User login
VIDEO: IL-17 antagonists continue reshaping psoriasis therapy in 2016
WAIKOLOA, HAWAII – What’s going to be the biggest development in the field of psoriasis in 2016?
Hint: Look for a repeat of 2015.
The first of the interleukin-17 antagonists, secukinumab, was the biggest development of 2015, explained Dr. Craig L. Leonardi, associate clinical professor of dermatology at Saint Louis University, St. Louis. “It’s always good when we get another mechanism of action.”
2016 should see the debut of another IL-17 antagonist, ixekizumab, which “seems to be more efficacious than secukinumab,” Dr. Leonardi said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – What’s going to be the biggest development in the field of psoriasis in 2016?
Hint: Look for a repeat of 2015.
The first of the interleukin-17 antagonists, secukinumab, was the biggest development of 2015, explained Dr. Craig L. Leonardi, associate clinical professor of dermatology at Saint Louis University, St. Louis. “It’s always good when we get another mechanism of action.”
2016 should see the debut of another IL-17 antagonist, ixekizumab, which “seems to be more efficacious than secukinumab,” Dr. Leonardi said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – What’s going to be the biggest development in the field of psoriasis in 2016?
Hint: Look for a repeat of 2015.
The first of the interleukin-17 antagonists, secukinumab, was the biggest development of 2015, explained Dr. Craig L. Leonardi, associate clinical professor of dermatology at Saint Louis University, St. Louis. “It’s always good when we get another mechanism of action.”
2016 should see the debut of another IL-17 antagonist, ixekizumab, which “seems to be more efficacious than secukinumab,” Dr. Leonardi said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Addressing Patient Concerns on Biologics for Psoriasis
What does your patient need to know at the first visit? Why is this information important?
At the first visit I discuss potential side effects. The ones that patients want to hear about are infection and cancer. Clinical studies do not seem to indicate an increased risk for infection. But is that what I tell the patient? No. I tell the patient that there may be a slightly increased risk for infection with biologics. I tell patients the prescribing information lists some rare and serious infections such as tuberculosis, hepatitis B reactivation, sepsis, invasive fungal infections, and opportunistic infections. I do say that if the patient is young and healthy, the risk is relatively low. I do tell the patient that we do check the things that we can check, such as screening for tuberculosis and hepatitis B virus.
Once a patient starts a biologic, I ask him/her to tell me or the primary care physician if he/she develops fevers, chills, weight loss, chronic cough, or an illness that lasts 1 week or more. I emphasize that high-risk patients—those who are elderly, have chronic kidney or liver disease, or have uncontrolled diabetes—should be more vigilant for signs of infection than otherwise-healthy patients.
The word cancer is scary for patients, so I mention that patients with psoriasis are more likely to develop lymphoma, which may be related to having psoriasis itself, not to therapies used to treat psoriasis. For tumor necrosis factor inhibitors, there is a warning about an increased risk for nonmelanoma skin cancers, which has been confirmed in some studies, so I tell patients that it is important to come in at least every 6 months to evaluate their psoriasis but also to check for skin cancers.
I also review with patients that many times their insurance company will require them to fail a traditional systemic therapy first before they can start a biologic. Also, monthly co-pays for a biologic likely will be higher than oral therapies they have used.
How do you keep patients compliant with treatment?
Patients usually want to be compliant on their own, as they will see how effective the biologic is and how clear their skin will become while on therapy. They will see that if they take a break for whatever reason (eg, ran out of medicine, went on vacation, loss of medical insurance), the psoriasis will return, perhaps with a vengeance.
I remind patients who are not compliant with biologic therapy that the body may produce antibodies against the biologic itself if there is a substantial break from therapy, which may make the biologic less effective over time.
If the patient wants to reduce the dose but not stop it completely, I recommend to increase the interval of the maintenance dosing by 1 day after each injection and see if the psoriasis slowly returns. If it does return, then he/she should reduce the interval of the maintenance dosing by 1 day and hold that interval.
What do you do if they refuse treatment?
I stress to patients that biologics are typically the best long-term treatment with the highest levels of effectiveness and safety for psoriasis. In the rare case of a patient refusing a biologic, I discuss other options such as oral therapy (eg, methotrexate, cyclosporine, acitretin, apremilast) or phototherapy. If the patient is a candidate for biologic therapy, topical therapy may not be adequate to treat a large body surface area affected.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation has recently published an updated patient booklet, “Systemic Medications for Psoriasis and Psoriatic Arthritis,” that I would encourage all patients to read for further information.
Suggested Reading
National Psoriasis Foundation. Systemic medications for psoriasis and psoriatic arthritis. https://www.psoriasis.org/sites/default/files/systemics_booklet.pdf. Accessed January 11, 2016.
What does your patient need to know at the first visit? Why is this information important?
At the first visit I discuss potential side effects. The ones that patients want to hear about are infection and cancer. Clinical studies do not seem to indicate an increased risk for infection. But is that what I tell the patient? No. I tell the patient that there may be a slightly increased risk for infection with biologics. I tell patients the prescribing information lists some rare and serious infections such as tuberculosis, hepatitis B reactivation, sepsis, invasive fungal infections, and opportunistic infections. I do say that if the patient is young and healthy, the risk is relatively low. I do tell the patient that we do check the things that we can check, such as screening for tuberculosis and hepatitis B virus.
Once a patient starts a biologic, I ask him/her to tell me or the primary care physician if he/she develops fevers, chills, weight loss, chronic cough, or an illness that lasts 1 week or more. I emphasize that high-risk patients—those who are elderly, have chronic kidney or liver disease, or have uncontrolled diabetes—should be more vigilant for signs of infection than otherwise-healthy patients.
The word cancer is scary for patients, so I mention that patients with psoriasis are more likely to develop lymphoma, which may be related to having psoriasis itself, not to therapies used to treat psoriasis. For tumor necrosis factor inhibitors, there is a warning about an increased risk for nonmelanoma skin cancers, which has been confirmed in some studies, so I tell patients that it is important to come in at least every 6 months to evaluate their psoriasis but also to check for skin cancers.
I also review with patients that many times their insurance company will require them to fail a traditional systemic therapy first before they can start a biologic. Also, monthly co-pays for a biologic likely will be higher than oral therapies they have used.
How do you keep patients compliant with treatment?
Patients usually want to be compliant on their own, as they will see how effective the biologic is and how clear their skin will become while on therapy. They will see that if they take a break for whatever reason (eg, ran out of medicine, went on vacation, loss of medical insurance), the psoriasis will return, perhaps with a vengeance.
I remind patients who are not compliant with biologic therapy that the body may produce antibodies against the biologic itself if there is a substantial break from therapy, which may make the biologic less effective over time.
If the patient wants to reduce the dose but not stop it completely, I recommend to increase the interval of the maintenance dosing by 1 day after each injection and see if the psoriasis slowly returns. If it does return, then he/she should reduce the interval of the maintenance dosing by 1 day and hold that interval.
What do you do if they refuse treatment?
I stress to patients that biologics are typically the best long-term treatment with the highest levels of effectiveness and safety for psoriasis. In the rare case of a patient refusing a biologic, I discuss other options such as oral therapy (eg, methotrexate, cyclosporine, acitretin, apremilast) or phototherapy. If the patient is a candidate for biologic therapy, topical therapy may not be adequate to treat a large body surface area affected.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation has recently published an updated patient booklet, “Systemic Medications for Psoriasis and Psoriatic Arthritis,” that I would encourage all patients to read for further information.
Suggested Reading
National Psoriasis Foundation. Systemic medications for psoriasis and psoriatic arthritis. https://www.psoriasis.org/sites/default/files/systemics_booklet.pdf. Accessed January 11, 2016.
What does your patient need to know at the first visit? Why is this information important?
At the first visit I discuss potential side effects. The ones that patients want to hear about are infection and cancer. Clinical studies do not seem to indicate an increased risk for infection. But is that what I tell the patient? No. I tell the patient that there may be a slightly increased risk for infection with biologics. I tell patients the prescribing information lists some rare and serious infections such as tuberculosis, hepatitis B reactivation, sepsis, invasive fungal infections, and opportunistic infections. I do say that if the patient is young and healthy, the risk is relatively low. I do tell the patient that we do check the things that we can check, such as screening for tuberculosis and hepatitis B virus.
Once a patient starts a biologic, I ask him/her to tell me or the primary care physician if he/she develops fevers, chills, weight loss, chronic cough, or an illness that lasts 1 week or more. I emphasize that high-risk patients—those who are elderly, have chronic kidney or liver disease, or have uncontrolled diabetes—should be more vigilant for signs of infection than otherwise-healthy patients.
The word cancer is scary for patients, so I mention that patients with psoriasis are more likely to develop lymphoma, which may be related to having psoriasis itself, not to therapies used to treat psoriasis. For tumor necrosis factor inhibitors, there is a warning about an increased risk for nonmelanoma skin cancers, which has been confirmed in some studies, so I tell patients that it is important to come in at least every 6 months to evaluate their psoriasis but also to check for skin cancers.
I also review with patients that many times their insurance company will require them to fail a traditional systemic therapy first before they can start a biologic. Also, monthly co-pays for a biologic likely will be higher than oral therapies they have used.
How do you keep patients compliant with treatment?
Patients usually want to be compliant on their own, as they will see how effective the biologic is and how clear their skin will become while on therapy. They will see that if they take a break for whatever reason (eg, ran out of medicine, went on vacation, loss of medical insurance), the psoriasis will return, perhaps with a vengeance.
I remind patients who are not compliant with biologic therapy that the body may produce antibodies against the biologic itself if there is a substantial break from therapy, which may make the biologic less effective over time.
If the patient wants to reduce the dose but not stop it completely, I recommend to increase the interval of the maintenance dosing by 1 day after each injection and see if the psoriasis slowly returns. If it does return, then he/she should reduce the interval of the maintenance dosing by 1 day and hold that interval.
What do you do if they refuse treatment?
I stress to patients that biologics are typically the best long-term treatment with the highest levels of effectiveness and safety for psoriasis. In the rare case of a patient refusing a biologic, I discuss other options such as oral therapy (eg, methotrexate, cyclosporine, acitretin, apremilast) or phototherapy. If the patient is a candidate for biologic therapy, topical therapy may not be adequate to treat a large body surface area affected.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation has recently published an updated patient booklet, “Systemic Medications for Psoriasis and Psoriatic Arthritis,” that I would encourage all patients to read for further information.
Suggested Reading
National Psoriasis Foundation. Systemic medications for psoriasis and psoriatic arthritis. https://www.psoriasis.org/sites/default/files/systemics_booklet.pdf. Accessed January 11, 2016.
Psoriasis for Seniors
The evaluation and treatment of psoriasis in older patients have long been issues of interest among clinicians. This population is at risk from comorbidities associated with psoriasis. In addition, the potential for increased side effects of therapies in this population has been a concern.
Takeshita et al1 recently published a study evaluating the prevalence of psoriasis and its treatments in the elderly population. The authors point out that despite major advances in the field of psoriasis, there are large gaps in knowledge among the increasing elderly population. The authors noted that this study is the first to evaluate the epidemiology and treatment of psoriasis in the US population using Medicare.1
Utilizing 8 different algorithms, claims-based psoriasis prevalence was calculated for 799,607 beneficiaries in the 2011 Medicare 5% sample (random 5% sample of Medicare beneficiaries) and was found to range from 0.51% to 1.23%. For the main analyses, a diagnosis of psoriasis was established by the presence of at least 2 inpatient or outpatient claims for psoriasis.1
The authors reported the following characteristics for the study population1: the mean age was 68.6 years; 43.2% of the participants were male; 88.8% were white; 5.1% were black; 2.2% were Hispanic; and 3.9% were other or unknown race. Regional distribution of residence was as follows: 24.0% in the northeast, 23.0% in the Midwest, 36.2% in the south, and 16.6% in the west. County-level mean per capita income was $40,115; 63.6% of beneficiaries qualified for Medicare based on age alone; 58.4% were not receiving a Medicare Part D low-income subsidy (LIS); and 19.0% were receiving Part D plans with enhanced alternative coverage. The most commonly coded comorbidities were cardiometabolic disorders (67.6% hypertension; 59.9% dyslipidemia; 32.4% diabetes); 23.5% had atherosclerotic outcomes. The prevalence of obesity was relatively low at 9.3% and the prevalence of psoriatic arthritis was 9.4%. Other comorbid diseases of interest included depression (17.1%), renal disease (9.8%), liver disease (5.1%), and inflammatory bowel disease (1.2%).1
The analysis of psoriatic therapy revealed that topical therapies were used by 76.6% of the total psoriasis sample, the majority of which were topical corticosteroids.1 Phototherapy was used by 7% and oral systemic medications were used by 14.3% (the majority received methotrexate). Biologics were received by 10.2%, and of those patients, 44.4% received etanercept, 34.2% adalimumab, 22.7% infliximab, and 7.9% ustekinumab.1
There were several interesting findings in the analysis.1 Oral systemic medications such as methotrexate were the most common therapies for moderate to severe psoriasis, followed by biologics.1 Associated comorbidities for which biologic therapy is indicated (ie, ankylosing spondylitis, inflammatory bowel disease, psoriatic arthritis) were associated with greater odds of receiving treatment with biologics. Individuals lacking LIS under the Part D plan had 70% lower odds of receiving biologics compared with those with LIS that allowed for lower out-of-pocket costs. The odds of having received biologics were 69% lower for black individuals compared to white patients.1
This study helps us to further understand the patterns of psoriasis and its treatment in the elderly population. Some of the findings are in line with our current thinking regarding comorbidities and therapies used, while other observations, such as a lower number of untreated patients than expected, are more surprising. Interestingly, this study identified potential financial and racial barriers to the receipt of biologic therapies. These barriers are important issues to address as we strive to better care for our psoriatic population.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use [published online July 27, 2015]. J Invest Dermatol. 2015;135:2955-2963. doi:10.1038/jid.2015.296.
The evaluation and treatment of psoriasis in older patients have long been issues of interest among clinicians. This population is at risk from comorbidities associated with psoriasis. In addition, the potential for increased side effects of therapies in this population has been a concern.
Takeshita et al1 recently published a study evaluating the prevalence of psoriasis and its treatments in the elderly population. The authors point out that despite major advances in the field of psoriasis, there are large gaps in knowledge among the increasing elderly population. The authors noted that this study is the first to evaluate the epidemiology and treatment of psoriasis in the US population using Medicare.1
Utilizing 8 different algorithms, claims-based psoriasis prevalence was calculated for 799,607 beneficiaries in the 2011 Medicare 5% sample (random 5% sample of Medicare beneficiaries) and was found to range from 0.51% to 1.23%. For the main analyses, a diagnosis of psoriasis was established by the presence of at least 2 inpatient or outpatient claims for psoriasis.1
The authors reported the following characteristics for the study population1: the mean age was 68.6 years; 43.2% of the participants were male; 88.8% were white; 5.1% were black; 2.2% were Hispanic; and 3.9% were other or unknown race. Regional distribution of residence was as follows: 24.0% in the northeast, 23.0% in the Midwest, 36.2% in the south, and 16.6% in the west. County-level mean per capita income was $40,115; 63.6% of beneficiaries qualified for Medicare based on age alone; 58.4% were not receiving a Medicare Part D low-income subsidy (LIS); and 19.0% were receiving Part D plans with enhanced alternative coverage. The most commonly coded comorbidities were cardiometabolic disorders (67.6% hypertension; 59.9% dyslipidemia; 32.4% diabetes); 23.5% had atherosclerotic outcomes. The prevalence of obesity was relatively low at 9.3% and the prevalence of psoriatic arthritis was 9.4%. Other comorbid diseases of interest included depression (17.1%), renal disease (9.8%), liver disease (5.1%), and inflammatory bowel disease (1.2%).1
The analysis of psoriatic therapy revealed that topical therapies were used by 76.6% of the total psoriasis sample, the majority of which were topical corticosteroids.1 Phototherapy was used by 7% and oral systemic medications were used by 14.3% (the majority received methotrexate). Biologics were received by 10.2%, and of those patients, 44.4% received etanercept, 34.2% adalimumab, 22.7% infliximab, and 7.9% ustekinumab.1
There were several interesting findings in the analysis.1 Oral systemic medications such as methotrexate were the most common therapies for moderate to severe psoriasis, followed by biologics.1 Associated comorbidities for which biologic therapy is indicated (ie, ankylosing spondylitis, inflammatory bowel disease, psoriatic arthritis) were associated with greater odds of receiving treatment with biologics. Individuals lacking LIS under the Part D plan had 70% lower odds of receiving biologics compared with those with LIS that allowed for lower out-of-pocket costs. The odds of having received biologics were 69% lower for black individuals compared to white patients.1
This study helps us to further understand the patterns of psoriasis and its treatment in the elderly population. Some of the findings are in line with our current thinking regarding comorbidities and therapies used, while other observations, such as a lower number of untreated patients than expected, are more surprising. Interestingly, this study identified potential financial and racial barriers to the receipt of biologic therapies. These barriers are important issues to address as we strive to better care for our psoriatic population.
The evaluation and treatment of psoriasis in older patients have long been issues of interest among clinicians. This population is at risk from comorbidities associated with psoriasis. In addition, the potential for increased side effects of therapies in this population has been a concern.
Takeshita et al1 recently published a study evaluating the prevalence of psoriasis and its treatments in the elderly population. The authors point out that despite major advances in the field of psoriasis, there are large gaps in knowledge among the increasing elderly population. The authors noted that this study is the first to evaluate the epidemiology and treatment of psoriasis in the US population using Medicare.1
Utilizing 8 different algorithms, claims-based psoriasis prevalence was calculated for 799,607 beneficiaries in the 2011 Medicare 5% sample (random 5% sample of Medicare beneficiaries) and was found to range from 0.51% to 1.23%. For the main analyses, a diagnosis of psoriasis was established by the presence of at least 2 inpatient or outpatient claims for psoriasis.1
The authors reported the following characteristics for the study population1: the mean age was 68.6 years; 43.2% of the participants were male; 88.8% were white; 5.1% were black; 2.2% were Hispanic; and 3.9% were other or unknown race. Regional distribution of residence was as follows: 24.0% in the northeast, 23.0% in the Midwest, 36.2% in the south, and 16.6% in the west. County-level mean per capita income was $40,115; 63.6% of beneficiaries qualified for Medicare based on age alone; 58.4% were not receiving a Medicare Part D low-income subsidy (LIS); and 19.0% were receiving Part D plans with enhanced alternative coverage. The most commonly coded comorbidities were cardiometabolic disorders (67.6% hypertension; 59.9% dyslipidemia; 32.4% diabetes); 23.5% had atherosclerotic outcomes. The prevalence of obesity was relatively low at 9.3% and the prevalence of psoriatic arthritis was 9.4%. Other comorbid diseases of interest included depression (17.1%), renal disease (9.8%), liver disease (5.1%), and inflammatory bowel disease (1.2%).1
The analysis of psoriatic therapy revealed that topical therapies were used by 76.6% of the total psoriasis sample, the majority of which were topical corticosteroids.1 Phototherapy was used by 7% and oral systemic medications were used by 14.3% (the majority received methotrexate). Biologics were received by 10.2%, and of those patients, 44.4% received etanercept, 34.2% adalimumab, 22.7% infliximab, and 7.9% ustekinumab.1
There were several interesting findings in the analysis.1 Oral systemic medications such as methotrexate were the most common therapies for moderate to severe psoriasis, followed by biologics.1 Associated comorbidities for which biologic therapy is indicated (ie, ankylosing spondylitis, inflammatory bowel disease, psoriatic arthritis) were associated with greater odds of receiving treatment with biologics. Individuals lacking LIS under the Part D plan had 70% lower odds of receiving biologics compared with those with LIS that allowed for lower out-of-pocket costs. The odds of having received biologics were 69% lower for black individuals compared to white patients.1
This study helps us to further understand the patterns of psoriasis and its treatment in the elderly population. Some of the findings are in line with our current thinking regarding comorbidities and therapies used, while other observations, such as a lower number of untreated patients than expected, are more surprising. Interestingly, this study identified potential financial and racial barriers to the receipt of biologic therapies. These barriers are important issues to address as we strive to better care for our psoriatic population.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use [published online July 27, 2015]. J Invest Dermatol. 2015;135:2955-2963. doi:10.1038/jid.2015.296.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use [published online July 27, 2015]. J Invest Dermatol. 2015;135:2955-2963. doi:10.1038/jid.2015.296.
House panel says Medicare biosimilar payment plan will stifle innovation
Questions linger as to whether federal health care payment policies will encourage – or stifle – the entry of more biosimilars into the marketplace.
Getting the biosimilar approval process right “doesn’t matter unless we get the charging/reimbursement process right,” Rep. Joe Barton (R-Tex.), chairmen emeritus of the House Energy and Commerce Committee, said Feb. 4 during a Health Subcommittee hearing. Without a workable reimbursement process “there’s no incentive to create the drug in the first place. Nobody’s going to do it.”
At issue is how the Centers for Medicare & Medicaid Services plans to pay for biosimilars. The 2016 Medicare Physician Fee Schedule establishes that biosimilars would receive the same reimbursement code as their biologic innovator product and be paid at a weighted average of the average sales price of all biosimilars in that code, plus 6% of the innovator product.
Rep. Frank Pallone (D-N.J.), ranking member of the subcommittee, expressed concern over the policy. “I worry that this inappropriately treats biosimilars like generic drugs and will disincentivize manufacturers from entering the biosimilars marketplace. ... Biosimilars are not generics. Each is its own unique product and biosimilars go through a much more stringent approval process.”
Sean Cavanaugh, deputy administrator and director of the CMS, testified that in terms of market forces, there are enough similarities between generic drugs and biosimilars to maintain that kind of similarity. He added, however, that the CMS is open to changing reimbursement methodology “if we thought that our payment policy was not accomplishing what we expected it to.”
Rep. Brett Guthrie (R-Ky.), vice chairman of the subcommittee, said the reimbursement scheme could lead to “inappropriate switching between biosimilars – switching to a lower cost that is not the same [and] a less vibrant biosimilar market altogether.”
Mr. Cavanaugh noted that the CMS spends more than $1 billion on each of the top six drugs it pays for, all of which are biologics. “I think that alone creates the opportunity for a very vibrant market in biosimilars,” he said.
In addition, he did not see switching as an issue.
“Physicians do not order biologics or other drug products by billing code,” Mr. Cavanaugh said. “Similarly, pharmacists do not derive what switchings they are allowed to do based on billing codes.”
Questions linger as to whether federal health care payment policies will encourage – or stifle – the entry of more biosimilars into the marketplace.
Getting the biosimilar approval process right “doesn’t matter unless we get the charging/reimbursement process right,” Rep. Joe Barton (R-Tex.), chairmen emeritus of the House Energy and Commerce Committee, said Feb. 4 during a Health Subcommittee hearing. Without a workable reimbursement process “there’s no incentive to create the drug in the first place. Nobody’s going to do it.”
At issue is how the Centers for Medicare & Medicaid Services plans to pay for biosimilars. The 2016 Medicare Physician Fee Schedule establishes that biosimilars would receive the same reimbursement code as their biologic innovator product and be paid at a weighted average of the average sales price of all biosimilars in that code, plus 6% of the innovator product.
Rep. Frank Pallone (D-N.J.), ranking member of the subcommittee, expressed concern over the policy. “I worry that this inappropriately treats biosimilars like generic drugs and will disincentivize manufacturers from entering the biosimilars marketplace. ... Biosimilars are not generics. Each is its own unique product and biosimilars go through a much more stringent approval process.”
Sean Cavanaugh, deputy administrator and director of the CMS, testified that in terms of market forces, there are enough similarities between generic drugs and biosimilars to maintain that kind of similarity. He added, however, that the CMS is open to changing reimbursement methodology “if we thought that our payment policy was not accomplishing what we expected it to.”
Rep. Brett Guthrie (R-Ky.), vice chairman of the subcommittee, said the reimbursement scheme could lead to “inappropriate switching between biosimilars – switching to a lower cost that is not the same [and] a less vibrant biosimilar market altogether.”
Mr. Cavanaugh noted that the CMS spends more than $1 billion on each of the top six drugs it pays for, all of which are biologics. “I think that alone creates the opportunity for a very vibrant market in biosimilars,” he said.
In addition, he did not see switching as an issue.
“Physicians do not order biologics or other drug products by billing code,” Mr. Cavanaugh said. “Similarly, pharmacists do not derive what switchings they are allowed to do based on billing codes.”
Questions linger as to whether federal health care payment policies will encourage – or stifle – the entry of more biosimilars into the marketplace.
Getting the biosimilar approval process right “doesn’t matter unless we get the charging/reimbursement process right,” Rep. Joe Barton (R-Tex.), chairmen emeritus of the House Energy and Commerce Committee, said Feb. 4 during a Health Subcommittee hearing. Without a workable reimbursement process “there’s no incentive to create the drug in the first place. Nobody’s going to do it.”
At issue is how the Centers for Medicare & Medicaid Services plans to pay for biosimilars. The 2016 Medicare Physician Fee Schedule establishes that biosimilars would receive the same reimbursement code as their biologic innovator product and be paid at a weighted average of the average sales price of all biosimilars in that code, plus 6% of the innovator product.
Rep. Frank Pallone (D-N.J.), ranking member of the subcommittee, expressed concern over the policy. “I worry that this inappropriately treats biosimilars like generic drugs and will disincentivize manufacturers from entering the biosimilars marketplace. ... Biosimilars are not generics. Each is its own unique product and biosimilars go through a much more stringent approval process.”
Sean Cavanaugh, deputy administrator and director of the CMS, testified that in terms of market forces, there are enough similarities between generic drugs and biosimilars to maintain that kind of similarity. He added, however, that the CMS is open to changing reimbursement methodology “if we thought that our payment policy was not accomplishing what we expected it to.”
Rep. Brett Guthrie (R-Ky.), vice chairman of the subcommittee, said the reimbursement scheme could lead to “inappropriate switching between biosimilars – switching to a lower cost that is not the same [and] a less vibrant biosimilar market altogether.”
Mr. Cavanaugh noted that the CMS spends more than $1 billion on each of the top six drugs it pays for, all of which are biologics. “I think that alone creates the opportunity for a very vibrant market in biosimilars,” he said.
In addition, he did not see switching as an issue.
“Physicians do not order biologics or other drug products by billing code,” Mr. Cavanaugh said. “Similarly, pharmacists do not derive what switchings they are allowed to do based on billing codes.”
FROM A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Biosimilar infliximab gains FDA Advisory Committee endorsement
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
SCCA2: Potential biomarker for psoriasis severity and treatment efficacy
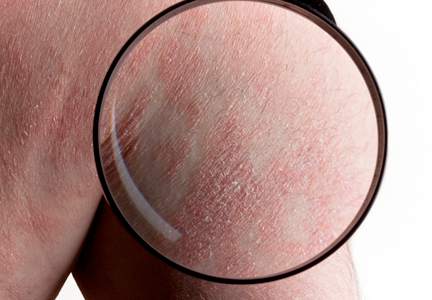
The level of serum squamous cell carcinoma antigen 2 – an isoform of SCCA that is widely used as a tumor marker for SCC – was significantly higher in both skin and serum of patients with psoriasis, compared with healthy volunteers in a recent study, correlating with psoriasis severity score and reflecting treatment efficacy.
The intensity of skin and serum levels of SCCA2 was correlated in patients with psoriasis, and the study results also suggest that Th17 cytokines (IL-17 and IL-22) induce SCCA 2 elevation in serum and skin. “Significant elevation of SCCA2 is associated with disease severity and reflects treatment efficacy. SCCA2 may be a useful biomarker in psoriasis, reflecting Th17-type inflammation – the main determinant of the severity of psoriasis,” wrote Dr. Yuko Watanabe of Yokohama (Japan) City University and associates (Br J Derm. 2016 Jan 29. doi: 10.1111/bjd.14426).
According to the investigators, the commonly used psoriasis area severity index (PASI) is suboptimal because it is not truly quantitative and varies among clinicians. Since there are several new treatment options for psoriasis, including biologics, the authors believe that a new serum marker for psoriasis is needed to evaluate disease activity and manage treatment.
The prospective, cross-sectional study included 123 patients with psoriasis and 25 healthy controls. Serum SCCA2 levels were significantly higher in patients with psoriasis, compared with controls: a median of 2.7 ng/mL versus 0.70 ng/mL, respectively (P <.0001). Serum SCCA2 levels were positively correlated with PASI for all patients with psoriasis, regardless of subtype. In 38 patients on monotherapy or combination therapy with drugs to treat psoriasis, SCCA2 levels decreased in the 35 patients whose PASI improved on treatment (P <.0001) and increased in the three cases of clinical deterioration.
The study was supported by a grant from Japan’s Ministry of Education, Culture, Sports, Science and Technology. The investigators declared that they had no conflicts of interest.
The level of serum squamous cell carcinoma antigen 2 – an isoform of SCCA that is widely used as a tumor marker for SCC – was significantly higher in both skin and serum of patients with psoriasis, compared with healthy volunteers in a recent study, correlating with psoriasis severity score and reflecting treatment efficacy.
The intensity of skin and serum levels of SCCA2 was correlated in patients with psoriasis, and the study results also suggest that Th17 cytokines (IL-17 and IL-22) induce SCCA 2 elevation in serum and skin. “Significant elevation of SCCA2 is associated with disease severity and reflects treatment efficacy. SCCA2 may be a useful biomarker in psoriasis, reflecting Th17-type inflammation – the main determinant of the severity of psoriasis,” wrote Dr. Yuko Watanabe of Yokohama (Japan) City University and associates (Br J Derm. 2016 Jan 29. doi: 10.1111/bjd.14426).
According to the investigators, the commonly used psoriasis area severity index (PASI) is suboptimal because it is not truly quantitative and varies among clinicians. Since there are several new treatment options for psoriasis, including biologics, the authors believe that a new serum marker for psoriasis is needed to evaluate disease activity and manage treatment.
The prospective, cross-sectional study included 123 patients with psoriasis and 25 healthy controls. Serum SCCA2 levels were significantly higher in patients with psoriasis, compared with controls: a median of 2.7 ng/mL versus 0.70 ng/mL, respectively (P <.0001). Serum SCCA2 levels were positively correlated with PASI for all patients with psoriasis, regardless of subtype. In 38 patients on monotherapy or combination therapy with drugs to treat psoriasis, SCCA2 levels decreased in the 35 patients whose PASI improved on treatment (P <.0001) and increased in the three cases of clinical deterioration.
The study was supported by a grant from Japan’s Ministry of Education, Culture, Sports, Science and Technology. The investigators declared that they had no conflicts of interest.
The level of serum squamous cell carcinoma antigen 2 – an isoform of SCCA that is widely used as a tumor marker for SCC – was significantly higher in both skin and serum of patients with psoriasis, compared with healthy volunteers in a recent study, correlating with psoriasis severity score and reflecting treatment efficacy.
The intensity of skin and serum levels of SCCA2 was correlated in patients with psoriasis, and the study results also suggest that Th17 cytokines (IL-17 and IL-22) induce SCCA 2 elevation in serum and skin. “Significant elevation of SCCA2 is associated with disease severity and reflects treatment efficacy. SCCA2 may be a useful biomarker in psoriasis, reflecting Th17-type inflammation – the main determinant of the severity of psoriasis,” wrote Dr. Yuko Watanabe of Yokohama (Japan) City University and associates (Br J Derm. 2016 Jan 29. doi: 10.1111/bjd.14426).
According to the investigators, the commonly used psoriasis area severity index (PASI) is suboptimal because it is not truly quantitative and varies among clinicians. Since there are several new treatment options for psoriasis, including biologics, the authors believe that a new serum marker for psoriasis is needed to evaluate disease activity and manage treatment.
The prospective, cross-sectional study included 123 patients with psoriasis and 25 healthy controls. Serum SCCA2 levels were significantly higher in patients with psoriasis, compared with controls: a median of 2.7 ng/mL versus 0.70 ng/mL, respectively (P <.0001). Serum SCCA2 levels were positively correlated with PASI for all patients with psoriasis, regardless of subtype. In 38 patients on monotherapy or combination therapy with drugs to treat psoriasis, SCCA2 levels decreased in the 35 patients whose PASI improved on treatment (P <.0001) and increased in the three cases of clinical deterioration.
The study was supported by a grant from Japan’s Ministry of Education, Culture, Sports, Science and Technology. The investigators declared that they had no conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
VIDEO: Humor, psychology turn noncompliance into better outcomes
GRAND CAYMAN – “Why say ‘noncompliant patient’? It’s redundant!”
That’s what Dr. Steven R. Feldman jokingly told an audience at this year’s Caribbean Dermatology Symposium, provided by the Global Academy for Medical Education.
In this video interview, Dr. Feldman, the director of the Psoriasis Treatment Center at Wake Forest University, Winston-Salem, N.C., recounts anecdotes that led him to understand what it takes to get patients to comply with treatment, also shares his tips for getting patients to take a more active role in healing what ails them, whether it be psoriasis or any other illness.
Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
GRAND CAYMAN – “Why say ‘noncompliant patient’? It’s redundant!”
That’s what Dr. Steven R. Feldman jokingly told an audience at this year’s Caribbean Dermatology Symposium, provided by the Global Academy for Medical Education.
In this video interview, Dr. Feldman, the director of the Psoriasis Treatment Center at Wake Forest University, Winston-Salem, N.C., recounts anecdotes that led him to understand what it takes to get patients to comply with treatment, also shares his tips for getting patients to take a more active role in healing what ails them, whether it be psoriasis or any other illness.
Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
GRAND CAYMAN – “Why say ‘noncompliant patient’? It’s redundant!”
That’s what Dr. Steven R. Feldman jokingly told an audience at this year’s Caribbean Dermatology Symposium, provided by the Global Academy for Medical Education.
In this video interview, Dr. Feldman, the director of the Psoriasis Treatment Center at Wake Forest University, Winston-Salem, N.C., recounts anecdotes that led him to understand what it takes to get patients to comply with treatment, also shares his tips for getting patients to take a more active role in healing what ails them, whether it be psoriasis or any other illness.
Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT THE CARIBBEAN DERMATOLOGY SYMPOSIUM
VIDEO: Ken Gordon’s pro tips on using biologics in psoriasis
GRAND CAYMAN – When considering the use of biologic therapy for psoriasis patients who are at greater risk of cancer or of serious infection, Dr. Kenneth B. Gordon advises clinicians to “look at the patient in front of you” and the impact the disease is having on them.
In an interview at the annual Caribbean Dermatology Symposium, Dr. Gordon, professor of dermatology at Northwestern University, Chicago, discusses the use of biologics to treat psoriasis in patients with a history of cancer, patients at an increased risk for serious infections (such as those with chronic renal disease or diabetes), as well as patients with HIV, who can have significant psoriasis.
He also provides some recommendations on counseling patients and discusses the use of biologics in children with psoriasis.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
This article was updated 1/31/2016.
GRAND CAYMAN – When considering the use of biologic therapy for psoriasis patients who are at greater risk of cancer or of serious infection, Dr. Kenneth B. Gordon advises clinicians to “look at the patient in front of you” and the impact the disease is having on them.
In an interview at the annual Caribbean Dermatology Symposium, Dr. Gordon, professor of dermatology at Northwestern University, Chicago, discusses the use of biologics to treat psoriasis in patients with a history of cancer, patients at an increased risk for serious infections (such as those with chronic renal disease or diabetes), as well as patients with HIV, who can have significant psoriasis.
He also provides some recommendations on counseling patients and discusses the use of biologics in children with psoriasis.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
This article was updated 1/31/2016.
GRAND CAYMAN – When considering the use of biologic therapy for psoriasis patients who are at greater risk of cancer or of serious infection, Dr. Kenneth B. Gordon advises clinicians to “look at the patient in front of you” and the impact the disease is having on them.
In an interview at the annual Caribbean Dermatology Symposium, Dr. Gordon, professor of dermatology at Northwestern University, Chicago, discusses the use of biologics to treat psoriasis in patients with a history of cancer, patients at an increased risk for serious infections (such as those with chronic renal disease or diabetes), as well as patients with HIV, who can have significant psoriasis.
He also provides some recommendations on counseling patients and discusses the use of biologics in children with psoriasis.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
This article was updated 1/31/2016.
AT THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Step therapy and biologics: An easier road ahead?
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
Price Explosion

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?