User login
Secukinumab receives FDA approval for psoriatic arthritis, ankylosing spondylitis
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
EADV: Family history of cardiovascular disease is key in psoriasis patients
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
AT THE EADV CONGRESS
Key clinical point: A family history of cardiovascular disease takes on extra importance in assessing cardiovascular risk in young adult psoriasis patients.
Major finding: Danes with mild or severe psoriasis plus a family history of cardiovascular disease were respectively 28% and 62% more likely to have an early cardiovascular event than the general population. In contrast, Danish psoriasis patients without a positive family history were not at increased risk of a cardiovascular event.
Data source: A population-based study of 2.7 million Danish young adults, including more than 30,000 diagnosed with psoriasis in their 20s, who were followed for 15 years.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was supported by Danish national research funding.
EADV: New oral psoriasis drug shows excellent safety
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.
Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
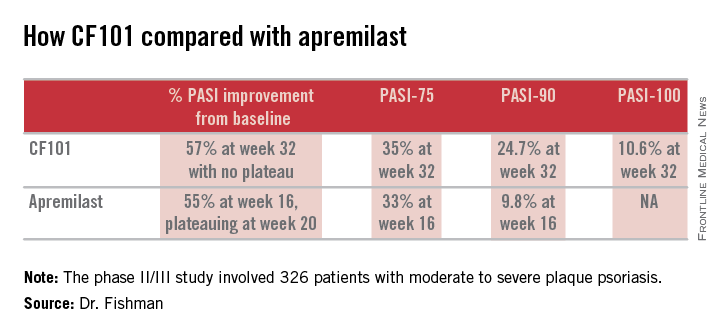
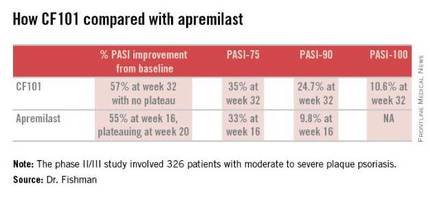
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.

Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.

Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
AT THE EADV CONGRESS
Key clinical point: The oral A3 adenosine receptor agonist CF1-1 shows promise as a potential first-line systemic therapy for moderate to severe plaque psoriasis.
Major finding: The PASI-75 response rate with the twice-daily 2-mg dose of oral CF101 was 35.3% at 32 weeks in a phase II/III study.
Data source: This was a randomized, double-blind, placebo-controlled, 32-week phase II/III study including 326 patients with moderate-to-severe plaque psoriasis.
Disclosures: The study was sponsored by Can-Fite BioPharma of Israel and presented by the Dr. Fishman, the company’s CEO.
Topical Psoriasis Therapies and Unmet Patient Needs: The Importance of Optimizing Methotrexate
Although a wide variety of treatment modalities exist for the management of psoriasis, nontreatment, undertreatment, and treatment dissatisfaction represent key clinical challenges. Careful tailoring of therapeutic regimens to meet individual patient needs and priorities may therefore be critical to improving treatment adherence and clinical outcomes. Importantly, systemic therapies such as methotrexate (MTX) may be particularly useful for individualizing patient treatment regimens and appear to be underutilized components of treatment optimization.
A majority of psoriasis patients have mild to moderate disease and many are treated primarily with topical medications. Although these treatments generally are safe and effective, practical limitations (eg, formulation, ease of application) may affect patients’ treatment adherence and satisfaction even in the context of high efficacy. Systemic therapies, although associated with a distinct set of risks and treatment challenges, may enable patients to overcome many of these limitations, particularly in patients with moderate to severe disease or impaired quality of life as well as those with an inadequate response to or dissatisfaction with topical treatments.
Systemic Treatment Options
Among the systemic treatment options for psoriasis, biologic therapies often are most highly regarded due to their strong efficacy, although traditional systemic therapies such as MTX, cyclosporine, and acitretin remain important treatment options and have an extensive history in the treatment of psoriasis. In addition to typically being required by insurance companies prior to the initiation of biologic therapies, traditional systemic therapies also may be preferred by patients because of the options for oral and subcutaneous administration and their relatively low costs. Furthermore, systemic therapies may be critical in patients for whom biologic therapies are relatively contraindicated, such as those with an increased risk of infection, history of malignancy, or hypersensitivity to any of the product’s ingredients.
Among traditional systemic therapies, MTX is one of the most frequently used psoriasis treatment worldwide and can be highly effective for even severe cases. Importantly, MTX is a valuable component of combination treatments for psoriasis and is frequently coadministered with topical and biologic agents and phototherapy, suggesting that MTX may be a particularly useful option to consider when adjusting a patient’s treatment regimen.
Benefits of Subcutaneous Methotrexate Administration
Methotrexate can be delivered either orally or parenterally, contributing to its compatibility with a wide variety of psoriasis treatment regimens and patient preferences. Administration is predominantly oral in the United States, but parenteral MTX (most commonly delivered subcutaneously) can confer important benefits and is used regularly in countries outside of the United States.
An important advantage of subcutaneous versus oral MTX is greater bioavailability, particularly at higher doses. In studies of healthy volunteers or patients with rheumatoid arthritis, the bioavailability of MTX following oral administration appears to plateau at doses of 15 mg or higher,1 whereas that of subcutaneous MTX appears to increase linearly at a wide range of doses and exceeds that of oral MTX at each dose examined.1,2 A switch from oral to subcutaneous MTX may therefore benefit patients experiencing suboptimal disease control.
Another important benefit of subcutanoues versus oral MTX is the potential for reduced intensity of gastrointestinal adverse events.2,3 In a study of patients with rheumatoid arthritis, those who received subcutaneous MTX reported less severe nausea, vomiting, abdominal pain, and diarrhea than those who received oral MTX,3 which may improve treatment adherence and potentially enable patients to tolerate higher doses. Because gastrointestinal adverse events are a common cause of MTX treatment discontinuation, a switch from oral to subcutaneous MTX may be an important strategy to enable more patients to benefit from this treatment option.
Subcutaneous MTX presents some potential challenges, including patients’ fear of needles and difficulties with drawing and administering an accurate drug dose using a vial, needle, and syringe. However, recent developments in autoinjector technology have produced MTX injection devices that largely mitigate many of these challenges. Methotrexate autoinjectors allow for the accurate administration of prespecified doses, and patients generally find them easy to use.4 Furthermore, MTX autoinjectors have been associated with low levels of adminsitration-site pain (median pain score on a visual analog scale, 1.0/100 mm in one study4), and the concealment of a needle from view may potentially lessen needle phobia.
Role of Subcutaneous Methotrexate in Patient Care
The types of patients expected to benefit most from subcutaneous MTX include those with moderate to severe psoriasis and those who have experienced dissatisfaction with topical medications or phototherapy. The increased bioavailability of subcutaneous MTX as well as the reduced intensity of gastrointestinal adverse events compared with oral MTX may enable patients to achieve a greater clinical response, and the systemic route of administration may improve treatment adherence and patient satisfaction among those who are dissatisfied with topical treatment regimens. Notably, an additional benefit of optimizing MTX treatment may be the potential to prevent or delay progression to biologic therapies, which may be an important goal of both patients and physicians to prevent higher health care costs.
Another principal role of subcutaneous MTX is as a component of combination therapy with topicals or other systemic therapies for either long-term care or periodic treatment of disease flares. Methotrexate is a frequent component of combination therapies, and subcutaneous administration may be preferable for many patients, particularly those who are already accustomed to injectable therapies (eg, biologic agents) and those who regularly visit a physician who can perform the injections (eg, for regular phototherapy treatments). Interestingly, the coadministration of MTX may be especially valuable in the context of biologic therapies, as concomitant MTX is associated with a reduced incidence of antidrug antibodies and may therefore enhance or prolong responses to biologic agents.
Final Thoughts
Because subcutaneous MTX is infrequently used for the treatment of psoriasis in the United States, increased awareness of its unique advantages may provide new opportunities for patients to tailor treatment regimens to meet individual needs and preferences. Treatment optimization across a broad range of patient characteristics may be critical to improving adherence and satisfaction in psoriasis patients and may be considered a major therapeutic goal.
Acknowledgment
Medical writing assistance was provided by Anna Abt, PhD, of ETHOS Health Communications in Newtown, Pennsylvania, with financial support from LEO Pharma.
- Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
- Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32:563-571.
- Rutkowska-Sak L, Rell-Bakalarska M, Lisowska B. Oral vs. subcutaneous low-dose methotrexate treatment in reducing gastrointestinal side effects. Reumatologia. 2009;47:207-211.
- Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20:256-260.
Although a wide variety of treatment modalities exist for the management of psoriasis, nontreatment, undertreatment, and treatment dissatisfaction represent key clinical challenges. Careful tailoring of therapeutic regimens to meet individual patient needs and priorities may therefore be critical to improving treatment adherence and clinical outcomes. Importantly, systemic therapies such as methotrexate (MTX) may be particularly useful for individualizing patient treatment regimens and appear to be underutilized components of treatment optimization.
A majority of psoriasis patients have mild to moderate disease and many are treated primarily with topical medications. Although these treatments generally are safe and effective, practical limitations (eg, formulation, ease of application) may affect patients’ treatment adherence and satisfaction even in the context of high efficacy. Systemic therapies, although associated with a distinct set of risks and treatment challenges, may enable patients to overcome many of these limitations, particularly in patients with moderate to severe disease or impaired quality of life as well as those with an inadequate response to or dissatisfaction with topical treatments.
Systemic Treatment Options
Among the systemic treatment options for psoriasis, biologic therapies often are most highly regarded due to their strong efficacy, although traditional systemic therapies such as MTX, cyclosporine, and acitretin remain important treatment options and have an extensive history in the treatment of psoriasis. In addition to typically being required by insurance companies prior to the initiation of biologic therapies, traditional systemic therapies also may be preferred by patients because of the options for oral and subcutaneous administration and their relatively low costs. Furthermore, systemic therapies may be critical in patients for whom biologic therapies are relatively contraindicated, such as those with an increased risk of infection, history of malignancy, or hypersensitivity to any of the product’s ingredients.
Among traditional systemic therapies, MTX is one of the most frequently used psoriasis treatment worldwide and can be highly effective for even severe cases. Importantly, MTX is a valuable component of combination treatments for psoriasis and is frequently coadministered with topical and biologic agents and phototherapy, suggesting that MTX may be a particularly useful option to consider when adjusting a patient’s treatment regimen.
Benefits of Subcutaneous Methotrexate Administration
Methotrexate can be delivered either orally or parenterally, contributing to its compatibility with a wide variety of psoriasis treatment regimens and patient preferences. Administration is predominantly oral in the United States, but parenteral MTX (most commonly delivered subcutaneously) can confer important benefits and is used regularly in countries outside of the United States.
An important advantage of subcutaneous versus oral MTX is greater bioavailability, particularly at higher doses. In studies of healthy volunteers or patients with rheumatoid arthritis, the bioavailability of MTX following oral administration appears to plateau at doses of 15 mg or higher,1 whereas that of subcutaneous MTX appears to increase linearly at a wide range of doses and exceeds that of oral MTX at each dose examined.1,2 A switch from oral to subcutaneous MTX may therefore benefit patients experiencing suboptimal disease control.
Another important benefit of subcutanoues versus oral MTX is the potential for reduced intensity of gastrointestinal adverse events.2,3 In a study of patients with rheumatoid arthritis, those who received subcutaneous MTX reported less severe nausea, vomiting, abdominal pain, and diarrhea than those who received oral MTX,3 which may improve treatment adherence and potentially enable patients to tolerate higher doses. Because gastrointestinal adverse events are a common cause of MTX treatment discontinuation, a switch from oral to subcutaneous MTX may be an important strategy to enable more patients to benefit from this treatment option.
Subcutaneous MTX presents some potential challenges, including patients’ fear of needles and difficulties with drawing and administering an accurate drug dose using a vial, needle, and syringe. However, recent developments in autoinjector technology have produced MTX injection devices that largely mitigate many of these challenges. Methotrexate autoinjectors allow for the accurate administration of prespecified doses, and patients generally find them easy to use.4 Furthermore, MTX autoinjectors have been associated with low levels of adminsitration-site pain (median pain score on a visual analog scale, 1.0/100 mm in one study4), and the concealment of a needle from view may potentially lessen needle phobia.
Role of Subcutaneous Methotrexate in Patient Care
The types of patients expected to benefit most from subcutaneous MTX include those with moderate to severe psoriasis and those who have experienced dissatisfaction with topical medications or phototherapy. The increased bioavailability of subcutaneous MTX as well as the reduced intensity of gastrointestinal adverse events compared with oral MTX may enable patients to achieve a greater clinical response, and the systemic route of administration may improve treatment adherence and patient satisfaction among those who are dissatisfied with topical treatment regimens. Notably, an additional benefit of optimizing MTX treatment may be the potential to prevent or delay progression to biologic therapies, which may be an important goal of both patients and physicians to prevent higher health care costs.
Another principal role of subcutaneous MTX is as a component of combination therapy with topicals or other systemic therapies for either long-term care or periodic treatment of disease flares. Methotrexate is a frequent component of combination therapies, and subcutaneous administration may be preferable for many patients, particularly those who are already accustomed to injectable therapies (eg, biologic agents) and those who regularly visit a physician who can perform the injections (eg, for regular phototherapy treatments). Interestingly, the coadministration of MTX may be especially valuable in the context of biologic therapies, as concomitant MTX is associated with a reduced incidence of antidrug antibodies and may therefore enhance or prolong responses to biologic agents.
Final Thoughts
Because subcutaneous MTX is infrequently used for the treatment of psoriasis in the United States, increased awareness of its unique advantages may provide new opportunities for patients to tailor treatment regimens to meet individual needs and preferences. Treatment optimization across a broad range of patient characteristics may be critical to improving adherence and satisfaction in psoriasis patients and may be considered a major therapeutic goal.
Acknowledgment
Medical writing assistance was provided by Anna Abt, PhD, of ETHOS Health Communications in Newtown, Pennsylvania, with financial support from LEO Pharma.
Although a wide variety of treatment modalities exist for the management of psoriasis, nontreatment, undertreatment, and treatment dissatisfaction represent key clinical challenges. Careful tailoring of therapeutic regimens to meet individual patient needs and priorities may therefore be critical to improving treatment adherence and clinical outcomes. Importantly, systemic therapies such as methotrexate (MTX) may be particularly useful for individualizing patient treatment regimens and appear to be underutilized components of treatment optimization.
A majority of psoriasis patients have mild to moderate disease and many are treated primarily with topical medications. Although these treatments generally are safe and effective, practical limitations (eg, formulation, ease of application) may affect patients’ treatment adherence and satisfaction even in the context of high efficacy. Systemic therapies, although associated with a distinct set of risks and treatment challenges, may enable patients to overcome many of these limitations, particularly in patients with moderate to severe disease or impaired quality of life as well as those with an inadequate response to or dissatisfaction with topical treatments.
Systemic Treatment Options
Among the systemic treatment options for psoriasis, biologic therapies often are most highly regarded due to their strong efficacy, although traditional systemic therapies such as MTX, cyclosporine, and acitretin remain important treatment options and have an extensive history in the treatment of psoriasis. In addition to typically being required by insurance companies prior to the initiation of biologic therapies, traditional systemic therapies also may be preferred by patients because of the options for oral and subcutaneous administration and their relatively low costs. Furthermore, systemic therapies may be critical in patients for whom biologic therapies are relatively contraindicated, such as those with an increased risk of infection, history of malignancy, or hypersensitivity to any of the product’s ingredients.
Among traditional systemic therapies, MTX is one of the most frequently used psoriasis treatment worldwide and can be highly effective for even severe cases. Importantly, MTX is a valuable component of combination treatments for psoriasis and is frequently coadministered with topical and biologic agents and phototherapy, suggesting that MTX may be a particularly useful option to consider when adjusting a patient’s treatment regimen.
Benefits of Subcutaneous Methotrexate Administration
Methotrexate can be delivered either orally or parenterally, contributing to its compatibility with a wide variety of psoriasis treatment regimens and patient preferences. Administration is predominantly oral in the United States, but parenteral MTX (most commonly delivered subcutaneously) can confer important benefits and is used regularly in countries outside of the United States.
An important advantage of subcutaneous versus oral MTX is greater bioavailability, particularly at higher doses. In studies of healthy volunteers or patients with rheumatoid arthritis, the bioavailability of MTX following oral administration appears to plateau at doses of 15 mg or higher,1 whereas that of subcutaneous MTX appears to increase linearly at a wide range of doses and exceeds that of oral MTX at each dose examined.1,2 A switch from oral to subcutaneous MTX may therefore benefit patients experiencing suboptimal disease control.
Another important benefit of subcutanoues versus oral MTX is the potential for reduced intensity of gastrointestinal adverse events.2,3 In a study of patients with rheumatoid arthritis, those who received subcutaneous MTX reported less severe nausea, vomiting, abdominal pain, and diarrhea than those who received oral MTX,3 which may improve treatment adherence and potentially enable patients to tolerate higher doses. Because gastrointestinal adverse events are a common cause of MTX treatment discontinuation, a switch from oral to subcutaneous MTX may be an important strategy to enable more patients to benefit from this treatment option.
Subcutaneous MTX presents some potential challenges, including patients’ fear of needles and difficulties with drawing and administering an accurate drug dose using a vial, needle, and syringe. However, recent developments in autoinjector technology have produced MTX injection devices that largely mitigate many of these challenges. Methotrexate autoinjectors allow for the accurate administration of prespecified doses, and patients generally find them easy to use.4 Furthermore, MTX autoinjectors have been associated with low levels of adminsitration-site pain (median pain score on a visual analog scale, 1.0/100 mm in one study4), and the concealment of a needle from view may potentially lessen needle phobia.
Role of Subcutaneous Methotrexate in Patient Care
The types of patients expected to benefit most from subcutaneous MTX include those with moderate to severe psoriasis and those who have experienced dissatisfaction with topical medications or phototherapy. The increased bioavailability of subcutaneous MTX as well as the reduced intensity of gastrointestinal adverse events compared with oral MTX may enable patients to achieve a greater clinical response, and the systemic route of administration may improve treatment adherence and patient satisfaction among those who are dissatisfied with topical treatment regimens. Notably, an additional benefit of optimizing MTX treatment may be the potential to prevent or delay progression to biologic therapies, which may be an important goal of both patients and physicians to prevent higher health care costs.
Another principal role of subcutaneous MTX is as a component of combination therapy with topicals or other systemic therapies for either long-term care or periodic treatment of disease flares. Methotrexate is a frequent component of combination therapies, and subcutaneous administration may be preferable for many patients, particularly those who are already accustomed to injectable therapies (eg, biologic agents) and those who regularly visit a physician who can perform the injections (eg, for regular phototherapy treatments). Interestingly, the coadministration of MTX may be especially valuable in the context of biologic therapies, as concomitant MTX is associated with a reduced incidence of antidrug antibodies and may therefore enhance or prolong responses to biologic agents.
Final Thoughts
Because subcutaneous MTX is infrequently used for the treatment of psoriasis in the United States, increased awareness of its unique advantages may provide new opportunities for patients to tailor treatment regimens to meet individual needs and preferences. Treatment optimization across a broad range of patient characteristics may be critical to improving adherence and satisfaction in psoriasis patients and may be considered a major therapeutic goal.
Acknowledgment
Medical writing assistance was provided by Anna Abt, PhD, of ETHOS Health Communications in Newtown, Pennsylvania, with financial support from LEO Pharma.
- Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
- Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32:563-571.
- Rutkowska-Sak L, Rell-Bakalarska M, Lisowska B. Oral vs. subcutaneous low-dose methotrexate treatment in reducing gastrointestinal side effects. Reumatologia. 2009;47:207-211.
- Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20:256-260.
- Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
- Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32:563-571.
- Rutkowska-Sak L, Rell-Bakalarska M, Lisowska B. Oral vs. subcutaneous low-dose methotrexate treatment in reducing gastrointestinal side effects. Reumatologia. 2009;47:207-211.
- Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20:256-260.
Bump in the Road
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
ACR: Etanercept during pregnancy doubles the odds of major malformations
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
AT THE ACR ANNUAL MEETING
Key clinical point: Although etanercept exposure was associated with more than twofold higher odds of major structural defects, there was no pattern to the defects and no biological plausibility to etanercept causing the defects.
Major finding: There were 33 major structural defects in children born to etanercept women versus 7 in a disease comparison group, translating to a more than doubling of risk with etanercept (OR, 2.37; 95% CI, 1.02-5.52).
Data source: Prospective investigation of 830 pregnant women.
Disclosures: The presenting investigator disclosed funding from 14 companies, including Amgen, the maker of etanercept, and AbbVie, the maker of adalimumab.
Methotrexate has a role in treating articular manifestations of psoriatic arthritis
Patients with psoriatic arthritis taking methotrexate demonstrated an improvement in peripheral joint disease, skin disease, enthesitis, dactylitis, and nail disease over 12 weeks in a subanalysis of methotrexate users in the TICOPA (Tight Control of Psoriatic Arthritis) study.
Out of the original 206 patients in the open-label, randomized, controlled TICOPA study, the subanalysis involved 188 who received methotrexate in its first 12 weeks. Substudy authors Dr. Laura C. Coates and Dr. Philip S. Helliwell of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) verified a maximum dose at 12 weeks of at least 15 mg/week in 175, 20 mg/week in 122, and 25 mg/week in 86 (J Rheumatol. Dec 15. doi: 10.3899/jrheum.150614).
The proportions of patients achieving American College of Rheumatology (ACR) outcomes at 12 weeks were 40.8% for ACR20, 18.8% for ACR50, and 8.6% for ACR70. A total of 22.4% achieved minimal disease activity, defined as meeting five of these seven criteria: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area and Severity Index (PASI) of 1 or less or body surface area involvement of 3 or less, patient pain visual analog score (VAS) of 15 or less, patient global disease activity VAS of 20 or less, health assessment questionnaire of 0.5 or less, and 0-1 tender entheseal points.
Other improvements that occurred at 12 weeks included 27.2% reaching a PASI75, a 62.7% drop in dactylitis incidence, a significant drop of –59.7 in Leeds dactylitis instrument median score, and a significant decrease in the proportion of patients with enthesitis (25.7%). However, the median change in enthesitis score was 0.
Response rates did not differ between patients receiving methotrexate 15 mg/week or higher doses, although there was generally a higher proportion who met various response criteria among those taking greater than 15 mg/week. However, the authors noted that there could be an underestimation of the dose effect because the design of the TICOPA study, which randomized patients to a protocol for tight control of psoriatic arthritis disease activity or standard care, introduced a bias by intentionally escalating treatment doses in patients who continue to have active disease.
The investigators advised that the study results be interpreted in the context of the open-label design of the study, and placed alongside the other observational studies that support its use in psoriatic arthritis.
No relevant conflicts of interest were disclosed.
Patients with psoriatic arthritis taking methotrexate demonstrated an improvement in peripheral joint disease, skin disease, enthesitis, dactylitis, and nail disease over 12 weeks in a subanalysis of methotrexate users in the TICOPA (Tight Control of Psoriatic Arthritis) study.
Out of the original 206 patients in the open-label, randomized, controlled TICOPA study, the subanalysis involved 188 who received methotrexate in its first 12 weeks. Substudy authors Dr. Laura C. Coates and Dr. Philip S. Helliwell of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) verified a maximum dose at 12 weeks of at least 15 mg/week in 175, 20 mg/week in 122, and 25 mg/week in 86 (J Rheumatol. Dec 15. doi: 10.3899/jrheum.150614).
The proportions of patients achieving American College of Rheumatology (ACR) outcomes at 12 weeks were 40.8% for ACR20, 18.8% for ACR50, and 8.6% for ACR70. A total of 22.4% achieved minimal disease activity, defined as meeting five of these seven criteria: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area and Severity Index (PASI) of 1 or less or body surface area involvement of 3 or less, patient pain visual analog score (VAS) of 15 or less, patient global disease activity VAS of 20 or less, health assessment questionnaire of 0.5 or less, and 0-1 tender entheseal points.
Other improvements that occurred at 12 weeks included 27.2% reaching a PASI75, a 62.7% drop in dactylitis incidence, a significant drop of –59.7 in Leeds dactylitis instrument median score, and a significant decrease in the proportion of patients with enthesitis (25.7%). However, the median change in enthesitis score was 0.
Response rates did not differ between patients receiving methotrexate 15 mg/week or higher doses, although there was generally a higher proportion who met various response criteria among those taking greater than 15 mg/week. However, the authors noted that there could be an underestimation of the dose effect because the design of the TICOPA study, which randomized patients to a protocol for tight control of psoriatic arthritis disease activity or standard care, introduced a bias by intentionally escalating treatment doses in patients who continue to have active disease.
The investigators advised that the study results be interpreted in the context of the open-label design of the study, and placed alongside the other observational studies that support its use in psoriatic arthritis.
No relevant conflicts of interest were disclosed.
Patients with psoriatic arthritis taking methotrexate demonstrated an improvement in peripheral joint disease, skin disease, enthesitis, dactylitis, and nail disease over 12 weeks in a subanalysis of methotrexate users in the TICOPA (Tight Control of Psoriatic Arthritis) study.
Out of the original 206 patients in the open-label, randomized, controlled TICOPA study, the subanalysis involved 188 who received methotrexate in its first 12 weeks. Substudy authors Dr. Laura C. Coates and Dr. Philip S. Helliwell of the Leeds Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England) verified a maximum dose at 12 weeks of at least 15 mg/week in 175, 20 mg/week in 122, and 25 mg/week in 86 (J Rheumatol. Dec 15. doi: 10.3899/jrheum.150614).
The proportions of patients achieving American College of Rheumatology (ACR) outcomes at 12 weeks were 40.8% for ACR20, 18.8% for ACR50, and 8.6% for ACR70. A total of 22.4% achieved minimal disease activity, defined as meeting five of these seven criteria: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area and Severity Index (PASI) of 1 or less or body surface area involvement of 3 or less, patient pain visual analog score (VAS) of 15 or less, patient global disease activity VAS of 20 or less, health assessment questionnaire of 0.5 or less, and 0-1 tender entheseal points.
Other improvements that occurred at 12 weeks included 27.2% reaching a PASI75, a 62.7% drop in dactylitis incidence, a significant drop of –59.7 in Leeds dactylitis instrument median score, and a significant decrease in the proportion of patients with enthesitis (25.7%). However, the median change in enthesitis score was 0.
Response rates did not differ between patients receiving methotrexate 15 mg/week or higher doses, although there was generally a higher proportion who met various response criteria among those taking greater than 15 mg/week. However, the authors noted that there could be an underestimation of the dose effect because the design of the TICOPA study, which randomized patients to a protocol for tight control of psoriatic arthritis disease activity or standard care, introduced a bias by intentionally escalating treatment doses in patients who continue to have active disease.
The investigators advised that the study results be interpreted in the context of the open-label design of the study, and placed alongside the other observational studies that support its use in psoriatic arthritis.
No relevant conflicts of interest were disclosed.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Methotrexate is a commonly prescribed drug in psoriatic arthritis, and while it may not be effective for all patients, it does have a role in the treatment of articular manifestations.
Major finding: The proportions of patients achieving American College of Rheumatology (ACR) outcomes at 12 weeks were 40.8% for ACR20, 18.8% for ACR50, and 8.6% for ACR70.
Data source: Open-label, observational study of 188 patients participating in the Tight Control of Psoriatic Arthritis (TICOPA) study.
Disclosures: No relevant conflicts of interest were disclosed.
More comorbidity with PsA form of spondyloarthritis
Psoriatic arthritis in patients with spondyloarthritis conveys an increased risk of comorbidities, especially cardiovascular and metabolic diseases, as compared with spondyloarthritis alone.
The association persisted even after adjusting for demographic factors, disease duration, and length of treatment, Dr. Naba Haque of the University of Leuven (Belgium) and associates wrote in the Journal of Rheumatology (2015 Dec 15. doi: 10.3899/jrheum.141359).
In an analysis of 518 patients in the spondyloarthritis database at the university, over half (54%) had comorbidities, and those with psoriatic spondyloarthropathy had significantly more comorbidities than those without psoriatic arthropathy (P less than .001). Cardiovascular comorbidities were most common, with an increased prevalence of hypertension, coronary artery disease, and hyperlipidemia in the patients with psoriatic spondyloarthropathy (odds ratios, 1.81, 1.39, and 1.60, respectively; P less than .001). The differences persisted after adjusting for confounders such as age, sex, disease duration, and treatment.
Twice as many patients with spondyloarthritis and psoriatic arthropathy met the criteria for metabolic syndrome as did patients without psoriatric arthropathy (10% vs. 5%, P = .03). The regression model showed a significant difference in the prevalence of diabetes in the PsA patients (OR, 1.35; 95% confidence interval, 1.17-1.56; P less than .001). Psoriatic arthropathy also was linked to an increased risk of cancer (12% vs. 6%; P less than .05); however, the authors wrote that “we can only state a possible positive association … this observation needs to be studied in further detail.”
The study was supported by an unrestricted grant from the Abbvie Chair for psoriatic arthritis research (Dr. Rik J. Lories and Dr. Kurt de Vlam).
Psoriatic arthritis in patients with spondyloarthritis conveys an increased risk of comorbidities, especially cardiovascular and metabolic diseases, as compared with spondyloarthritis alone.
The association persisted even after adjusting for demographic factors, disease duration, and length of treatment, Dr. Naba Haque of the University of Leuven (Belgium) and associates wrote in the Journal of Rheumatology (2015 Dec 15. doi: 10.3899/jrheum.141359).
In an analysis of 518 patients in the spondyloarthritis database at the university, over half (54%) had comorbidities, and those with psoriatic spondyloarthropathy had significantly more comorbidities than those without psoriatic arthropathy (P less than .001). Cardiovascular comorbidities were most common, with an increased prevalence of hypertension, coronary artery disease, and hyperlipidemia in the patients with psoriatic spondyloarthropathy (odds ratios, 1.81, 1.39, and 1.60, respectively; P less than .001). The differences persisted after adjusting for confounders such as age, sex, disease duration, and treatment.
Twice as many patients with spondyloarthritis and psoriatic arthropathy met the criteria for metabolic syndrome as did patients without psoriatric arthropathy (10% vs. 5%, P = .03). The regression model showed a significant difference in the prevalence of diabetes in the PsA patients (OR, 1.35; 95% confidence interval, 1.17-1.56; P less than .001). Psoriatic arthropathy also was linked to an increased risk of cancer (12% vs. 6%; P less than .05); however, the authors wrote that “we can only state a possible positive association … this observation needs to be studied in further detail.”
The study was supported by an unrestricted grant from the Abbvie Chair for psoriatic arthritis research (Dr. Rik J. Lories and Dr. Kurt de Vlam).
Psoriatic arthritis in patients with spondyloarthritis conveys an increased risk of comorbidities, especially cardiovascular and metabolic diseases, as compared with spondyloarthritis alone.
The association persisted even after adjusting for demographic factors, disease duration, and length of treatment, Dr. Naba Haque of the University of Leuven (Belgium) and associates wrote in the Journal of Rheumatology (2015 Dec 15. doi: 10.3899/jrheum.141359).
In an analysis of 518 patients in the spondyloarthritis database at the university, over half (54%) had comorbidities, and those with psoriatic spondyloarthropathy had significantly more comorbidities than those without psoriatic arthropathy (P less than .001). Cardiovascular comorbidities were most common, with an increased prevalence of hypertension, coronary artery disease, and hyperlipidemia in the patients with psoriatic spondyloarthropathy (odds ratios, 1.81, 1.39, and 1.60, respectively; P less than .001). The differences persisted after adjusting for confounders such as age, sex, disease duration, and treatment.
Twice as many patients with spondyloarthritis and psoriatic arthropathy met the criteria for metabolic syndrome as did patients without psoriatric arthropathy (10% vs. 5%, P = .03). The regression model showed a significant difference in the prevalence of diabetes in the PsA patients (OR, 1.35; 95% confidence interval, 1.17-1.56; P less than .001). Psoriatic arthropathy also was linked to an increased risk of cancer (12% vs. 6%; P less than .05); however, the authors wrote that “we can only state a possible positive association … this observation needs to be studied in further detail.”
The study was supported by an unrestricted grant from the Abbvie Chair for psoriatic arthritis research (Dr. Rik J. Lories and Dr. Kurt de Vlam).
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: People with psoriatic arthritis (PsA) have a significantly increased risk of cardiovascular and metabolic diseases, compared with people with non-PsA forms of spondyloarthritis (SpA).
Major finding: After correcting for risk factors, spondyloarthritis plus psoriatic arthritis was linked with an increased prevalence of hypertension, coronary artery disease, and hyperlipidemia (OR, 1.81, 1.39, and 1.60 respectively, P less than .001).
Data source: Cross-sectional study of 518 spondyloarthritis patients in a database at a university rheumatology department.
Disclosures: The study was supported by an unrestricted grant from the Abbvie Chair for psoriatic arthritis research (Dr. Lories and Dr. de Vlam).
Secukinumab cut ankylosing spondylitis symptoms in MEASURE trials
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Interleukin-17A may play a role in ankylosing spondylitis, and secukinumab may prove to be an effective therapy for these patients.
Major finding: The primary endpoint of Assessment of Spondyloarthritis International Society (ASAS20) response rates at week 16 was met in both secukinumab groups in MEASURE 1 and in the group that received 150 mg of secukinumab subcutaneously in MEASURE 2.
Data source: Two double-blind, phase III studies: MEASURE 1 involving 371 patients with AS and MEASURE 2 involving 371 patients.
Disclosures: The studies were funded by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
Aerosol foam product found effective for psoriasis vulgaris
An alcohol-free aerosol foam that contains a fixed combination of calcipotriene 0.005% plus betamethasone dipropionate 0.064% provided rapid itch relief and was well tolerated in patients with psoriasis vulgaris, according to results from a randomized, phase III study.
In earlier phase II studies, the product, which is being developed by Denmark-based LEO Pharma, was shown to be effective and had a safe tolerability profile, with no clinically relevant impact on the hypothalamic-pituitary-adrenal axis or on calcium metabolism. In an effort to confirm the efficacy and safety profile seen in the phase II trials, researchers led by Dr. Craig Leonardi, a dermatologist at Saint Louis (Mo.) University, performed the phase III study to compare the aerosol foam product with vehicle when applied once daily for up to 4 weeks in patients with psoriasis vulgaris.
Reporting in the December 2015 issue of the Journal of Drugs in Dermatology, Dr. Leonardi and his associates at 27 outpatient sites enrolled 426 patients aged 18 years and older between June and October of 2013 in a trial known as PSO-FAST (Cal/BD Foam in Psoriasis Vulgaris, a Four-Week, Vehicle-Controlled, Efficacy, and Safety Trial). The primary outcome was the proportion of patients who achieved treatment success at week 4, based on the physician’s global assessment, which was defined as clear or almost clear (for patients with at least moderate disease at baseline) or clear (for patients who had mild disease at baseline). Secondary outcomes included a modified (excluding head) Psoriasis Area and Severity Index (mPASI) and patient’s assessment of itch based on a visual analog scale(J. Drugs Dermatol. 2015;14[12]:1468-77).
Of the 426 patients, 323 received the aerosol foam product, while 103 received the vehicle. Their median age was 51 years, 59% were male, and their mean body mass index was 32.3 kg/m2. At week 4, the researchers found that significantly more patients in the aerosol foam group achieved treatment success, compared with those in the vehicle group (53.3% vs. 4.8%, respectively; odds ratio 30.3; P less than .001).
In addition, the mean mPASI score was significantly lower among patients in the aerosol foam group, compared with those in the vehicle group (a score of 2.0 vs. 5.5, for an adjusted difference of –3.3; P less than .001).
Among the 96% of patients who reported any level of itch at baseline, 36.8% in the aerosol foam group reported a 70% reduction in itch at day 3, compared with 24% of those in the vehicle group (OR 1.9; P = .018).
“This trial also demonstrated itch alleviation led to significant reductions in sleep loss, with 70.8% of patients using Cal/BD aerosol foam reporting a 70% reduction in itch-related sleep loss by week 4,” the researchers wrote.
No clinically significant changes in mean albumin-corrected serum calcium or urinary calcium to creatinine ratio was observed in either group. The researchers concluded that the Cal/DB aerosol foam product “may be a beneficial treatment option for those patients who are candidates for therapy with a superpotent steroid, but desire a therapeutic safety profile similar to that of a less potent steroid.”
An alcohol-free aerosol foam that contains a fixed combination of calcipotriene 0.005% plus betamethasone dipropionate 0.064% provided rapid itch relief and was well tolerated in patients with psoriasis vulgaris, according to results from a randomized, phase III study.
In earlier phase II studies, the product, which is being developed by Denmark-based LEO Pharma, was shown to be effective and had a safe tolerability profile, with no clinically relevant impact on the hypothalamic-pituitary-adrenal axis or on calcium metabolism. In an effort to confirm the efficacy and safety profile seen in the phase II trials, researchers led by Dr. Craig Leonardi, a dermatologist at Saint Louis (Mo.) University, performed the phase III study to compare the aerosol foam product with vehicle when applied once daily for up to 4 weeks in patients with psoriasis vulgaris.
Reporting in the December 2015 issue of the Journal of Drugs in Dermatology, Dr. Leonardi and his associates at 27 outpatient sites enrolled 426 patients aged 18 years and older between June and October of 2013 in a trial known as PSO-FAST (Cal/BD Foam in Psoriasis Vulgaris, a Four-Week, Vehicle-Controlled, Efficacy, and Safety Trial). The primary outcome was the proportion of patients who achieved treatment success at week 4, based on the physician’s global assessment, which was defined as clear or almost clear (for patients with at least moderate disease at baseline) or clear (for patients who had mild disease at baseline). Secondary outcomes included a modified (excluding head) Psoriasis Area and Severity Index (mPASI) and patient’s assessment of itch based on a visual analog scale(J. Drugs Dermatol. 2015;14[12]:1468-77).
Of the 426 patients, 323 received the aerosol foam product, while 103 received the vehicle. Their median age was 51 years, 59% were male, and their mean body mass index was 32.3 kg/m2. At week 4, the researchers found that significantly more patients in the aerosol foam group achieved treatment success, compared with those in the vehicle group (53.3% vs. 4.8%, respectively; odds ratio 30.3; P less than .001).
In addition, the mean mPASI score was significantly lower among patients in the aerosol foam group, compared with those in the vehicle group (a score of 2.0 vs. 5.5, for an adjusted difference of –3.3; P less than .001).
Among the 96% of patients who reported any level of itch at baseline, 36.8% in the aerosol foam group reported a 70% reduction in itch at day 3, compared with 24% of those in the vehicle group (OR 1.9; P = .018).
“This trial also demonstrated itch alleviation led to significant reductions in sleep loss, with 70.8% of patients using Cal/BD aerosol foam reporting a 70% reduction in itch-related sleep loss by week 4,” the researchers wrote.
No clinically significant changes in mean albumin-corrected serum calcium or urinary calcium to creatinine ratio was observed in either group. The researchers concluded that the Cal/DB aerosol foam product “may be a beneficial treatment option for those patients who are candidates for therapy with a superpotent steroid, but desire a therapeutic safety profile similar to that of a less potent steroid.”
An alcohol-free aerosol foam that contains a fixed combination of calcipotriene 0.005% plus betamethasone dipropionate 0.064% provided rapid itch relief and was well tolerated in patients with psoriasis vulgaris, according to results from a randomized, phase III study.
In earlier phase II studies, the product, which is being developed by Denmark-based LEO Pharma, was shown to be effective and had a safe tolerability profile, with no clinically relevant impact on the hypothalamic-pituitary-adrenal axis or on calcium metabolism. In an effort to confirm the efficacy and safety profile seen in the phase II trials, researchers led by Dr. Craig Leonardi, a dermatologist at Saint Louis (Mo.) University, performed the phase III study to compare the aerosol foam product with vehicle when applied once daily for up to 4 weeks in patients with psoriasis vulgaris.
Reporting in the December 2015 issue of the Journal of Drugs in Dermatology, Dr. Leonardi and his associates at 27 outpatient sites enrolled 426 patients aged 18 years and older between June and October of 2013 in a trial known as PSO-FAST (Cal/BD Foam in Psoriasis Vulgaris, a Four-Week, Vehicle-Controlled, Efficacy, and Safety Trial). The primary outcome was the proportion of patients who achieved treatment success at week 4, based on the physician’s global assessment, which was defined as clear or almost clear (for patients with at least moderate disease at baseline) or clear (for patients who had mild disease at baseline). Secondary outcomes included a modified (excluding head) Psoriasis Area and Severity Index (mPASI) and patient’s assessment of itch based on a visual analog scale(J. Drugs Dermatol. 2015;14[12]:1468-77).
Of the 426 patients, 323 received the aerosol foam product, while 103 received the vehicle. Their median age was 51 years, 59% were male, and their mean body mass index was 32.3 kg/m2. At week 4, the researchers found that significantly more patients in the aerosol foam group achieved treatment success, compared with those in the vehicle group (53.3% vs. 4.8%, respectively; odds ratio 30.3; P less than .001).
In addition, the mean mPASI score was significantly lower among patients in the aerosol foam group, compared with those in the vehicle group (a score of 2.0 vs. 5.5, for an adjusted difference of –3.3; P less than .001).
Among the 96% of patients who reported any level of itch at baseline, 36.8% in the aerosol foam group reported a 70% reduction in itch at day 3, compared with 24% of those in the vehicle group (OR 1.9; P = .018).
“This trial also demonstrated itch alleviation led to significant reductions in sleep loss, with 70.8% of patients using Cal/BD aerosol foam reporting a 70% reduction in itch-related sleep loss by week 4,” the researchers wrote.
No clinically significant changes in mean albumin-corrected serum calcium or urinary calcium to creatinine ratio was observed in either group. The researchers concluded that the Cal/DB aerosol foam product “may be a beneficial treatment option for those patients who are candidates for therapy with a superpotent steroid, but desire a therapeutic safety profile similar to that of a less potent steroid.”
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: A foam product containing calcipotriene 0.005% plus betamethasone dipropionate 0.064% was found to be safe and effective for patients with psoriasis vulgaris.
Major finding: At week 4, a significantly greater number of patients in the aerosol foam group achieved treatment success, compared with those in the vehicle group (53.3% vs. 4.8%, respectively; OR 30.3; P less than .001).
Data source: A randomized, phase III trial compared an aerosol foam product containing calcipotriene 0.005% plus betamethasone dipropionate 0.064% with vehicle, applied once daily for up to 4 weeks in 426 patients with psoriasis vulgaris.
Disclosures: Dr. Leonardi reported that he has been a consultant for LEO Pharma. All of the other authors disclosed having current or former ties to LEO Pharma, including two who are currently employed by the company. Dr. Leonardi and many of the other study authors also reported having numerous ties to other pharmaceutical companies.