User login
Fighting back against psoriasis bullies
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
VIDEO: Tune in to psoriasis patients’ quality of life
LAS VEGAS – Physicians often fail to predict the impact of disease on quality of life in their psoriasis patients, which can help guide treatment, Joel Gelfand, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
“This is true across all medical conditions,” said Dr. Gelfand, professor of dermatology, at the University of Pennsylvania, Philadelphia. For example, some patients with psoriasis may have extensive disease, but it doesn’t bother them, and therefore they may need less treatment, he pointed out.
In his practice, patients with psoriasis are asked to rate physical symptoms (including flaking and itching) and emotional symptoms (including anxiety and depression) related to their disease on a scale of 0 to 10, with 10 being the worst. “The higher those scores are, the more aggressive I’ll be in treating them,” he said. Patient scores can be tracked over time, to review progress with their chosen treatment, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Janssen, Lilly, Novartis, Celgene, Merck, Sanofi, Pfizer, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Physicians often fail to predict the impact of disease on quality of life in their psoriasis patients, which can help guide treatment, Joel Gelfand, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
“This is true across all medical conditions,” said Dr. Gelfand, professor of dermatology, at the University of Pennsylvania, Philadelphia. For example, some patients with psoriasis may have extensive disease, but it doesn’t bother them, and therefore they may need less treatment, he pointed out.
In his practice, patients with psoriasis are asked to rate physical symptoms (including flaking and itching) and emotional symptoms (including anxiety and depression) related to their disease on a scale of 0 to 10, with 10 being the worst. “The higher those scores are, the more aggressive I’ll be in treating them,” he said. Patient scores can be tracked over time, to review progress with their chosen treatment, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Janssen, Lilly, Novartis, Celgene, Merck, Sanofi, Pfizer, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Physicians often fail to predict the impact of disease on quality of life in their psoriasis patients, which can help guide treatment, Joel Gelfand, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
“This is true across all medical conditions,” said Dr. Gelfand, professor of dermatology, at the University of Pennsylvania, Philadelphia. For example, some patients with psoriasis may have extensive disease, but it doesn’t bother them, and therefore they may need less treatment, he pointed out.
In his practice, patients with psoriasis are asked to rate physical symptoms (including flaking and itching) and emotional symptoms (including anxiety and depression) related to their disease on a scale of 0 to 10, with 10 being the worst. “The higher those scores are, the more aggressive I’ll be in treating them,” he said. Patient scores can be tracked over time, to review progress with their chosen treatment, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Janssen, Lilly, Novartis, Celgene, Merck, Sanofi, Pfizer, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At SDEF LAS VEGAS DERMATOLOGY SEMINAR
Update on New Drugs in Dermatology
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
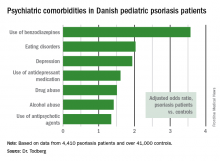
Pediatric psoriasis linked to multiple psychiatric comorbidities
VIENNA – Psoriasis in children and adolescents is associated with significantly increased risk of a variety of psychiatric comorbidities, according to a large Danish national study.
This finding has important public health implications. Psoriasis is a common skin disease, and 30% of cases have their onset in childhood or adolescence, Tanja Todberg, MD, observed at the annual congress of the European Academy of Dermatology and Venereology.
Diagnosis of psoriasis was based upon medical records and documentation that at least a second prescription for a topical vitamin D derivative had been filled. Those agents are the overwhelming choice as first-line therapy in the pediatric population, explained Dr. Todberg of the University of Copenhagen.
The pediatric psoriasis patients proved to be at significantly increased risk of being diagnosed with depression, eating disorders, drug abuse, and alcohol abuse. They were also more likely than controls to be prescribed antidepressants, antipsychotic agents, and benzodiazepines. That was every prespecified psychiatric outcome that Dr. Todberg and her coinvestigators included in the study except for one: anxiety disorders occurred at a similar rate in the pediatric psoriasis patients and controls.
Dr. Todberg reported having no financial conflicts of interest regarding this study, which was supported by Danish medical research funding.
[email protected]
VIENNA – Psoriasis in children and adolescents is associated with significantly increased risk of a variety of psychiatric comorbidities, according to a large Danish national study.
This finding has important public health implications. Psoriasis is a common skin disease, and 30% of cases have their onset in childhood or adolescence, Tanja Todberg, MD, observed at the annual congress of the European Academy of Dermatology and Venereology.
Diagnosis of psoriasis was based upon medical records and documentation that at least a second prescription for a topical vitamin D derivative had been filled. Those agents are the overwhelming choice as first-line therapy in the pediatric population, explained Dr. Todberg of the University of Copenhagen.
The pediatric psoriasis patients proved to be at significantly increased risk of being diagnosed with depression, eating disorders, drug abuse, and alcohol abuse. They were also more likely than controls to be prescribed antidepressants, antipsychotic agents, and benzodiazepines. That was every prespecified psychiatric outcome that Dr. Todberg and her coinvestigators included in the study except for one: anxiety disorders occurred at a similar rate in the pediatric psoriasis patients and controls.
Dr. Todberg reported having no financial conflicts of interest regarding this study, which was supported by Danish medical research funding.
[email protected]
VIENNA – Psoriasis in children and adolescents is associated with significantly increased risk of a variety of psychiatric comorbidities, according to a large Danish national study.
This finding has important public health implications. Psoriasis is a common skin disease, and 30% of cases have their onset in childhood or adolescence, Tanja Todberg, MD, observed at the annual congress of the European Academy of Dermatology and Venereology.
Diagnosis of psoriasis was based upon medical records and documentation that at least a second prescription for a topical vitamin D derivative had been filled. Those agents are the overwhelming choice as first-line therapy in the pediatric population, explained Dr. Todberg of the University of Copenhagen.
The pediatric psoriasis patients proved to be at significantly increased risk of being diagnosed with depression, eating disorders, drug abuse, and alcohol abuse. They were also more likely than controls to be prescribed antidepressants, antipsychotic agents, and benzodiazepines. That was every prespecified psychiatric outcome that Dr. Todberg and her coinvestigators included in the study except for one: anxiety disorders occurred at a similar rate in the pediatric psoriasis patients and controls.
Dr. Todberg reported having no financial conflicts of interest regarding this study, which was supported by Danish medical research funding.
[email protected]
AT THE EADV CONGRESS
Key clinical point:
Major finding: Danish pediatric patients with psoriasis were significantly more at risk of developing a range of psychiatric disorders than were control subjects.
Data source: This was a retrospective study of prospectively collected registry data on all 4,410 Danish children and adolescents diagnosed with psoriasis during 1997-2012 and more than 41,000 Danish controls matched for age, sex, and calendar year.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which was supported by Danish medical research funds.
Infliximab biosimilar posts mostly reassuring data in Norway’s NOR-SWITCH study
WASHINGTON – Data from the first randomized trial of switching from an originator biologic to a biosimilar of the originator indicate that the infliximab biosimilar Remsima is no different from the infliximab originator Remicade in the rate of disease worsening over 1 year across a combination of all its approved indications.
The outcomes of the Norwegian, double-blind, noninferiority trial, called NOR-SWITCH, indicate similar rates of disease worsening across patients switched to Remsima and those who stayed on Remicade. However, exploratory group analyses conducted on the different disease subgroups in the trial (Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriasis, and psoriatic arthritis) showed a potentially concerning level of disease worsening among Crohn’s disease patients on Remsima with a confidence interval that nearly fell entirely within the range favoring Remicade.
In the United States, Remsima, also known as CT-P13, is marketed by Pfizer as Inflectra.
The trial randomized 482 patients who were on stable treatment with Remicade for at least 6 months for any of the six indications for which Remicade and Remsima are approved to either stay on Remicade or switch to Remsima with the same dosing regimen for 52 weeks. Overall, patients had a mean age of about 48 years and 36%-41% were female. They had a mean disease duration of about 17 years and had been taking Remicade for a mean of nearly 7 years.
The primary endpoint was disease worsening during follow-up, according to worsening in disease-specific composite measures and/or a consensus between an investigator and a patient that led to a major change in treatment. The investigators made an assumption of 30% disease worsening across all the indications for the trial’s power calculation, based on available literature and observational data.
Disease worsening occurred in 26.2% of patients who stayed on Remicade and 29.6% of patients who switched to Remsima, based on a per-protocol analysis of 202 Remicade and 206 Remsima patients. The 95% confidence interval of the adjusted treatment difference of –4.4% was –12.7% to 3.9%, which was within the pre-specified noninferiority margin of 15%.
Exploratory subgroup analyses of the different disease subgroups showed no statistically significant differences between the two treatments in disease worsening. However, in Crohn’s disease patients, who formed the largest subgroup in the study at 155 patients, the adjusted treatment difference was –14.3% (21.2% with disease worsening for Remicade and 36.5% for Remsima) with a 95% CI of –29.3% to 0.7%.
It’s difficult to discern whether the 95% confidence interval seen in the Crohn’s disease subgroup is a part of the natural variation one would expect to see in a subgroup analysis of different diseases or if there might be a true signal for disease worsening in the Crohn’s disease patients who took Remsima. “The problem is that it’s in the largest subgroup that has no other data. If this had been in rheumatoid arthritis, that would be different,” coauthor Inge C. Olsen, PhD, a biostatistician at Diakonhjemmet Hospital, said in an interview. “All the registry trials were done in RA and spondyloarthritis patients. ... That’s an issue, but with regards to the [NOR-SWITCH] study, it’s very clear that you have no power to show anything in the subgroup analysis, and they are exploratory analyses and are not answering any hypothesis.” Currently, there are no plans to follow up on these results in another study, he said.
Other issues that the NOR-SWITCH study does not answer are the outcomes of switching back and forth between Remicade and Remsima, switching from one infliximab biosimilar to another infliximab biosimilar, and switching from other originator biologics to their biosimilars.
“Is that feasible? Is that safe? Will it retain efficacy? We don’t know. There’s a real need for those studies to be done,” Dr. Goll said.
In Norway, the remaining patients who had not switched yet from Remicade to Remsima are now doing so based on the trial’s results, Dr. Goll said. The cost of Remsima in Norway was about 75% less than Remicade in 2015 and about 60% less in 2016, she noted.
It’s still an open question what the results of the NOR-SWITCH trial might indicate for how clinicians in the United States will use Inflectra and other biosimilars, according to John J. Cush, MD, professor of medicine and rheumatology at Baylor University, Dallas.
“I think the real problem here is that it’s nice to know that [CT-P13] wasn’t inferior, but when you get into the weeds and you look at the details, those of us who may not have a lot of certainty about this might worry about this, especially when there are three new biosimilars approved in the United States: Amjevita, which is an adalimumab biosimilar; Erelzi, which is an etanercept biosimilar; and Inflectra’s about to be launched as an infliximab biosimilar,” Dr. Cush said during a session reviewing selected abstracts from the meeting. “When this NOR-SWITCH study was done in Norway, it’s a 70% savings over the original product. The new ones being introduced over here [in the United States] start at about 15%. I’m less motivated with that degree of savings to want to take some chances on my patients. So we need a little bit more certainty; we need to feel better about the cost savings to patients and health care overall. Confidence in biosimilars is what’s going to sell biosimilars. We’re a long way from confidence still.”
NOR-SWITCH was funded by the Norwegian government. Some of the investigators disclosed relationships with Pfizer and/or Celltrion, which separately market CT-P13 in different parts of the world.
[email protected]
WASHINGTON – Data from the first randomized trial of switching from an originator biologic to a biosimilar of the originator indicate that the infliximab biosimilar Remsima is no different from the infliximab originator Remicade in the rate of disease worsening over 1 year across a combination of all its approved indications.
The outcomes of the Norwegian, double-blind, noninferiority trial, called NOR-SWITCH, indicate similar rates of disease worsening across patients switched to Remsima and those who stayed on Remicade. However, exploratory group analyses conducted on the different disease subgroups in the trial (Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriasis, and psoriatic arthritis) showed a potentially concerning level of disease worsening among Crohn’s disease patients on Remsima with a confidence interval that nearly fell entirely within the range favoring Remicade.
In the United States, Remsima, also known as CT-P13, is marketed by Pfizer as Inflectra.
The trial randomized 482 patients who were on stable treatment with Remicade for at least 6 months for any of the six indications for which Remicade and Remsima are approved to either stay on Remicade or switch to Remsima with the same dosing regimen for 52 weeks. Overall, patients had a mean age of about 48 years and 36%-41% were female. They had a mean disease duration of about 17 years and had been taking Remicade for a mean of nearly 7 years.
The primary endpoint was disease worsening during follow-up, according to worsening in disease-specific composite measures and/or a consensus between an investigator and a patient that led to a major change in treatment. The investigators made an assumption of 30% disease worsening across all the indications for the trial’s power calculation, based on available literature and observational data.
Disease worsening occurred in 26.2% of patients who stayed on Remicade and 29.6% of patients who switched to Remsima, based on a per-protocol analysis of 202 Remicade and 206 Remsima patients. The 95% confidence interval of the adjusted treatment difference of –4.4% was –12.7% to 3.9%, which was within the pre-specified noninferiority margin of 15%.
Exploratory subgroup analyses of the different disease subgroups showed no statistically significant differences between the two treatments in disease worsening. However, in Crohn’s disease patients, who formed the largest subgroup in the study at 155 patients, the adjusted treatment difference was –14.3% (21.2% with disease worsening for Remicade and 36.5% for Remsima) with a 95% CI of –29.3% to 0.7%.
It’s difficult to discern whether the 95% confidence interval seen in the Crohn’s disease subgroup is a part of the natural variation one would expect to see in a subgroup analysis of different diseases or if there might be a true signal for disease worsening in the Crohn’s disease patients who took Remsima. “The problem is that it’s in the largest subgroup that has no other data. If this had been in rheumatoid arthritis, that would be different,” coauthor Inge C. Olsen, PhD, a biostatistician at Diakonhjemmet Hospital, said in an interview. “All the registry trials were done in RA and spondyloarthritis patients. ... That’s an issue, but with regards to the [NOR-SWITCH] study, it’s very clear that you have no power to show anything in the subgroup analysis, and they are exploratory analyses and are not answering any hypothesis.” Currently, there are no plans to follow up on these results in another study, he said.
Other issues that the NOR-SWITCH study does not answer are the outcomes of switching back and forth between Remicade and Remsima, switching from one infliximab biosimilar to another infliximab biosimilar, and switching from other originator biologics to their biosimilars.
“Is that feasible? Is that safe? Will it retain efficacy? We don’t know. There’s a real need for those studies to be done,” Dr. Goll said.
In Norway, the remaining patients who had not switched yet from Remicade to Remsima are now doing so based on the trial’s results, Dr. Goll said. The cost of Remsima in Norway was about 75% less than Remicade in 2015 and about 60% less in 2016, she noted.
It’s still an open question what the results of the NOR-SWITCH trial might indicate for how clinicians in the United States will use Inflectra and other biosimilars, according to John J. Cush, MD, professor of medicine and rheumatology at Baylor University, Dallas.
“I think the real problem here is that it’s nice to know that [CT-P13] wasn’t inferior, but when you get into the weeds and you look at the details, those of us who may not have a lot of certainty about this might worry about this, especially when there are three new biosimilars approved in the United States: Amjevita, which is an adalimumab biosimilar; Erelzi, which is an etanercept biosimilar; and Inflectra’s about to be launched as an infliximab biosimilar,” Dr. Cush said during a session reviewing selected abstracts from the meeting. “When this NOR-SWITCH study was done in Norway, it’s a 70% savings over the original product. The new ones being introduced over here [in the United States] start at about 15%. I’m less motivated with that degree of savings to want to take some chances on my patients. So we need a little bit more certainty; we need to feel better about the cost savings to patients and health care overall. Confidence in biosimilars is what’s going to sell biosimilars. We’re a long way from confidence still.”
NOR-SWITCH was funded by the Norwegian government. Some of the investigators disclosed relationships with Pfizer and/or Celltrion, which separately market CT-P13 in different parts of the world.
[email protected]
WASHINGTON – Data from the first randomized trial of switching from an originator biologic to a biosimilar of the originator indicate that the infliximab biosimilar Remsima is no different from the infliximab originator Remicade in the rate of disease worsening over 1 year across a combination of all its approved indications.
The outcomes of the Norwegian, double-blind, noninferiority trial, called NOR-SWITCH, indicate similar rates of disease worsening across patients switched to Remsima and those who stayed on Remicade. However, exploratory group analyses conducted on the different disease subgroups in the trial (Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriasis, and psoriatic arthritis) showed a potentially concerning level of disease worsening among Crohn’s disease patients on Remsima with a confidence interval that nearly fell entirely within the range favoring Remicade.
In the United States, Remsima, also known as CT-P13, is marketed by Pfizer as Inflectra.
The trial randomized 482 patients who were on stable treatment with Remicade for at least 6 months for any of the six indications for which Remicade and Remsima are approved to either stay on Remicade or switch to Remsima with the same dosing regimen for 52 weeks. Overall, patients had a mean age of about 48 years and 36%-41% were female. They had a mean disease duration of about 17 years and had been taking Remicade for a mean of nearly 7 years.
The primary endpoint was disease worsening during follow-up, according to worsening in disease-specific composite measures and/or a consensus between an investigator and a patient that led to a major change in treatment. The investigators made an assumption of 30% disease worsening across all the indications for the trial’s power calculation, based on available literature and observational data.
Disease worsening occurred in 26.2% of patients who stayed on Remicade and 29.6% of patients who switched to Remsima, based on a per-protocol analysis of 202 Remicade and 206 Remsima patients. The 95% confidence interval of the adjusted treatment difference of –4.4% was –12.7% to 3.9%, which was within the pre-specified noninferiority margin of 15%.
Exploratory subgroup analyses of the different disease subgroups showed no statistically significant differences between the two treatments in disease worsening. However, in Crohn’s disease patients, who formed the largest subgroup in the study at 155 patients, the adjusted treatment difference was –14.3% (21.2% with disease worsening for Remicade and 36.5% for Remsima) with a 95% CI of –29.3% to 0.7%.
It’s difficult to discern whether the 95% confidence interval seen in the Crohn’s disease subgroup is a part of the natural variation one would expect to see in a subgroup analysis of different diseases or if there might be a true signal for disease worsening in the Crohn’s disease patients who took Remsima. “The problem is that it’s in the largest subgroup that has no other data. If this had been in rheumatoid arthritis, that would be different,” coauthor Inge C. Olsen, PhD, a biostatistician at Diakonhjemmet Hospital, said in an interview. “All the registry trials were done in RA and spondyloarthritis patients. ... That’s an issue, but with regards to the [NOR-SWITCH] study, it’s very clear that you have no power to show anything in the subgroup analysis, and they are exploratory analyses and are not answering any hypothesis.” Currently, there are no plans to follow up on these results in another study, he said.
Other issues that the NOR-SWITCH study does not answer are the outcomes of switching back and forth between Remicade and Remsima, switching from one infliximab biosimilar to another infliximab biosimilar, and switching from other originator biologics to their biosimilars.
“Is that feasible? Is that safe? Will it retain efficacy? We don’t know. There’s a real need for those studies to be done,” Dr. Goll said.
In Norway, the remaining patients who had not switched yet from Remicade to Remsima are now doing so based on the trial’s results, Dr. Goll said. The cost of Remsima in Norway was about 75% less than Remicade in 2015 and about 60% less in 2016, she noted.
It’s still an open question what the results of the NOR-SWITCH trial might indicate for how clinicians in the United States will use Inflectra and other biosimilars, according to John J. Cush, MD, professor of medicine and rheumatology at Baylor University, Dallas.
“I think the real problem here is that it’s nice to know that [CT-P13] wasn’t inferior, but when you get into the weeds and you look at the details, those of us who may not have a lot of certainty about this might worry about this, especially when there are three new biosimilars approved in the United States: Amjevita, which is an adalimumab biosimilar; Erelzi, which is an etanercept biosimilar; and Inflectra’s about to be launched as an infliximab biosimilar,” Dr. Cush said during a session reviewing selected abstracts from the meeting. “When this NOR-SWITCH study was done in Norway, it’s a 70% savings over the original product. The new ones being introduced over here [in the United States] start at about 15%. I’m less motivated with that degree of savings to want to take some chances on my patients. So we need a little bit more certainty; we need to feel better about the cost savings to patients and health care overall. Confidence in biosimilars is what’s going to sell biosimilars. We’re a long way from confidence still.”
NOR-SWITCH was funded by the Norwegian government. Some of the investigators disclosed relationships with Pfizer and/or Celltrion, which separately market CT-P13 in different parts of the world.
[email protected]
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Disease worsening occurred in 26.2% of patients who stayed on Remicade and 29.6% of patients who switched to Remsima, based on a per-protocol analysis.
Data source: The multicenter, double-blind, randomized NOR-SWITCH trial of 482 patients.
Disclosures: The trial was funded by the Norwegian government. Some of the investigators disclosed relationships with Pfizer and/or Celltrion, which separately market CT-P13 in different parts of the world.
Debunking Psoriasis Myths: Do Psoriasis Therapies Cause Depression?
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
VIDEO: Consider comorbidities when preparing patients for systemic psoriasis therapy
LAS VEGAS – Clinicians should consider the increased risk for multiple comorbidities in their patients with psoriasis, Joel M. Gelfand, MD, said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“These are patients who should undergo the type of age-appropriate screening that any patient should have,” including checks for blood pressure and diabetes, said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia.
In terms of preparing for systemic psoriasis therapy, “there are lots of things we can do to lower the risk of having bad outcomes,” including age-appropriate cancer screening such as colonoscopy and mammography, he added. Vaccination is also an important strategy to help reduce the risk of potential side effects related to immunosuppression, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Clinicians should consider the increased risk for multiple comorbidities in their patients with psoriasis, Joel M. Gelfand, MD, said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“These are patients who should undergo the type of age-appropriate screening that any patient should have,” including checks for blood pressure and diabetes, said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia.
In terms of preparing for systemic psoriasis therapy, “there are lots of things we can do to lower the risk of having bad outcomes,” including age-appropriate cancer screening such as colonoscopy and mammography, he added. Vaccination is also an important strategy to help reduce the risk of potential side effects related to immunosuppression, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Clinicians should consider the increased risk for multiple comorbidities in their patients with psoriasis, Joel M. Gelfand, MD, said in a video interview at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“These are patients who should undergo the type of age-appropriate screening that any patient should have,” including checks for blood pressure and diabetes, said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia.
In terms of preparing for systemic psoriasis therapy, “there are lots of things we can do to lower the risk of having bad outcomes,” including age-appropriate cancer screening such as colonoscopy and mammography, he added. Vaccination is also an important strategy to help reduce the risk of potential side effects related to immunosuppression, he noted.
Dr. Gelfand disclosed relationships with multiple companies including AbbVie, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sanofi, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: Biosimilars show promise and progress
LAS VEGAS – There is reason to be optimistic about biosimilars for treating psoriasis, Bruce E. Strober, MD, PhD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
To date, the Food and Drug Administration has approved three biosimilar versions of agents used to treat psoriasis: adalimumab, infliximab, and etanercept.
“The biosimilar development and approval pathway is quite rigorous,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington, in a video interview. Although none are yet available, “I can say with high confidence that biosimilar molecules are very comparable,” to the reference molecules, he said.
The impact of biosimilars on the market in the United States in terms of cost and access remains to be seen, Dr, Strober added.
However, “I do believe the science and the clinical trials process and the regulatory process are good,” and that, if clinicians had to use biosimilars for their patients, the patient experience would be safe and effective, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There is reason to be optimistic about biosimilars for treating psoriasis, Bruce E. Strober, MD, PhD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
To date, the Food and Drug Administration has approved three biosimilar versions of agents used to treat psoriasis: adalimumab, infliximab, and etanercept.
“The biosimilar development and approval pathway is quite rigorous,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington, in a video interview. Although none are yet available, “I can say with high confidence that biosimilar molecules are very comparable,” to the reference molecules, he said.
The impact of biosimilars on the market in the United States in terms of cost and access remains to be seen, Dr, Strober added.
However, “I do believe the science and the clinical trials process and the regulatory process are good,” and that, if clinicians had to use biosimilars for their patients, the patient experience would be safe and effective, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There is reason to be optimistic about biosimilars for treating psoriasis, Bruce E. Strober, MD, PhD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
To date, the Food and Drug Administration has approved three biosimilar versions of agents used to treat psoriasis: adalimumab, infliximab, and etanercept.
“The biosimilar development and approval pathway is quite rigorous,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington, in a video interview. Although none are yet available, “I can say with high confidence that biosimilar molecules are very comparable,” to the reference molecules, he said.
The impact of biosimilars on the market in the United States in terms of cost and access remains to be seen, Dr, Strober added.
However, “I do believe the science and the clinical trials process and the regulatory process are good,” and that, if clinicians had to use biosimilars for their patients, the patient experience would be safe and effective, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Optimize anti–TNF-alpha therapy for psoriasis
While anti–tumor necrosis factor (TNF)-alpha medications have shown effectiveness for psoriasis, ongoing issues include optimizing therapy, managing treatment in special populations, and comparing the drugs with newer therapies, according to Dr. Francisco A. Kerdel.
Among the concerns regarding optimizing anti–TNF-alpha therapy are loss of response, continuous versus intermittent dosing, combination therapy, and long-term safety and efficacy, Dr. Kerdel, professor and vice-chair of the department of dermatology, Florida International University, Miami, said in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, “no evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis,” Dr. Kerdel noted.
Patient factors that impact the choice and potential response of anti–TNF-alpha therapy include not only physical issues such as weight, disease activity, and comorbidities, but also patient preferences for simpler dosing regimens or a desire to avoid injections, he said.
Despite these issues, there is currently no consensus on and no guidelines for switching treatments after failure with an anti–TNF-alpha agent, he noted.
Other questions associated with anti–TNF-alpha agents in psoriasis include whether the loading dose recommended by most drug labels is necessary, according to Dr. Kerdel. “In practice, many clinicians omit the loading dosage and initiate therapy with maintenance doses,” he said. In addition, “physicians should use clinical judgment when screening and monitoring patients taking biologic agents.”
From a dermatologist perspective, the top unmet therapeutic needs in the treatment of psoriasis were improved efficacy, improved tolerability, improved long-term safety, another oral treatment option, and a new mechanism of action, based on a survey that included 391 dermatologists (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
As psoriasis treatments evolve, other issues include compiling real-world, postmarketing data on biosimilars; monitoring patients for the emergence of significant adverse events; and searching for biomarkers to help target biologics to specific patients and psoriasis subtypes, Dr. Kerdel added.
SDEF and this news organization are owned by the same parent company.
Dr. Kerdel disclosed being a speaker for AbbVie, Amgen, Celgene, Galderma, and Janssen; conducting research for those 5 companies and for Novartis, Pfizer, and Valeant; he is also on the board of Celgene.
While anti–tumor necrosis factor (TNF)-alpha medications have shown effectiveness for psoriasis, ongoing issues include optimizing therapy, managing treatment in special populations, and comparing the drugs with newer therapies, according to Dr. Francisco A. Kerdel.
Among the concerns regarding optimizing anti–TNF-alpha therapy are loss of response, continuous versus intermittent dosing, combination therapy, and long-term safety and efficacy, Dr. Kerdel, professor and vice-chair of the department of dermatology, Florida International University, Miami, said in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, “no evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis,” Dr. Kerdel noted.
Patient factors that impact the choice and potential response of anti–TNF-alpha therapy include not only physical issues such as weight, disease activity, and comorbidities, but also patient preferences for simpler dosing regimens or a desire to avoid injections, he said.
Despite these issues, there is currently no consensus on and no guidelines for switching treatments after failure with an anti–TNF-alpha agent, he noted.
Other questions associated with anti–TNF-alpha agents in psoriasis include whether the loading dose recommended by most drug labels is necessary, according to Dr. Kerdel. “In practice, many clinicians omit the loading dosage and initiate therapy with maintenance doses,” he said. In addition, “physicians should use clinical judgment when screening and monitoring patients taking biologic agents.”
From a dermatologist perspective, the top unmet therapeutic needs in the treatment of psoriasis were improved efficacy, improved tolerability, improved long-term safety, another oral treatment option, and a new mechanism of action, based on a survey that included 391 dermatologists (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
As psoriasis treatments evolve, other issues include compiling real-world, postmarketing data on biosimilars; monitoring patients for the emergence of significant adverse events; and searching for biomarkers to help target biologics to specific patients and psoriasis subtypes, Dr. Kerdel added.
SDEF and this news organization are owned by the same parent company.
Dr. Kerdel disclosed being a speaker for AbbVie, Amgen, Celgene, Galderma, and Janssen; conducting research for those 5 companies and for Novartis, Pfizer, and Valeant; he is also on the board of Celgene.
While anti–tumor necrosis factor (TNF)-alpha medications have shown effectiveness for psoriasis, ongoing issues include optimizing therapy, managing treatment in special populations, and comparing the drugs with newer therapies, according to Dr. Francisco A. Kerdel.
Among the concerns regarding optimizing anti–TNF-alpha therapy are loss of response, continuous versus intermittent dosing, combination therapy, and long-term safety and efficacy, Dr. Kerdel, professor and vice-chair of the department of dermatology, Florida International University, Miami, said in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
However, “no evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis,” Dr. Kerdel noted.
Patient factors that impact the choice and potential response of anti–TNF-alpha therapy include not only physical issues such as weight, disease activity, and comorbidities, but also patient preferences for simpler dosing regimens or a desire to avoid injections, he said.
Despite these issues, there is currently no consensus on and no guidelines for switching treatments after failure with an anti–TNF-alpha agent, he noted.
Other questions associated with anti–TNF-alpha agents in psoriasis include whether the loading dose recommended by most drug labels is necessary, according to Dr. Kerdel. “In practice, many clinicians omit the loading dosage and initiate therapy with maintenance doses,” he said. In addition, “physicians should use clinical judgment when screening and monitoring patients taking biologic agents.”
From a dermatologist perspective, the top unmet therapeutic needs in the treatment of psoriasis were improved efficacy, improved tolerability, improved long-term safety, another oral treatment option, and a new mechanism of action, based on a survey that included 391 dermatologists (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
As psoriasis treatments evolve, other issues include compiling real-world, postmarketing data on biosimilars; monitoring patients for the emergence of significant adverse events; and searching for biomarkers to help target biologics to specific patients and psoriasis subtypes, Dr. Kerdel added.
SDEF and this news organization are owned by the same parent company.
Dr. Kerdel disclosed being a speaker for AbbVie, Amgen, Celgene, Galderma, and Janssen; conducting research for those 5 companies and for Novartis, Pfizer, and Valeant; he is also on the board of Celgene.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: IL-23 inhibitors on the upswing
LAS VEGAS – Interleukin-23 (IL-23) inhibitors, currently in the pipeline for treating psoriasis, are showing great promise for the treatment of the disease, Bruce E. Strober, MD, PhD, said in a video interview at the Skin Disease Education Foundation’s Las Vegas Dermatology Seminar.
“There likely will be at least three, if not four, IL-23 inhibitors, all biologics, approved within the next 5 years,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut Health Center, Farmington. “The IL-23 inhibitors are specific, and they seem to be delivering as good or better efficacy than ustekinumab,” with the potential for longer dosing intervals, depending on the patient, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Interleukin-23 (IL-23) inhibitors, currently in the pipeline for treating psoriasis, are showing great promise for the treatment of the disease, Bruce E. Strober, MD, PhD, said in a video interview at the Skin Disease Education Foundation’s Las Vegas Dermatology Seminar.
“There likely will be at least three, if not four, IL-23 inhibitors, all biologics, approved within the next 5 years,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut Health Center, Farmington. “The IL-23 inhibitors are specific, and they seem to be delivering as good or better efficacy than ustekinumab,” with the potential for longer dosing intervals, depending on the patient, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – Interleukin-23 (IL-23) inhibitors, currently in the pipeline for treating psoriasis, are showing great promise for the treatment of the disease, Bruce E. Strober, MD, PhD, said in a video interview at the Skin Disease Education Foundation’s Las Vegas Dermatology Seminar.
“There likely will be at least three, if not four, IL-23 inhibitors, all biologics, approved within the next 5 years,” said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut Health Center, Farmington. “The IL-23 inhibitors are specific, and they seem to be delivering as good or better efficacy than ustekinumab,” with the potential for longer dosing intervals, depending on the patient, he noted.
Dr. Strober disclosed relationships with multiple companies including AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, GlaxoSmithKline, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR