User login
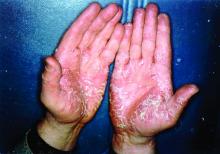
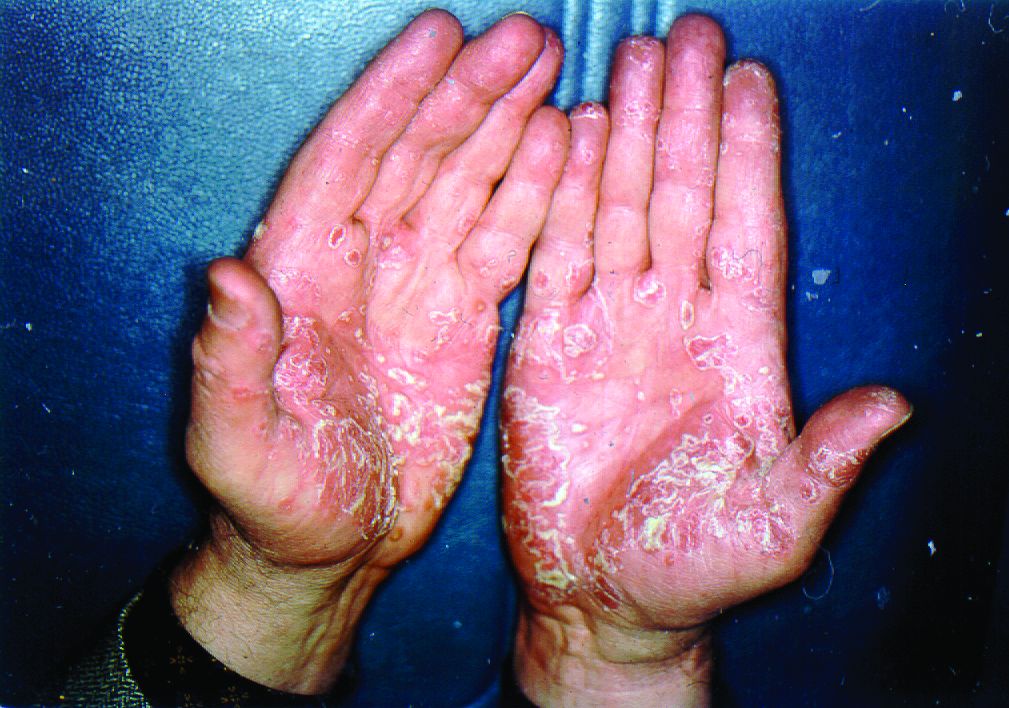
Pushing the Limits: Developing a New Standard of Care for Psoriasis
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
Concurrent Anticytokine Biologics for the Management of Severe Hidradenitis Suppurativa: Are They Safe and Effective?
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
Dysregulated immune responses including elevations in the inflammatory cytokines tumor necrosis factor (TNF),1-4 IL- 1 β ,3 and IL-12/235-7 have been identified in hidradenitis suppurativa (HS). Targeted biologic agents may offer an opportunity to intervene in specific aberrant inflammatory pathways to effectively treat HS while minimizing a dverse effects (AEs). There is growing evidence, however, that treatment of HS with a single biologic agent is not effective in all patients.6,8-17 The TNF antagonist adalimumab has been shown to achieve clinical response in approximately 50% of patients (N = 633). 18
The administration of concurrent biologics may offer the potential for improved disease control through synergistic targeting of multiple inflammatory pathways, particularly for severe and recalcitrant HS. This approach may be effective given insights from mechanistic studies suggesting the involvement of multiple inflammatory pathways in the disease pathogenesis.3,21 Concurrent anticytokine biologics have been used safely and effectively in other inflammatory diseases; for example, combination therapy with TNF and IL-12/23 antagonists have resulted in near-complete to complete resolution of severe psoriatic skin and joint disease without AEs.22-24
An increased risk for infection without increased efficacy associated with the use of concurrent anticytokine biologics for treatment of rheumatoid arthritis (RA) has raised concerns about the safety of this therapeutic approach. In a study of concurrent etanercept and anakinra therapy for RA (N=244), the combined therapy was not more efficacious than etanercept alone (American College of Rheumatology 50% response at week 24: etanercept 25 mg twice weekly, 41%; etanercept 25 mg twice weekly plus anakinra 100 mg once daily, 31%; etanercept 25 mg once weekly plus anakinra 100 mg once daily, 39% [P=.914]).25 Combination therapy also was associated with a higher overall incidence of serious AEs, serious infections requiring antibiotics or hospitalizations, and serious infections leading to study withdrawal. Reported infections included pneumonia, cellulitis, herpes zoster, pneumonitis, and pyelonephritis, but no opportunistic infections or tuberculosis were reported. A single case of lymphoma was reported in the full-dose etanercept plus anakinra group; however, the association with therapy is unclear, as RA itself is associated with an increased risk of malignancy.25
Although these results are notable, caution must be exercised in extrapolating safety and efficacy data for treatment with concurrent biologics from the RA literature for management of HS for several reasons. First, RA is an autoimmune disease that is associated with an increased risk for genitourinary and bronchopulmonary infections and septic arthritis, even in the absence of treatment with steroids and immunomodulatory drugs.26,27 Increased risk for development of lymphoma, lung cancer, and nonmelanoma skin cancer also has been associated with RA.28,29 The exact etiology of this increased risk is unknown, but it is thought to relate to immunologic disturbances and chronic systemic inflammation associated with RA.29 Furthermore, RA disease characteristics and comorbidities that may contribute to an increased risk for infection and malignancy include advanced age as well as a history of leukopenia, chronic lung disease, diabetes mellitus, alcoholism, and/or smoking.30 Infection and malignancy risk in RA also may be compounded by immunomodulatory therapies.31,32
Conversely, although microbes are believed to play an important role in HS initiation and progression, HS is neither considered an infectious disease nor associated with an increased risk for infection.33 Increased malignancy risk generally is not reported with HS, and systematic therapeutic trials of biologic therapies for HS have been notable for an absence of infectious or malignant AEs compared to placebo.12,14,16,18,19 From a mechanistic standpoint, data suggest that HS may be fundamentally distinct from RA and other autoimmune diseases; therefore, it may not be appropriate to extrapolate safety data from the latter to guide therapeutic strategies for the former.
The concept that different inflammatory diseases harbor distinct risks for comorbidities and AEs associated with medications is further supported by data from patients with PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne), a monogenic autoinflammatory disease characterized by inflammasome activation and subsequent increased signaling via IL-1.34
We have safely and effectively treated 2 patients with severe HS with extended courses of concurrent TNF and IL-1 antagonists. Both patients had previously failed treatment with multiple therapeutic interventions, including topical and systemic antibiotics, disease-modifying antirheumatic drugs, hormonal therapy, biologic monotherapy with several targeted agents, and wide local excision. In the setting of concurrent certolizumab plus anakinra in the first patient and adalimumab plus anakinra in the second, both patients reported reduced drainage, pain, and number of disease flares. Both patients also were maintained on extended treatment courses (11 months and 2 years, respectively) without evidence of infection or malignancy.
Concurrent biologics may be safe and effective in managing recalcitrant HS; however, large prospective studies are needed to confirm these anecdotal findings. As our understanding of HS pathogenesis expands, novel and more effective therapeutic options will be developed. Until then, concurrent biologics may be a potential option for patients with severe recalcitrant HS.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
- Jemec GB. Predicting response to anti-TNF-alpha treatment in hidradenitis suppurativa. Br J Dermatol. 2013;168:233.
- Sbidian E, Hotz C, Seneschal J, et al. Antitumour necrosis factor-α therapy for hidradenitis suppurativa: results from a national cohort study between 2000 and 2013 [published online December 22, 2015]. Br J Dermatol. 2016;174:667-670.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β [published online May 17, 2011]. Br J Dermatol. 2011;164:1292-1298.
- van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: a systematic review. J Dermatolog Treat. 2013;24:392-404.
- Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626-627.
- Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911-914.
- Santos-Peréz MI, García-Rodicio S, Del Olmo-Revuelto MA, et al. Ustekinumab for hidradenitis suppurativa: a case report [published online December 3, 2013]. Actas Dermosifiliogr. 2014;105:720-722.
- Amano M, Grant A, Kerdel FA. A prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativa. Int J Dermatol. 2010;49:950-955.
- Blanco R, Gonzalez-Lopez MA, Gonzalez-Vela MC, et al. Disparate results in studies of adalimumab in the treatment of hidradenitis suppurativa: comment on the article by Amano et al. Int J Dermatol. 2013;52:380-381.
- Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
- Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
- Leslie KS, Tripathi SV, Nguyen TV, et al. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:243-251.
- Menis D, Maronas-Jimenez L, Delgado-Marquez AM, et al. Two cases of severe hidradenitis suppurativa with failure of anakinra therapy [published online January 22, 2015]. Br J Dermatol. 2015;172:810-811.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Zarchi K, Dufour DN, Jemec GB. Successful treatment of severe hidradenitis suppurativa with anakinra. JAMA Dermatol. 2013;149:1192-1194.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Hoffman LK, Ghias MH, Garg A, et al. Major gaps in understanding and treatment of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:86-92.
- Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798.
- Torre KM, Payette MJ. Combination biologic therapy for the treatment of severe palmoplantar pustulosis. JAAD Case Rep. 2017;3:240-242.
- Babalola O, Lakdawala N, Strober BE. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: a case report. JAAD Case Rep. 2015;1:3-4.
- Cuchacovich R, Garcia-Valladares I, Espinoza LR. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J Rheumatol. 2012;39:187-193.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412-1419.
- Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. 1971;14:135-137.
- Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293.
- Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64:1414-1420.
- Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis [published online April 23, 2008]. Arthritis Res Ther. 2008;10:R45.
- Doran MF, Crowson CS, Pond GR, et al. Predictors of infection inrheumatoid arthritis. Arthritis Rheum. 2002;46:2294-2300.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Smith EJ, Allantaz F, Bennett L, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics. 2010;11:519-527.
Do Psoriasis Patients Engage In Vigorous Physical Activity?
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Practice Points
- Psoriasis is associated with comorbid disease conditions, including cardiovascular disease.
- Regular physical activity is known to decrease the risk of developing cardiovascular disease.
- Patients with psoriasis would likely benefit from regular participation in vigorous physical activity to help reduce the risk of developing cardiovascular disease.
Phase 3 trials show halobetasol/tazarotene lotion works for psoriasis
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Online psoriasis consultations shown equivalent to office visits
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
REPORTING FROM AAD 18
Key clinical point: Online physician telemonitoring of psoriasis patients was equivalent to in-person management.
Major finding: After 1 year, changes in the Psoriasis Area and Severity Index in the two arms met the prespecified definition of equivalence.
Study details: A multicenter, randomized trial with 296 psoriasis patients in which outcomes were compared for online monitoring and in-person examinations.
Disclosures: Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 18, abstract 6730.
VIDEO: PPACMAN aims to advance the combined rheum-derm clinic approach in the community
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM AAD 18
Could guselkumab be a disease-modifying agent in plaque psoriasis?
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
REPORTING FROM AAD 2018
Key clinical point: Guselkumab shows some signs of having a disease-modifying effect in moderate to severe psoriasis after 28 weeks of treatment.
Major finding: A total of 37% of patients who withdrew from guselkumab at 28 weeks had still maintained PASI 90 improvement over baseline at 48 weeks.
Study details: An analysis of randomization to drug continuation vs. withdrawal in 375 patients with PASI 90 response to guselkumab in the VOYAGE 2 trial.
Disclosures: Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
Source: Gordon K et al. AAD 2018 Abstract 6748.
Ustekinumab quells aortic inflammation in patients with severe psoriasis
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email [email protected].
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email [email protected].
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email [email protected].
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
REPORTING FROM AAD 2018
Key clinical point:
Major finding: Aortic inflammation decreased by 6.6% in those taking ustekinumab, but increased by 12.1% in those taking placebo.
Study details: The randomized trial comprised 43 patients.
Disclosures: The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand made no financial disclosures.
Source: Gelfand J et al. AAD 2018 Abstract 6645.
VIDEO: With new therapies available, it’s the ‘decade of eczema,’ researcher says
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
REPORTING FROM AAD 18
Biologics have best chance of achieving PASI 90 in psoriasis
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS