User login
Medicare payments set for infliximab biosimilar Inflectra
Payment for the infliximab biosimilar drug Inflectra will now be covered by Medicare, the drug’s manufacturer, Pfizer, said in an announcement.
The Centers for Medicare & Medicaid Services (CMS) included Inflectra (infliximab-dyyb) in its January 2017 Average Sales Price pricing file, which went into effect Jan. 1, 2017. Pfizer said that Inflectra is priced at a 15% discount to the current wholesale acquisition cost for the infliximab originator Remicade, but this price does not include discounts to payers, providers, distributors, and other purchasing organizations.
For the first quarter of 2017, the payment limit set by the CMS for Inflectra is $100.306 per 10-mg unit and $82.218 for Remicade.
Various national and regional wholesalers across the country began receiving shipments of Inflectra in November 2016, according to Pfizer.
In conjunction with the availability of Inflectra, Pfizer announced its enCompass program, “a comprehensive reimbursement service and patient support program offering coding and reimbursement support for providers, copay assistance to eligible patients who have commercial insurance that covers Inflectra, and financial assistance for eligible uninsured and underinsured patients.”
The FDA approved Inflectra in April 2016 for all of the same indications as Remicade: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis, and ulcerative colitis.
Payment for the infliximab biosimilar drug Inflectra will now be covered by Medicare, the drug’s manufacturer, Pfizer, said in an announcement.
The Centers for Medicare & Medicaid Services (CMS) included Inflectra (infliximab-dyyb) in its January 2017 Average Sales Price pricing file, which went into effect Jan. 1, 2017. Pfizer said that Inflectra is priced at a 15% discount to the current wholesale acquisition cost for the infliximab originator Remicade, but this price does not include discounts to payers, providers, distributors, and other purchasing organizations.
For the first quarter of 2017, the payment limit set by the CMS for Inflectra is $100.306 per 10-mg unit and $82.218 for Remicade.
Various national and regional wholesalers across the country began receiving shipments of Inflectra in November 2016, according to Pfizer.
In conjunction with the availability of Inflectra, Pfizer announced its enCompass program, “a comprehensive reimbursement service and patient support program offering coding and reimbursement support for providers, copay assistance to eligible patients who have commercial insurance that covers Inflectra, and financial assistance for eligible uninsured and underinsured patients.”
The FDA approved Inflectra in April 2016 for all of the same indications as Remicade: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis, and ulcerative colitis.
Payment for the infliximab biosimilar drug Inflectra will now be covered by Medicare, the drug’s manufacturer, Pfizer, said in an announcement.
The Centers for Medicare & Medicaid Services (CMS) included Inflectra (infliximab-dyyb) in its January 2017 Average Sales Price pricing file, which went into effect Jan. 1, 2017. Pfizer said that Inflectra is priced at a 15% discount to the current wholesale acquisition cost for the infliximab originator Remicade, but this price does not include discounts to payers, providers, distributors, and other purchasing organizations.
For the first quarter of 2017, the payment limit set by the CMS for Inflectra is $100.306 per 10-mg unit and $82.218 for Remicade.
Various national and regional wholesalers across the country began receiving shipments of Inflectra in November 2016, according to Pfizer.
In conjunction with the availability of Inflectra, Pfizer announced its enCompass program, “a comprehensive reimbursement service and patient support program offering coding and reimbursement support for providers, copay assistance to eligible patients who have commercial insurance that covers Inflectra, and financial assistance for eligible uninsured and underinsured patients.”
The FDA approved Inflectra in April 2016 for all of the same indications as Remicade: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis, and ulcerative colitis.
Initial suboptimal responders to secukinumab usually bloom later
VIENNA – When the occasional patient on secukinumab for moderate to severe psoriasis fails to achieve a PASI 75 response initially, don’t despair: Continuing treatment with the biologic usually gets them over that bar, Christopher E. Griffiths, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A new secondary pooled analysis of four phase III, 52-week, pivotal clinical trials of secukinumab (Cosentyx) indicates that more than three-quarters of initial suboptimal responders will go on to achieve a PASI 75 response. Moreover, more than one-third will have a PASI 90 response by week 16, which is sustained through week 52. And almost one in five slow responders will have a PASI 100 response – clear skin – at week 52, according to Dr. Griffiths, professor of dermatology at the University of Manchester, England.
He presented a secondary analysis of four phase III studies: ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis), FEATURE (First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks), FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis), and JUNCTURE (Judging the Efficacy of Secukinumab in Patients With Psoriasis Using AutoiNjector: a Clinical Trial Evaluating Treatment Results). The analysis was conducted to provide additional perspective on the product labeling statement that treatment discontinuation should be considered in patients who haven’t responded to secukinumab by week 16.
The four studies featured a total of 2,405 patients with moderate to severe psoriasis on secukinumab at the approved dosing schedule.
The key findings: At week 12 – the primary endpoint in the four trials – only 5.2% of patients on secukinumab had not achieved a PASI 75 response. Yet just 4 weeks later, at week 16, 56% of this group had managed to get there. Seventy-seven percent of early non- or partial responders achieved a PASI 75 response at some point during weeks 13-52, and 55% had a PASI 75 response at 52 weeks.
Thirty-five percent of early poor responders achieved PASI 90 at 16 weeks and 37% at 52 weeks. Twelve percent of patients who didn’t get to PASI 75 at 12 weeks had a PASI 100 response by 16 weeks, and nearly 18% did by week 52.
This analysis was supported by secukinumab manufacturer Novartis. Dr. Griffiths reported receiving research funds from and serving as a consultant to Novartis and numerous other pharmaceutical companies.
VIENNA – When the occasional patient on secukinumab for moderate to severe psoriasis fails to achieve a PASI 75 response initially, don’t despair: Continuing treatment with the biologic usually gets them over that bar, Christopher E. Griffiths, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A new secondary pooled analysis of four phase III, 52-week, pivotal clinical trials of secukinumab (Cosentyx) indicates that more than three-quarters of initial suboptimal responders will go on to achieve a PASI 75 response. Moreover, more than one-third will have a PASI 90 response by week 16, which is sustained through week 52. And almost one in five slow responders will have a PASI 100 response – clear skin – at week 52, according to Dr. Griffiths, professor of dermatology at the University of Manchester, England.
He presented a secondary analysis of four phase III studies: ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis), FEATURE (First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks), FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis), and JUNCTURE (Judging the Efficacy of Secukinumab in Patients With Psoriasis Using AutoiNjector: a Clinical Trial Evaluating Treatment Results). The analysis was conducted to provide additional perspective on the product labeling statement that treatment discontinuation should be considered in patients who haven’t responded to secukinumab by week 16.
The four studies featured a total of 2,405 patients with moderate to severe psoriasis on secukinumab at the approved dosing schedule.
The key findings: At week 12 – the primary endpoint in the four trials – only 5.2% of patients on secukinumab had not achieved a PASI 75 response. Yet just 4 weeks later, at week 16, 56% of this group had managed to get there. Seventy-seven percent of early non- or partial responders achieved a PASI 75 response at some point during weeks 13-52, and 55% had a PASI 75 response at 52 weeks.
Thirty-five percent of early poor responders achieved PASI 90 at 16 weeks and 37% at 52 weeks. Twelve percent of patients who didn’t get to PASI 75 at 12 weeks had a PASI 100 response by 16 weeks, and nearly 18% did by week 52.
This analysis was supported by secukinumab manufacturer Novartis. Dr. Griffiths reported receiving research funds from and serving as a consultant to Novartis and numerous other pharmaceutical companies.
VIENNA – When the occasional patient on secukinumab for moderate to severe psoriasis fails to achieve a PASI 75 response initially, don’t despair: Continuing treatment with the biologic usually gets them over that bar, Christopher E. Griffiths, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A new secondary pooled analysis of four phase III, 52-week, pivotal clinical trials of secukinumab (Cosentyx) indicates that more than three-quarters of initial suboptimal responders will go on to achieve a PASI 75 response. Moreover, more than one-third will have a PASI 90 response by week 16, which is sustained through week 52. And almost one in five slow responders will have a PASI 100 response – clear skin – at week 52, according to Dr. Griffiths, professor of dermatology at the University of Manchester, England.
He presented a secondary analysis of four phase III studies: ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis), FEATURE (First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks), FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis), and JUNCTURE (Judging the Efficacy of Secukinumab in Patients With Psoriasis Using AutoiNjector: a Clinical Trial Evaluating Treatment Results). The analysis was conducted to provide additional perspective on the product labeling statement that treatment discontinuation should be considered in patients who haven’t responded to secukinumab by week 16.
The four studies featured a total of 2,405 patients with moderate to severe psoriasis on secukinumab at the approved dosing schedule.
The key findings: At week 12 – the primary endpoint in the four trials – only 5.2% of patients on secukinumab had not achieved a PASI 75 response. Yet just 4 weeks later, at week 16, 56% of this group had managed to get there. Seventy-seven percent of early non- or partial responders achieved a PASI 75 response at some point during weeks 13-52, and 55% had a PASI 75 response at 52 weeks.
Thirty-five percent of early poor responders achieved PASI 90 at 16 weeks and 37% at 52 weeks. Twelve percent of patients who didn’t get to PASI 75 at 12 weeks had a PASI 100 response by 16 weeks, and nearly 18% did by week 52.
This analysis was supported by secukinumab manufacturer Novartis. Dr. Griffiths reported receiving research funds from and serving as a consultant to Novartis and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Most psoriasis patients who don’t achieve a PASI 75 response by week 12 on secukinumab will do so by week 16 and will maintain that response through week 52.
Data source: A pooled secondary analysis of PASI response rates in four phase III randomized clinical trials of secukinumab featuring 2,405 patients with moderate to severe psoriasis who were on the biologic for 52 weeks, including the 119 who did not achieve a PASI 75 response by week 12.
Disclosures: This analysis of four phase III clinical trials was sponsored by Novartis, as were the trials. The presenter reported receiving research funding from and serving as a consultant to Novartis and other pharmaceutical companies.
Secukinumab tames severe scalp psoriasis
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: 53% of patients with chronic moderate to severe scalp psoriasis experienced at least a 90% improvement after 12 weeks on secukinumab, compared with 2% of controls.
Data source: This prospective, double-blind, phase IIIb clinical trial randomized 102 patients with moderate to severe scalp psoriasis to secukinumab or placebo.
Disclosures: The study was sponsored by Novartis. The presenter reported that his academic department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
Ixekizumab proves highly effective for palmoplantar, scalp psoriasis
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Addressing Patient Concerns About Treatment Safety Data
Latest ixekizumab safety data called ‘very reassuring’
VIENNA – Updated longer-term safety data for ixekizumab in patients with moderate to severe plaque psoriasis continue to show no new safety signals, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The safety database now includes 4,213 psoriasis patients on ixekizumab (Taltz) for a total of 7,843 patient-years of regular ongoing follow-up in seven different phase I-III clinical trials. And with a large group of patients now having been on the novel humanized monoclonal antibody targeting interleukin-17A for 2 years and smaller numbers out to 5 years, there have been no surprises, according to Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
The key trends in the safety analysis are that the number of patients with an adverse event resulting in ixekizumab discontinuation is very low, yet adverse event rates are declining over time.
“This is pretty common in clinical trials,” according to the dermatologist. “In those first 12 weeks of a study you are seeing the patients very frequently, asking them very detailed questions, and we often pick up adverse events more frequently as a result. Upper respiratory infections are a good example: In the first month a patient will remember what happened last week. But if you haven’t seen a patient in 3 months they might not remember that 10 weeks ago they had a little cold. That’s why we tend to see URI rates go down over time. Now, if you see adverse events go up over time – especially for things like malignancy – then there is certainly cause for concern that there’s a cumulative problem with toxicity. That is clearly not a problem with this drug.”
Turning to selected adverse events of interest, Dr. Kimball noted that 2.1% of patients have experienced oral candidiasis while on ixekizumab.
“Oral Candida infection is one of the known side effects with this drug. It doesn’t happen very frequently, and to date, the infections have been very manageable, but it is something you want to have in your mind because it does happen,” she noted.
Serious infections have occurred in 105 patients, 2.5% of those on ixekizumab. Major adverse cardiovascular events have occurred in 1.0%, nonmelanoma skin cancers in 0.7%, and other cancers in 1.1%. Of note, only 5 patients (0.1%) have developed Crohn’s disease, 10 have been diagnosed with ulcerative colitis, and there have been no completed suicides.
The safety follow-up is ongoing.
The safety registry is supported by Eli Lilly, which markets ixekizumab. Dr. Kimball reported receiving research funding from and serving as a consultant to Eli Lilly and numerous other pharmaceutical companies.
VIENNA – Updated longer-term safety data for ixekizumab in patients with moderate to severe plaque psoriasis continue to show no new safety signals, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The safety database now includes 4,213 psoriasis patients on ixekizumab (Taltz) for a total of 7,843 patient-years of regular ongoing follow-up in seven different phase I-III clinical trials. And with a large group of patients now having been on the novel humanized monoclonal antibody targeting interleukin-17A for 2 years and smaller numbers out to 5 years, there have been no surprises, according to Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
The key trends in the safety analysis are that the number of patients with an adverse event resulting in ixekizumab discontinuation is very low, yet adverse event rates are declining over time.
“This is pretty common in clinical trials,” according to the dermatologist. “In those first 12 weeks of a study you are seeing the patients very frequently, asking them very detailed questions, and we often pick up adverse events more frequently as a result. Upper respiratory infections are a good example: In the first month a patient will remember what happened last week. But if you haven’t seen a patient in 3 months they might not remember that 10 weeks ago they had a little cold. That’s why we tend to see URI rates go down over time. Now, if you see adverse events go up over time – especially for things like malignancy – then there is certainly cause for concern that there’s a cumulative problem with toxicity. That is clearly not a problem with this drug.”
Turning to selected adverse events of interest, Dr. Kimball noted that 2.1% of patients have experienced oral candidiasis while on ixekizumab.
“Oral Candida infection is one of the known side effects with this drug. It doesn’t happen very frequently, and to date, the infections have been very manageable, but it is something you want to have in your mind because it does happen,” she noted.
Serious infections have occurred in 105 patients, 2.5% of those on ixekizumab. Major adverse cardiovascular events have occurred in 1.0%, nonmelanoma skin cancers in 0.7%, and other cancers in 1.1%. Of note, only 5 patients (0.1%) have developed Crohn’s disease, 10 have been diagnosed with ulcerative colitis, and there have been no completed suicides.
The safety follow-up is ongoing.
The safety registry is supported by Eli Lilly, which markets ixekizumab. Dr. Kimball reported receiving research funding from and serving as a consultant to Eli Lilly and numerous other pharmaceutical companies.
VIENNA – Updated longer-term safety data for ixekizumab in patients with moderate to severe plaque psoriasis continue to show no new safety signals, Alexa B. Kimball, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The safety database now includes 4,213 psoriasis patients on ixekizumab (Taltz) for a total of 7,843 patient-years of regular ongoing follow-up in seven different phase I-III clinical trials. And with a large group of patients now having been on the novel humanized monoclonal antibody targeting interleukin-17A for 2 years and smaller numbers out to 5 years, there have been no surprises, according to Dr. Kimball, professor of dermatology at Harvard Medical School, Boston.
The key trends in the safety analysis are that the number of patients with an adverse event resulting in ixekizumab discontinuation is very low, yet adverse event rates are declining over time.
“This is pretty common in clinical trials,” according to the dermatologist. “In those first 12 weeks of a study you are seeing the patients very frequently, asking them very detailed questions, and we often pick up adverse events more frequently as a result. Upper respiratory infections are a good example: In the first month a patient will remember what happened last week. But if you haven’t seen a patient in 3 months they might not remember that 10 weeks ago they had a little cold. That’s why we tend to see URI rates go down over time. Now, if you see adverse events go up over time – especially for things like malignancy – then there is certainly cause for concern that there’s a cumulative problem with toxicity. That is clearly not a problem with this drug.”
Turning to selected adverse events of interest, Dr. Kimball noted that 2.1% of patients have experienced oral candidiasis while on ixekizumab.
“Oral Candida infection is one of the known side effects with this drug. It doesn’t happen very frequently, and to date, the infections have been very manageable, but it is something you want to have in your mind because it does happen,” she noted.
Serious infections have occurred in 105 patients, 2.5% of those on ixekizumab. Major adverse cardiovascular events have occurred in 1.0%, nonmelanoma skin cancers in 0.7%, and other cancers in 1.1%. Of note, only 5 patients (0.1%) have developed Crohn’s disease, 10 have been diagnosed with ulcerative colitis, and there have been no completed suicides.
The safety follow-up is ongoing.
The safety registry is supported by Eli Lilly, which markets ixekizumab. Dr. Kimball reported receiving research funding from and serving as a consultant to Eli Lilly and numerous other pharmaceutical companies.
THE EADV CONGRESS
Psoriasis and Internal Disease: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Psoriasis Treatment Considerations in Military Patients: Unique Patients, Unique Drugs
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
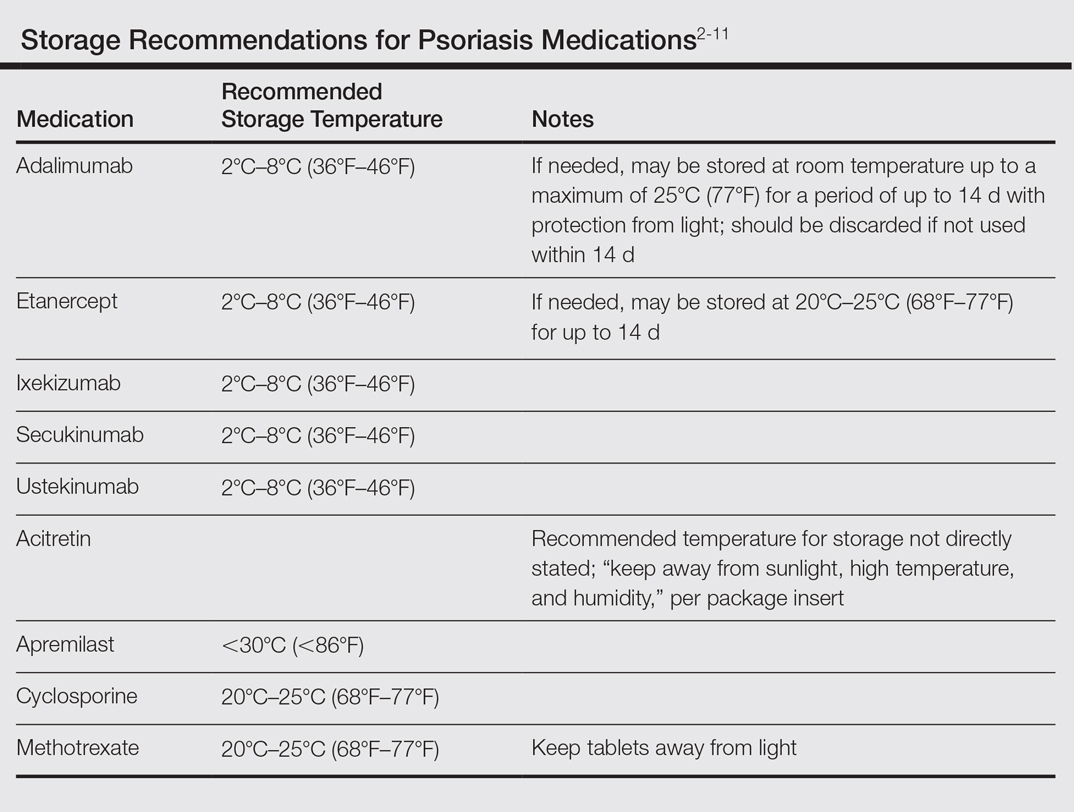
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
Psoriasis is a common dermatologic problem with nearly 5% prevalence in the United States. There is a bimodal distribution with peak onset between 20 and 30 years of age and 50 and 60 years, which means that this condition can arise before, during, or after military service.1 Unfortunately, for many prospective recruits psoriasis is a medically disqualifying condition that can prevent entry into active duty unless a medical waiver is granted. For active-duty military, new-onset psoriasis and its treatment can impair affected service members’ ability to perform mission-critical work and can prevent them from deploying to remote or austere locations. In this way, psoriasis presents a unique challenge for active-duty service members.
Many therapies are available that can effectively treat psoriasis, but these treatments often carry a side-effect profile that limits their use during travel or in austere settings. Herein, we discuss the unique challenges of treating psoriasis patients who are in the military at a time when global mobility is critical to mission success. Although in some ways these challenges truly are unique to the military population, we strongly believe that similar but perhaps underappreciated challenges exist in the civilian sector. Close examination of these challenges may reveal that alternative treatment choices are sometimes indicated for reasons beyond just efficacy, side-effect profile, and cost.
Treatment Considerations
The medical treatment of psoriasis has undergone substantial change in recent decades. Before the turn of the century, the mainstays of medical treatment were steroids, methotrexate, and phototherapy. Today, a wide array of biologics and other systemic drugs are altering the impact of psoriasis in our society. With so many treatment options currently available, the question becomes, “Which one is best for my patient?” Immediate considerations are efficacy versus side effects as well as cost; however, in military dermatology, the ability to store, transport, and administer the treatment can be just as important.
Although these problems may at first seem unique to active-duty military members, they also affect a substantial segment of the civilian sector. Take for instance the government contractor who deploys in support of military contingency actions, or the foreign aid workers, international businessmen, and diplomats around the world. In fact, any person who travels extensively might have difficulty carrying and storing their medications (Table) or encounter barriers that prevent routine access to care. Travel also may increase the risk of exposure to virulent pathogens such as Mycobacterium tuberculosis, which may further limit treatment options. This group of world travelers together comprises a minority of psoriasis patients who may be better treated with novel agents rather than with what might be considered the standard of care in a domestic setting.
Options for Care
Methotrexate
In many ways, methotrexate is the gold standard of psoriasis treatment. It is a first-line medication for many patients because it is typically well tolerated, has well-established efficacy, is easy to administer, and is relatively inexpensive.12 Although it is easy to store, transport, and administer, it requires regular laboratory monitoring at 3-month intervals or more frequently with dosage changes. It also is contraindicated in women of childbearing age who plan to become pregnant, which can be a considerable hindrance in the young active-duty population.
Cyclosporine
Cyclosporine is another inexpensive medication that can produce excellent results in the treatment of psoriasis.1,12 Although long-term use of cyclosporine in transplant patients has been well studied, its use for the treatment of dermatologic conditions is usually limited to 1 year. The need for monthly blood pressure checks and at least quarterly laboratory monitoring means it is not an optimal choice for a deployed service member.
Acitretin
Acitretin is another systemic medication with an established track record in psoriasis treatment. Although close follow-up and laboratory monitoring is required for both males and females, use of this medication can have a greater effect on women of childbearing age, as it is absolutely contraindicated in any female trying to conceive.13 In addition, acitretin is stored in fat cells, and traces of the drug can be found in the blood for up to 3 years. During this period, patients are advised to strictly avoid pregnancy and are even restricted from donating blood.13 Given these concerns, acitretin is not always a reasonable treatment option for the military service member.
Biologics
Biologics are the newest agents in the treatment of psoriasis. They require less laboratory monitoring and can provide excellent results. Adalimumab is a reasonable first-line biologic treatment for some patients. We find the laboratory monitoring is minimally obtrusive, side effects usually are limited, and the efficacy is great enough that most patients elect to continue treatment. Unfortunately, adalimumab has some major drawbacks in our specific use scenario in that it requires nearly continuous refrigeration and is never to exceed 25°C (77°F), it has a relatively close-interval dosing schedule, and it can cause immunosuppression. However, for short trips to nonaustere locations with an acceptable risk for pathogenic exposure, adalimumab may remain a viable option for many travelers, as it can be stored at room temperature for up to 14 days.2 Ustekinumab also is a reasonable choice for many travelers because dosing is every 12 weeks and it carries a lower risk of immunosuppression.2,3 Ustekinumab, however, has the major drawback of high cost.12 Newer IL-17A inhibitors such as secukinumab or ixekizumab also can offer excellent results, but long-term infection rates have not been reported. Overall, the infection rates are comparable to ustekinumab.14,15 After the loading phase, secukinumab is dosed monthly and logistically could still pose a problem due to the need for continued refrigeration.14
Apremilast
Although it is not the best first-line treatment for every patient, apremilast carries 3 distinct advantages in treating the military patient population: (1) laboratory monitoring is required only once per year, (2) it is easy to store, and (3) it is easy to administer. However, the major downside is that apremilast is less effective than other systemic agents in the treatment of psoriasis.16 As with other systemic drugs, adjunctive topical treatment can provide additional therapeutic effects, and for many patients, this combined approach is sufficient to reach their therapeutic goals.
For these reasons, in the special case of deployable, active-duty military members we often consider starting treatment with apremilast versus other systemic agents. As with all systemic psoriasis treatments, we generally advise patients to return 16 weeks after initiating treatment to assess efficacy and evaluate their deployment status. Although apremilast may take longer to reach full efficacy than many other systemic agents, one clinical trial suggested this time frame is sufficient to evaluate response to treatment.16 After this initial assessment, we revert to yearly monitoring, and the patient is usually cleared to deploy with minimal restrictions.
Final Considerations
The manifestation of psoriasis is different in every patient, and military service poses additional treatment challenges. For all of our military patients, we recommend an initial period of close follow-up after starting any new systemic agent, which is necessary to ensure the treatment is effective and well tolerated and also that we are good stewards of our resources. Once efficacy is established and side effects remain tolerable, we generally endorse continued treatment without specific travel or work restrictions.
We are cognizant of the unique nature of military service, and all too often we find ourselves trying to practice good medicine in bad places. As military physicians, we serve a population that is eager to do their job and willing to make incredible sacrifices to do so. After considering the wide range of circumstances unique to the military, our responsibility as providers is to do our best to improve service members’ quality of life as they carry out their missions.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Stelara [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
- Humira [package insert]. North Chicago, IL: AbbVie Inc; 2007.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
- Otezla [package insert]. Summit, NJ: Celgene Corporation; 2014.
- Enbrel [package insert]. Thousand Oaks, CA: Amgen; 2015.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Methotrexate [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc; 2016.
- Gengraf [package insert]. North Chicago, IL: Abbvie Inc; 2015.
- Acitretin [package insert]. Mason, OH: Prasco Laboratories; 2015.
- Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146:46-54.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis [published online June 8, 2016]. N Engl J Med. 2016;375:345-356.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, anoral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
Practice Points
- Establishing goals of treatment with each patient is a critical step in treating the patient rather than the diagnosis.
- A good social history can reveal job-related impact of disease and potential logistical roadblocks to treatment.
- Efficacy must be weighed against the burden of logistical constraints for each patient; potential issues include difficulty complying with follow-up visits, access to laboratory monitoring, exposure to pathogens, and adequacy of medication transport and storage.
Spot Psoriatic Arthritis Early in Psoriasis Patients
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
Study finds etanercept biosimilar safe and effective in patients with severe plaque psoriasis
An experimental biological agent, GP2015, demonstrated equivalent efficacy and comparable safety to etanercept for moderate to severe plaque psoriasis in a manufacturer-sponsored study, according to a report published online in the British Journal of Dermatology.
These findings should contribute to the confirmation of GP2015 as an etanercept (Enbrel) biosimilar for this patient population. Proposed biosimilars such as GP2015 are follow-on versions of authorized biological products, and regulatory authorities require that they demonstrate similarity with a large body of physiochemical and clinical data before they can be confirmed as biosimilars, said Christopher Griffiths, MD, of the Dermatology Centre, Salford Royal Hospital, University of Manchester (England), and his associates.
To contribute such data, Dr. Griffiths and his associates studied the efficacy and safety of GP2015 compared with etanercept, a tumor necrosis factor blocker, in the 2-year EGALITY trial. They assessed 531 adults at 74 medical centers in 11 European countries and South Africa, all of whom had active but stable chronic plaque psoriasis affecting 10% or more of their body surface area and Psoriasis Area and Severity Index (PASI) scores of 10 or more.
The study participants were randomly assigned in approximately equal numbers in a double-blind fashion to self administer GP2015 or etanercept subcutaneously, twice weekly for 12 weeks. Those who achieved at least a 50% improvement in PASI score were then randomized to continue the same treatment once weekly or to undergo a series of three treatment “switches” between GP2015 and etanercept at 6-week intervals until week 30. During an extension phase of the study, participants could then continue to receive the same treatment they had ended on, until week 52.
The primary efficacy endpoint – the percentage of patients who showed at least a 75% improvement from baseline in PASI score (PASI 75) at 12 weeks – was 73.4% with GP2015 and 75.7% with etanercept, which demonstrated therapeutic equivalence. A secondary efficacy end point – mean change in PASI score from baseline to week 12 – also was similar between the two study drugs. In all treatment groups, both PASI mean scores and change in PASI scores over time were comparable between the two study groups, regardless of switching between the two agents. In addition, in all groups, PASI 75 and PASI 90 scores increased gradually over time until week 30, and then remained stable through week 52, the investigators said (Br J Dermatol. 2016. doi: 10.1111/bjd.15152).
The proportion of patients with at least one treatment-emergent adverse event was similar in the GP2015 and etanercept groups who did not switch agents (59.8% and 57.3%, respectively), and was also similar among those who did switch agents between GP2015 (61%) and etanercept (59.4%).
The rates of serious adverse events and of adverse events that led to withdrawal from the study were similar across all treatment groups. Immunogenicity of GP2015 was similar to etanercept.
However, the rate of “adverse events of special interest” – those referred to in special warnings and precautions on the etanercept label – was markedly higher for continued GP2015 than for continued etanercept (11.0% vs. 4.7%) and for switched GP2015 than for switched etanercept (11.0% vs. 5.2%). One patient who took etanercept throughout the trial developed malignant melanoma.
The results of the study “confirm biosimilarity that was established with all previous analytical comparisons to the reference product in that equivalent efficacy was demonstrated as well as similar safety and immunogenicity of GP2015” with etanercept, “in a highly sensitive, generally immune-competent population,” the authors concluded.
The study was funded by Hexal AG, a Sandoz company, which was involved in the study design, data collection and analysis, and manuscript preparation. Dr. Griffiths reported ties to AbbVie, BMS. Galderma, Janssen, Leo-Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, and UCB Pharma, and his associates reported ties to numerous industry sources.
An experimental biological agent, GP2015, demonstrated equivalent efficacy and comparable safety to etanercept for moderate to severe plaque psoriasis in a manufacturer-sponsored study, according to a report published online in the British Journal of Dermatology.
These findings should contribute to the confirmation of GP2015 as an etanercept (Enbrel) biosimilar for this patient population. Proposed biosimilars such as GP2015 are follow-on versions of authorized biological products, and regulatory authorities require that they demonstrate similarity with a large body of physiochemical and clinical data before they can be confirmed as biosimilars, said Christopher Griffiths, MD, of the Dermatology Centre, Salford Royal Hospital, University of Manchester (England), and his associates.
To contribute such data, Dr. Griffiths and his associates studied the efficacy and safety of GP2015 compared with etanercept, a tumor necrosis factor blocker, in the 2-year EGALITY trial. They assessed 531 adults at 74 medical centers in 11 European countries and South Africa, all of whom had active but stable chronic plaque psoriasis affecting 10% or more of their body surface area and Psoriasis Area and Severity Index (PASI) scores of 10 or more.
The study participants were randomly assigned in approximately equal numbers in a double-blind fashion to self administer GP2015 or etanercept subcutaneously, twice weekly for 12 weeks. Those who achieved at least a 50% improvement in PASI score were then randomized to continue the same treatment once weekly or to undergo a series of three treatment “switches” between GP2015 and etanercept at 6-week intervals until week 30. During an extension phase of the study, participants could then continue to receive the same treatment they had ended on, until week 52.
The primary efficacy endpoint – the percentage of patients who showed at least a 75% improvement from baseline in PASI score (PASI 75) at 12 weeks – was 73.4% with GP2015 and 75.7% with etanercept, which demonstrated therapeutic equivalence. A secondary efficacy end point – mean change in PASI score from baseline to week 12 – also was similar between the two study drugs. In all treatment groups, both PASI mean scores and change in PASI scores over time were comparable between the two study groups, regardless of switching between the two agents. In addition, in all groups, PASI 75 and PASI 90 scores increased gradually over time until week 30, and then remained stable through week 52, the investigators said (Br J Dermatol. 2016. doi: 10.1111/bjd.15152).
The proportion of patients with at least one treatment-emergent adverse event was similar in the GP2015 and etanercept groups who did not switch agents (59.8% and 57.3%, respectively), and was also similar among those who did switch agents between GP2015 (61%) and etanercept (59.4%).
The rates of serious adverse events and of adverse events that led to withdrawal from the study were similar across all treatment groups. Immunogenicity of GP2015 was similar to etanercept.
However, the rate of “adverse events of special interest” – those referred to in special warnings and precautions on the etanercept label – was markedly higher for continued GP2015 than for continued etanercept (11.0% vs. 4.7%) and for switched GP2015 than for switched etanercept (11.0% vs. 5.2%). One patient who took etanercept throughout the trial developed malignant melanoma.
The results of the study “confirm biosimilarity that was established with all previous analytical comparisons to the reference product in that equivalent efficacy was demonstrated as well as similar safety and immunogenicity of GP2015” with etanercept, “in a highly sensitive, generally immune-competent population,” the authors concluded.
The study was funded by Hexal AG, a Sandoz company, which was involved in the study design, data collection and analysis, and manuscript preparation. Dr. Griffiths reported ties to AbbVie, BMS. Galderma, Janssen, Leo-Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, and UCB Pharma, and his associates reported ties to numerous industry sources.
An experimental biological agent, GP2015, demonstrated equivalent efficacy and comparable safety to etanercept for moderate to severe plaque psoriasis in a manufacturer-sponsored study, according to a report published online in the British Journal of Dermatology.
These findings should contribute to the confirmation of GP2015 as an etanercept (Enbrel) biosimilar for this patient population. Proposed biosimilars such as GP2015 are follow-on versions of authorized biological products, and regulatory authorities require that they demonstrate similarity with a large body of physiochemical and clinical data before they can be confirmed as biosimilars, said Christopher Griffiths, MD, of the Dermatology Centre, Salford Royal Hospital, University of Manchester (England), and his associates.
To contribute such data, Dr. Griffiths and his associates studied the efficacy and safety of GP2015 compared with etanercept, a tumor necrosis factor blocker, in the 2-year EGALITY trial. They assessed 531 adults at 74 medical centers in 11 European countries and South Africa, all of whom had active but stable chronic plaque psoriasis affecting 10% or more of their body surface area and Psoriasis Area and Severity Index (PASI) scores of 10 or more.
The study participants were randomly assigned in approximately equal numbers in a double-blind fashion to self administer GP2015 or etanercept subcutaneously, twice weekly for 12 weeks. Those who achieved at least a 50% improvement in PASI score were then randomized to continue the same treatment once weekly or to undergo a series of three treatment “switches” between GP2015 and etanercept at 6-week intervals until week 30. During an extension phase of the study, participants could then continue to receive the same treatment they had ended on, until week 52.
The primary efficacy endpoint – the percentage of patients who showed at least a 75% improvement from baseline in PASI score (PASI 75) at 12 weeks – was 73.4% with GP2015 and 75.7% with etanercept, which demonstrated therapeutic equivalence. A secondary efficacy end point – mean change in PASI score from baseline to week 12 – also was similar between the two study drugs. In all treatment groups, both PASI mean scores and change in PASI scores over time were comparable between the two study groups, regardless of switching between the two agents. In addition, in all groups, PASI 75 and PASI 90 scores increased gradually over time until week 30, and then remained stable through week 52, the investigators said (Br J Dermatol. 2016. doi: 10.1111/bjd.15152).
The proportion of patients with at least one treatment-emergent adverse event was similar in the GP2015 and etanercept groups who did not switch agents (59.8% and 57.3%, respectively), and was also similar among those who did switch agents between GP2015 (61%) and etanercept (59.4%).
The rates of serious adverse events and of adverse events that led to withdrawal from the study were similar across all treatment groups. Immunogenicity of GP2015 was similar to etanercept.
However, the rate of “adverse events of special interest” – those referred to in special warnings and precautions on the etanercept label – was markedly higher for continued GP2015 than for continued etanercept (11.0% vs. 4.7%) and for switched GP2015 than for switched etanercept (11.0% vs. 5.2%). One patient who took etanercept throughout the trial developed malignant melanoma.
The results of the study “confirm biosimilarity that was established with all previous analytical comparisons to the reference product in that equivalent efficacy was demonstrated as well as similar safety and immunogenicity of GP2015” with etanercept, “in a highly sensitive, generally immune-competent population,” the authors concluded.
The study was funded by Hexal AG, a Sandoz company, which was involved in the study design, data collection and analysis, and manuscript preparation. Dr. Griffiths reported ties to AbbVie, BMS. Galderma, Janssen, Leo-Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, and UCB Pharma, and his associates reported ties to numerous industry sources.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: GP2015, a proposed etanercept biosimilar, demonstrated equivalent efficacy and comparable safety to etanercept for severe plaque psoriasis.
Major finding: The primary efficacy end point – the percentage of patients who showed at least a 75% improvement from baseline in PASI score at 12 weeks – was 73.4% with GP2015 and 75.7% with etanercept.
Data source: An international randomized double-blind study of 531 patients with moderate to severe chronic plaque psoriasis.
Disclosures: Hexal AG, a Sandoz company, funded the study. Hexal AG was involved in the study design, data collection and analysis, and manuscript preparation. Dr. Griffiths reported ties to AbbVie, BMS, Galderma, Janssen, Leo Pharma, Lilly, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, and UCB Pharma, and his associates reported ties to numerous industry sources.