potential flags for COVID-19.
In an April 14 Morbidity and Mortality Weekly report from the CDC on COVID-19 infections in healthcare workers, of the 5000 who reported symptoms, 750 (16%) wrote “loss of smell or taste” as an “other” symptom.
Meanwhile, the COVID Symptom Tracker smartphone app, a joint effort by Massachusetts General Hospital, Boston, Stanford (Calif.) University, and King’s College, London, which as of press time, was monitoring some 2.5 million people, has had similar findings.
In a preprint publication on 400,000 people reporting one or more symptoms between March 24 and 29 on the tracker, 18% had lost their sense of smell or taste — more than the 10% who reported fever, but far less than the 53% who reported fatigue.
Only 1702 of the 400,000 had received a COVID-19 test.
Of those, 579 had tested positive and 1123 were negative.
The organizers estimated that of those who were positive, 59% reported losing smell or taste, compared with just 18% who tested negative.
“When combined with other symptoms, people with loss of smell and taste appear to be three times more likely to have contracted COVID-19 according to our data,” said Tim Spector, MD, a genetic epidemiologist at King’s College and the app’s lead researcher, on the symptom tracker’s website.
These people “should therefore self-isolate for 7 days to reduce the spread of the disease,” he urged.
Anosmia Is the Initial Symptom in Many Patients With COVID-19
The AAO-HNS also began collecting data from physicians and patients on March 25 through its Web-based 16-question symptom tracking tool.
It has received more than 500 reports of sudden taste or smell loss, said Denneny.
In a report on the first 237 responses, published in Otolaryngology-Head and Neck Surgery, anosmia (profound loss of smell) was found in 73% of subjects before a COVID-19 diagnosis and was the initial symptom in 27% of those subjects.
That latter determination “was the single most important finding,” said Denneny, noting it shows that smell and taste loss are “a sentinel symptom.”
Anosmia led to testing in only 40% of the cases.
Half of the reports came from otolaryngologists, but a large number came from other medical specialties, especially from family medicine.
Just 2% of reports came from patients in that first group, which was based on responses through April 3.
Denneny said that more reports are now coming in from patients, which he attributes to widespread media coverage about the loss of taste and smell.
It’s still not entirely clear why SARS-CoV-2 might inhibit taste or smell. More common viruses like influenza and other coronaviruses can also cause smell and taste loss.
So far, it seems like the sensory recovery is faster for SARS-CoV-2 than the other viruses, which suggests a potentially different mechanism of action, said Yan. Patients she surveyed at UC San Diego recovered the senses within a few weeks to a month, compared to months or a year with the more common viruses.
Yan’s study was partially supported by the National Institutes of Health. Sedaghat has reported no relevant financial relationships. The COVID Symptom Tracker is supported by Zoe Global Limited and has received grants from the Wellcome Trust, Medical Research Council/British Heart Foundation, and Biological Informative Markers for Stratification of Hypertension.
This article first appeared on Medscape.com.










