User login
‘Cytokine converter’ cells alleviate psoriasis in mice
Implantation of cells designed to sense the presence of proinflammatory cytokines and subsequently produce and deliver anti-inflammatory molecules prevented flares of psoriasis in mice, according to Lina Schukur of the department of Biosystems Science and Engineering, ETH Zurich, in Basel, Switzerland, and associates.
The investigators designed and engineered human cells that they explain “sequentially detected elevated TNF [tumor necrosis factor] and IL-22 [interleukin-22] levels from a psoriatic flare and, in response, produced therapeutic doses of IL-4 [interleukin-4] and IL-10 [interleukin-10].” TNF and IL-22 are proinflammatory cytokines, which have been found to be upregulated in patients with active psoriasis; and IL-4 and IL-10 are immunomodulatory cytokines that have been shown to induce rapid improvements in people with psoriasis in phase II clinical trials, they noted.
The researchers implanted these designer cells in mice with psoriasis-like lesions, which showed that this “antipsoriatic cytokine converter network” was able to improve skin lesions “and restore dermal tissue morphology.” They also found that the cells were responsive in blood samples from psoriasis patients, which suggested that the cytokine converter “is sufficiently sensitive to detect circulating TNF and IL-22 in humans.”
“These synthetic circuits, which program designer cells to process complex metabolic information, open the door to autonomously prevent, attenuate, or reset acute or chronic medical conditions without constant injections of drugs or cumbersome dosing schedules, and thus provide a new opportunity for personalized medicine,” the investigators wrote.
An editor’s summary of the study states that, “in demonstrating that the converter cells were responsive to blood from psoriasis patients, the authors suggest that synthetic biology may be ready to autonomously flip therapeutic switches in people and later take on other diseases with defined disease indicators.”
The full study, published on Dec. 16, 2015, is available in Science Translational Medicine at http://stm.sciencemag.org/content/7/318/318ra201.
Implantation of cells designed to sense the presence of proinflammatory cytokines and subsequently produce and deliver anti-inflammatory molecules prevented flares of psoriasis in mice, according to Lina Schukur of the department of Biosystems Science and Engineering, ETH Zurich, in Basel, Switzerland, and associates.
The investigators designed and engineered human cells that they explain “sequentially detected elevated TNF [tumor necrosis factor] and IL-22 [interleukin-22] levels from a psoriatic flare and, in response, produced therapeutic doses of IL-4 [interleukin-4] and IL-10 [interleukin-10].” TNF and IL-22 are proinflammatory cytokines, which have been found to be upregulated in patients with active psoriasis; and IL-4 and IL-10 are immunomodulatory cytokines that have been shown to induce rapid improvements in people with psoriasis in phase II clinical trials, they noted.
The researchers implanted these designer cells in mice with psoriasis-like lesions, which showed that this “antipsoriatic cytokine converter network” was able to improve skin lesions “and restore dermal tissue morphology.” They also found that the cells were responsive in blood samples from psoriasis patients, which suggested that the cytokine converter “is sufficiently sensitive to detect circulating TNF and IL-22 in humans.”
“These synthetic circuits, which program designer cells to process complex metabolic information, open the door to autonomously prevent, attenuate, or reset acute or chronic medical conditions without constant injections of drugs or cumbersome dosing schedules, and thus provide a new opportunity for personalized medicine,” the investigators wrote.
An editor’s summary of the study states that, “in demonstrating that the converter cells were responsive to blood from psoriasis patients, the authors suggest that synthetic biology may be ready to autonomously flip therapeutic switches in people and later take on other diseases with defined disease indicators.”
The full study, published on Dec. 16, 2015, is available in Science Translational Medicine at http://stm.sciencemag.org/content/7/318/318ra201.
Implantation of cells designed to sense the presence of proinflammatory cytokines and subsequently produce and deliver anti-inflammatory molecules prevented flares of psoriasis in mice, according to Lina Schukur of the department of Biosystems Science and Engineering, ETH Zurich, in Basel, Switzerland, and associates.
The investigators designed and engineered human cells that they explain “sequentially detected elevated TNF [tumor necrosis factor] and IL-22 [interleukin-22] levels from a psoriatic flare and, in response, produced therapeutic doses of IL-4 [interleukin-4] and IL-10 [interleukin-10].” TNF and IL-22 are proinflammatory cytokines, which have been found to be upregulated in patients with active psoriasis; and IL-4 and IL-10 are immunomodulatory cytokines that have been shown to induce rapid improvements in people with psoriasis in phase II clinical trials, they noted.
The researchers implanted these designer cells in mice with psoriasis-like lesions, which showed that this “antipsoriatic cytokine converter network” was able to improve skin lesions “and restore dermal tissue morphology.” They also found that the cells were responsive in blood samples from psoriasis patients, which suggested that the cytokine converter “is sufficiently sensitive to detect circulating TNF and IL-22 in humans.”
“These synthetic circuits, which program designer cells to process complex metabolic information, open the door to autonomously prevent, attenuate, or reset acute or chronic medical conditions without constant injections of drugs or cumbersome dosing schedules, and thus provide a new opportunity for personalized medicine,” the investigators wrote.
An editor’s summary of the study states that, “in demonstrating that the converter cells were responsive to blood from psoriasis patients, the authors suggest that synthetic biology may be ready to autonomously flip therapeutic switches in people and later take on other diseases with defined disease indicators.”
The full study, published on Dec. 16, 2015, is available in Science Translational Medicine at http://stm.sciencemag.org/content/7/318/318ra201.
FROM SCIENCE TRANSLATIONAL MEDICINE
Poor response to third anti-TNF agent seen in most psoriatic arthritis patients
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti-TNF agents are likely to respond poorly, if at all, to a third one.
Major finding: 47% of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers.
Data source: A prospective, open-label, longitudinal study involving 217 patients who switched anti-TNF therapy once and 57 who switched twice during a 9-year period.
Disclosures: This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Genetic differences may help predict progression of PsC to PsA
Dermatology researchers have uncovered differences in the genetic architecture of psoriatic arthritis (PsA) and cutaneous psoriasis (PsC).
Dr. Philip E. Stuart of the University of Michigan, Ann Arbor, and his colleagues from 32 institutions in the United States, Europe, and Canada, carried out a genome-wide association study (GWAS) of 1,430 patients with psoriatic arthritis and 1,417 healthy controls. They then combined results of their GWAS with five published studies of psoriasis associations (three GWASs and two targeted studies), comprising 3,061 psoriatic arthritis patients, 3,110 cutaneous-only psoriasis patients, and 9,273 psoriasis vulgaris patients, and 13,670 healthy controls of European descent.
The work, published in the American Journal of Human Genetics, identified five regions of significance in the PsA GWAS. These were all in known risk regions for PsA, with the strongest signals near HLA-B, IL12B, TRAF3IP2, TNIP1, and TYK2.
Looking across all studies, researchers detected 10 regions associated with PsA and 11 with PsC at genome-wide significance. Several of these association signals (IFNLR1, IFIH1, NFKBIA for PsA, and TJFRSF9, LCE3C/B, TRAF3IP2, IL23A, and NFKBIA for PsC) had not previously achieved such significance. Researchers also identified a genetic variant associated with psoriasis vulgaris (PsV) near CDKAL1, and other variants that were more strongly associated with either PsA or PsC.
“These results provide insights into the pathogenic similarities and differences between PsC and PsA,” the authors wrote. Identifying the causative variants driving the observed associations “will aid prediction and therapy of psoriasis and its cutaneous and joint manifestations.”
Read the article in the American Journal of Human Genetics (2015 Dec 3;97:816-36).
Dermatology researchers have uncovered differences in the genetic architecture of psoriatic arthritis (PsA) and cutaneous psoriasis (PsC).
Dr. Philip E. Stuart of the University of Michigan, Ann Arbor, and his colleagues from 32 institutions in the United States, Europe, and Canada, carried out a genome-wide association study (GWAS) of 1,430 patients with psoriatic arthritis and 1,417 healthy controls. They then combined results of their GWAS with five published studies of psoriasis associations (three GWASs and two targeted studies), comprising 3,061 psoriatic arthritis patients, 3,110 cutaneous-only psoriasis patients, and 9,273 psoriasis vulgaris patients, and 13,670 healthy controls of European descent.
The work, published in the American Journal of Human Genetics, identified five regions of significance in the PsA GWAS. These were all in known risk regions for PsA, with the strongest signals near HLA-B, IL12B, TRAF3IP2, TNIP1, and TYK2.
Looking across all studies, researchers detected 10 regions associated with PsA and 11 with PsC at genome-wide significance. Several of these association signals (IFNLR1, IFIH1, NFKBIA for PsA, and TJFRSF9, LCE3C/B, TRAF3IP2, IL23A, and NFKBIA for PsC) had not previously achieved such significance. Researchers also identified a genetic variant associated with psoriasis vulgaris (PsV) near CDKAL1, and other variants that were more strongly associated with either PsA or PsC.
“These results provide insights into the pathogenic similarities and differences between PsC and PsA,” the authors wrote. Identifying the causative variants driving the observed associations “will aid prediction and therapy of psoriasis and its cutaneous and joint manifestations.”
Read the article in the American Journal of Human Genetics (2015 Dec 3;97:816-36).
Dermatology researchers have uncovered differences in the genetic architecture of psoriatic arthritis (PsA) and cutaneous psoriasis (PsC).
Dr. Philip E. Stuart of the University of Michigan, Ann Arbor, and his colleagues from 32 institutions in the United States, Europe, and Canada, carried out a genome-wide association study (GWAS) of 1,430 patients with psoriatic arthritis and 1,417 healthy controls. They then combined results of their GWAS with five published studies of psoriasis associations (three GWASs and two targeted studies), comprising 3,061 psoriatic arthritis patients, 3,110 cutaneous-only psoriasis patients, and 9,273 psoriasis vulgaris patients, and 13,670 healthy controls of European descent.
The work, published in the American Journal of Human Genetics, identified five regions of significance in the PsA GWAS. These were all in known risk regions for PsA, with the strongest signals near HLA-B, IL12B, TRAF3IP2, TNIP1, and TYK2.
Looking across all studies, researchers detected 10 regions associated with PsA and 11 with PsC at genome-wide significance. Several of these association signals (IFNLR1, IFIH1, NFKBIA for PsA, and TJFRSF9, LCE3C/B, TRAF3IP2, IL23A, and NFKBIA for PsC) had not previously achieved such significance. Researchers also identified a genetic variant associated with psoriasis vulgaris (PsV) near CDKAL1, and other variants that were more strongly associated with either PsA or PsC.
“These results provide insights into the pathogenic similarities and differences between PsC and PsA,” the authors wrote. Identifying the causative variants driving the observed associations “will aid prediction and therapy of psoriasis and its cutaneous and joint manifestations.”
Read the article in the American Journal of Human Genetics (2015 Dec 3;97:816-36).
FROM AMERICAN JOURNAL OF HUMAN GENETICS
EADV: Pursuing ‘clear’ in psoriasis worthwhile, expert says
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
AT THE EADV CONGRESS
Key clinical point: A Physician Global Assessment of “clear” is worth striving for in psoriasis patients because it represents a clinically important lesser level of disease than “almost clear.”
Major finding: Fourteen percent of psoriasis patients with an on-treatment physician assessment of “almost clear” had at least moderately impaired health care quality of life, compared with 6.4% of those rated “clear.”
Data source: A secondary pooled analysis of quality of life–related outcomes among psoriasis patients rated “clear” as opposed to “almost clear” at week 12 of three phase III double-blind randomized trials of the interleukin-17 inhibitor brodalumab.
Disclosures: The presenter reported receiving research support from Amgen, which sponsored the study.
Anti-TNF therapy can continue for IBD patients with skin lesions
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
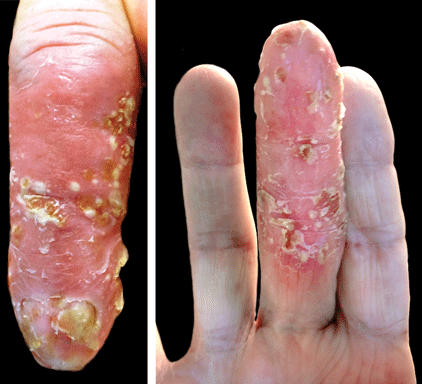
Scaly Plaque With Pustules and Anonychia on the Middle Finger
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
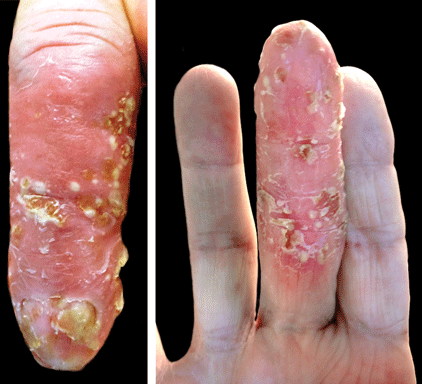
A 69-year-old man presented to our dermatology clinic with a persistent rash on the right middle finger of 5 years’ duration (left). Physical examination revealed a well-demarcated scaly plaque with pustules and anonychia localized to the right middle finger (right). Fungal and bacterial cultures revealed sterile pustules. The patient was successfully treated with an occluded superpotent topical steroid alternating with a topical vitamin D analogue.
Psoriasis cohort reveals high arthritis risk
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Incidence of psoriatic arthritis is higher among psoriasis patients than previously estimated.
Major finding: Annual incidence rate was 2.7 (95% confidence interval 2.1, 3.6) PsA cases per 100 psoriasis patients; significant predictors included disease severity, nail pitting, low education, and uveitis.
Data source: A prospective cohort study of 464 psoriasis patients without arthritis at baseline, followed for 8 years.
Disclosures: Krembil Foundation, Canadian Institutes of Health Research, and The Arthritis Society sponsored the study.
EADV: New long-term data on biologics for pediatric psoriasis ‘encouraging’
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
EADV: Pediatric psoriasis called ‘grossly undertreated’
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
EXPERT ANALYSIS FROM THE EADV CONGRESS
ACR: Cardiovascular risk factors in psoriatic diseases are common, often go untreated
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Patients with psoriatic diseases have high rates of modifiable cardiovascular risk factors that often go untreated, based on two large studies.
Major finding: These risk factors were untreated about one-third to one-half of the time.
Data source: The first study included 1,327 patients with psoriatic arthritis and 927 patients with psoriasis identified through the International Psoriasis & Arthritis Research Team (IPART). The second study analyzed data from The Health Improvement Network, including 211,832 patients with psoriatic disease and more than 1.3 million controls.
Disclosures: The IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. The second study was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. Dr. Eder and Dr. Jafri had no disclosures.