User login
Psoriasis patients often have history of childhood trauma
reported Maria Luigia Crosta, of Catholic University of the Sacred Heart, Rome, and her associates.
Other studies have shown that among dermatologic disorders, psoriasis has the highest link to psychiatric illness such as mood, anxiety, and personality disorders, and that patients with psoriasis have an increased risk of suicidal ideation, the investigators said.
“Improving resilience with a multidisciplinary approach and an early psychological intervention could facilitate the management of psoriasis, by promoting the establishment of a stronger therapeutic alliance and a better acceptance of disease. Programs for psoriasis patients should focus on self-motivation and strengthening of self-efficacy,” Dr. Crosta and her associates concluded.
SOURCE: Crosta ML et al. J Psychosom Res. 2018;106:25-8.
reported Maria Luigia Crosta, of Catholic University of the Sacred Heart, Rome, and her associates.
Other studies have shown that among dermatologic disorders, psoriasis has the highest link to psychiatric illness such as mood, anxiety, and personality disorders, and that patients with psoriasis have an increased risk of suicidal ideation, the investigators said.
“Improving resilience with a multidisciplinary approach and an early psychological intervention could facilitate the management of psoriasis, by promoting the establishment of a stronger therapeutic alliance and a better acceptance of disease. Programs for psoriasis patients should focus on self-motivation and strengthening of self-efficacy,” Dr. Crosta and her associates concluded.
SOURCE: Crosta ML et al. J Psychosom Res. 2018;106:25-8.
reported Maria Luigia Crosta, of Catholic University of the Sacred Heart, Rome, and her associates.
Other studies have shown that among dermatologic disorders, psoriasis has the highest link to psychiatric illness such as mood, anxiety, and personality disorders, and that patients with psoriasis have an increased risk of suicidal ideation, the investigators said.
“Improving resilience with a multidisciplinary approach and an early psychological intervention could facilitate the management of psoriasis, by promoting the establishment of a stronger therapeutic alliance and a better acceptance of disease. Programs for psoriasis patients should focus on self-motivation and strengthening of self-efficacy,” Dr. Crosta and her associates concluded.
SOURCE: Crosta ML et al. J Psychosom Res. 2018;106:25-8.
FROM THE JOURNAL OF PSYCHOSOMATIC RESEARCH
FDA approves certolizumab label update for pregnancy, breastfeeding
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
FDA approves IL-23 antagonist for plaque psoriasis
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
in adults who are eligible for systemic therapy or phototherapy, according to a statement from Sun Pharma.
Tildrakizumab is administered at a dose of 100 mg, subcutaneously, at weeks 0 and 4, then every 12 weeks. Approval is based on data from two phase 3, identically designed clinical trials, reSURFACE1 and reSURFACE2. Both studies were multicenter, randomized, double-blind, and placebo controlled. In the studies, 926 patients received tildrakizumab (616 patients) or placebo (310 patients).
The effectiveness of tildrakizumab extended beyond 12 weeks, with 74% of patients achieving a PASI 75 at 28 weeks after three doses. This percentage grew to 84% at week 64 in patients who continued treatment. Similar results were observed with PGA scores, with 69% of patients who had a PGA score of 0 or 1 at 12 weeks maintaining that score at week 28.
Tildrakizumab has been associated with serious side effects, including serious allergic reactions including skin rash, swelling of the face and mouth, trouble breathing, and chest tightness. It may also increase patient susceptibility to infection. It is approved with a Medication Guide for patients, explaining the potential risks associated with treatment.
Tildrakizumab will be marketed as Ilumya.
Sun Pharma is working with the FDA on postapproval commitments, and once that has been completed, they will have a better idea of when it will become available, according to a spokesperson for the manufacturer. The cost is not yet available.
PASI responses with biologics similar among white, nonwhite individuals, study finds
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
MIAMI – Skin clearance rates among people with moderate to severe plaque psoriasis treated with brodalumab were superior to clearance rates among those treated with ustekinumab in a study that also provided comparisons between white and nonwhite patients.
In the study, presented in a poster at the 2018 Orlando Dermatology Aesthetic and Clinical Conference, there were no significant difference in overall efficacy, safety, or health-related quality of life outcomes between white and nonwhite patients treated with either biologic.
Additional analyses specific to patients with skin of color can be beneficial, Amy McMichael, MD, one of the investigators, said in an interview. “Patients with skin of color experience differences in psoriasis-related symptoms,” noted Dr. McMichael, chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C. “Greater degrees of skin involvement have been shown in African-American patients, as have differences in erythema, scaling, dyspigmentation, and plaque thickness.”
She and her colleagues evaluated 1,849 participants in phase 3 brodalumab clinical trials, which included ustekinumab-treated patients as a comparison group. Approximately 10% of the AMAGINE-2 and AMAGINE-3 study populations were skin of color participants. The results reported at the meeting were from their ad hoc study of 12-week induction findings from the 52-week clinical trials.
At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved a Psoriasis Area and Severity Index (PASI) 75. At the same time, 86% of white and 88% of nonwhite patients treated with brodalumab achieved the same outcome. Similarly, PASI 90 and PASI 100 scores did not differ significantly between the 1,667 white and 182 skin of color participants.
The two biologics act on different aspects of the molecular pathway involved in psoriasis. Brodalumab (Siliq) specifically blocks the interleukin-17 receptor, whereas other biologics used to treat psoriasis, including ustekinumab (Stelara), an IL-12 and -23 antagonist, target upstream molecules in the inflammatory pathway. Dr. McMichael said, “The superior skin clearance rates seen in patients treated with brodalumab may be due to its target being a receptor as opposed to a ligand.”
Treatment-emergent adverse event rates were similar between the white and nonwhite patients. Treatment-emergent adverse events were reported in 58% and 57% of the white brodalumab and ustekinumab groups, respectively, and in 53% and 47% of the nonwhite brodalumab and ustekinumab groups, respectively. Serious adverse events occurred in 1.2% and 1.1% of the white brodalumab and ustekinumab cohorts, respectively, and in 1.7% and 0% of the nonwhite participants, respectively.
The investigators also assessed health-related quality of life and again found outcomes were similar between white and nonwhite participants. For example, among those treated with brodalumab, 80% of white and 78% of nonwhite patients achieved a score improvement of 5 or greater on the Dermatology Life Quality Index. Of those randomized to ustekinumab, 76% of white patients and 73% of nonwhite patients achieved the same outcome.
“We plan to perform a further analysis evaluating the Dr. McMichael said. Additionally, a population-based study to investigate treatment patterns in patients with psoriasis across racial and socioeconomic groups could also shed light on how patients with skin of color manage their psoriasis, she added.
Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
SOURCE: McMichael A et al. ODAC 2018.
REPORTING FROM ODAC 2018
Key clinical point: Responses to brodalumab and ustekinumab were comparable in nonwhite and white patients with psoriasis.
Major finding: At week 12, 70% of white and 63% of nonwhite participants treated with ustekinumab achieved PASI 75, a nonsignificant difference.
Study details: An ad hoc comparison of week 12 phase 3 study data in 1,849 patients including 182 patients with skin of color.
Disclosures: Dr. McMichael’s disclosures include having been an investigator for Allergan, Incyte, and Samumed; and a consultant to Aclaris, Galderma, IntraDerm, Johnson & Johnson, Merz, Pfizer, and Procter & Gamble.
Source: McMichael A et al. ODAC 2018.
Study highlights need to investigate psoriasis treatment outcomes in skin of color patients
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
MIAMI – Psoriasis often presents differently in skin of color patients, but an unanswered question remains: Does response to treatment with an agent like a fixed-dose combination foam also differ by ethnicity?
Researchers at Mount Sinai St. Luke’s Hospital in New York addressed this question using phase 2 and 3 study data for 1,104 people with psoriasis, about half of whom were randomized to topical treatment with calcipotriene and betamethasone dipropionate foam 0.005%/0.064% (Enstilar); the rest received a single component or vehicle only. The data were obtained from LEO Pharma, the product’s manufacturer.
“We were very interested in knowing if there was any difference in efficacy between the specific ethnic groups – the skin of color and non–skin of color patients,” said Bridget Kaufman, MD, a dermatopharmacology fellow at Mount Sinai St. Luke’s Hospital. “So we went back to look at the data to see if there was any difference in side effects or efficacy between ethnic groups.”
Strength in numbers?
The three randomized, pooled clinical studies included many ethnic groups. However, only 6.5% of participants were black and even fewer were Asian, American Indian, or native Hawaiian, Dr. Kaufman said. “It’s hard to see meaningful differences when you don’t have a substantial skin of color population.”
As a result, no significant associations emerged from the pooled data. “That is the main take-home message of this study: We don’t have a great understanding now of the difference in efficacy between white and nonwhite ethnic groups,” Dr. Kaufman said.
The researchers defined treatment success at 4 weeks as a two-point improvement to “clear” or “almost clear” on the Investigator Global Assessment of psoriasis. Of the adult participants with chronic plaque psoriasis randomized to the combination foam product, 54% of the white patients; 30% of black patients; 69% of Asian patients; and one of the two Hawaiian/Pacific Islander patients achieved treatment success after 4 weeks of topical treatment.
“All subgroups analyzed had a good response to treatment at 4 weeks. Numerically it appears African Americans in particular did not do quite as well, but we can’t [definitively] draw that conclusion,” Dr. Kaufman said in an interview.
More data, please
The study is just the first step in investigating the efficacy of this particular product in diverse ethnic groups, Dr. Kaufman added. “That really emphasizes the importance of studying these medications in skin of color populations in particular.”
More guidance on psoriasis in skin of color patients is available in a published review article by Dr. Kaufman and Andrew F. Alexis, MD, director of the Skin of Color Center at Mount Sinai St. Luke’s and Mount Sinai West, New York (Am J Clin Dermatol. 2017 Dec 5. doi: 10.1007/s40257-017-0332-7).
Enstilar manufacturer LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
[email protected]
SOURCE: Kaufman B et al. ODAC 2018
REPORTING FROM ODAC 2018
Key clinical point: There were no significant differences in response to calcipotriene and betamethasone dipropionate foam in phase 2 and 3 trials, possibly because of small percentages of skin of color participants in these studies.
Major finding: Treatment success rates at 4 weeks were 30% among black patients, 54% among white patients, and 69% among Asian patients, but not enough skin of color patients were enrolled for differences to reach statistical significance.
Study details: A retrospective analysis of pooled phase 2 and phase 3 studies with 1,104 participants with psoriasis.
Disclosures: LEO Pharma supplied the clinical data but did not fund the study. Dr. Kaufman had no relevant financial disclosures.
Source: Kaufman B et al. ODAC 2018.
Xeljanz: FDA panel recommends ulcerative colitis indication
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study, two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2), a 53-week, phase 3 maintenance trial (OCTAVE Sustain), and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.*
Patients who did not achieve remission, but showed some clinical response (decrease in Mayo score of at least 3 points), were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5 mg and 10 mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior TNF-blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients as part of an open-label extension study who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection (HZ), “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair and vice chair Jean-Pierre Raufman, MD, and Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
*This article was updated on March 12, 2018.
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study, two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2), a 53-week, phase 3 maintenance trial (OCTAVE Sustain), and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.*
Patients who did not achieve remission, but showed some clinical response (decrease in Mayo score of at least 3 points), were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5 mg and 10 mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior TNF-blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients as part of an open-label extension study who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection (HZ), “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair and vice chair Jean-Pierre Raufman, MD, and Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
*This article was updated on March 12, 2018.
SILVER SPRING, MD. – Federal advisors to the Food and Drug Administration on March 8 voted unanimously to recommend approval of an additional indication for tofacitinib (Xeljanz), this time for ulcerative colitis.
Members of the Gastrointestinal Drugs Advisory Committee unanimously voted to recommend two different dosing regimens: 10 mg twice daily for 16 weeks in patients who have not experienced a therapeutic benefit after 8 weeks of treatment, as well as 10 mg twice daily for patients who have an inadequate or loss of response to TNF-blocker therapy, based on the results of several phase 3 clinical trials.
The recommended ulcerative colitis (UC) indication was based on the OCTAVE trials (N Engl J Med 2017;376:1723-36), including a phase 2 study, two identical phase 3 induction trials (OCTAVE Induction 1 and OCTAVE Induction 2), a 53-week, phase 3 maintenance trial (OCTAVE Sustain), and an open-label extension study.
The induction trials enrolled a total of 1,139 patients with moderate to severe UC. Patients in both studies were administered tofacitinib 10 mg twice daily or placebo and were assessed after 8 weeks to judge clinical response. Patients in both studies displayed notable remission rates (18.5% and 16.6%), compared with placebo, according to Eric Maller, MD, executive director of the UC development program at Pfizer.*
Patients who did not achieve remission, but showed some clinical response (decrease in Mayo score of at least 3 points), were then enrolled in the 53-week OCTAVE Sustain, where they were randomized to receive tofacitinib 10 mg twice daily, 5 mg twice daily, or placebo.
During maintenance treatment, both 5 mg and 10 mg doses demonstrated substantial treatment benefits, with 32.4% and 41.0% of patients achieving remission, an increase of 22.0% and 30.7%, compared with placebo, respectively.
As part of the maintenance study, Pfizer analyzed patients with or without prior TNF-blocker failure. This analysis revealed that patients who had previously failed TNF-blocker therapy experienced a greater treatment benefit than those who had not. While the benefit was noticeable in both dosage groups, patients taking the 10-mg dose experienced the greatest benefit, with 70% increase in remission rates, 39% increase in mucosal healing, and 75% increase in steroid-free remission among baseline remitters, compared with patients in the 5-mg group, Dr. Maller said.
Researchers also looked at a subgroup of 295 patients as part of an open-label extension study who had no clinical response to tofacitinib 10 mg twice daily after 8 weeks and subsequently treated them for an additional 8 weeks. After the additional 8 weeks of treatment, over half (51.2%) displayed clinical responses and 8.6% were in remission.
“This is a desperate patient population. These are impressive results,” stated Darrell Pardi, MD, vice chair of the advisory committee and professor of medicine at the Mayo Clinic, Rochester, Minn.
Serious adverse events were seen in 4% of tofacitinib-treated patients in the induction trials, compared with 6% of placebo-treated, according to Lesley Hanes, MD, medical officer with the FDA Center for Drug Evaluation and Research.
Adverse events appeared to be dose dependent, with risk of deaths and malignancies (excluding nonmelanoma skin cancer), opportunistic infections, herpes zoster infection (HZ), “possible” drug-induced liver injury, and cardiovascular and thromboembolic events more common with the 10-mg dose, Dr. Hanes said.
“I think the safety concerns, though, they are dose dependent, the difference between the 5 [mg] and 10 [mg] were not large,” according to Dr. Pardi. “Several of these are mitigatable by dermatologic exam or, hopefully, a vaccine.”
Several of the advisory committee members submitted conflict of interest waivers. Chair and vice chair Jean-Pierre Raufman, MD, and Darrell Pardi, MD, disclosed funding from competing pharmaceutical manufacturers.
*This article was updated on March 12, 2018.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Pediatric Psoriasis: An Interview With Nanette B. Silverberg, MD
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
What causes psoriasis in children?
Psoriasis is a chronic immune-mediated inflammatory skin disease with a genetic predisposition (Eichenfield et al). Similar to many inflammatory skin diseases, school-aged children have a greater predisposition before or in early adolescence. As with adult disease, pediatric psoriasis has a complex pathogenesis largely related to aberrant immune response to triggers such as infections (eg, streptococcal pharyngitis, perianal streptococcal dermatitis, upper respiratory viral infections), trauma (ie, Koebner phenomenon), stress, and obesity.
What are the emerging data and recommendations on screening for comorbidities in children with psoriasis?
Similar to psoriasis in adults, obesity and the metabolic syndrome are a true association with pediatric psoriasis that has been discussed in the literature (Eichenfield et al). Although many children with psoriasis have obesity as a potential comorbidity, the risk of cardiovascular comorbidities independent of obesity is high in pediatric psoriasis including elevated lipids, hypertension, polycystic ovaries, nonalcoholic liver disease, and elevated liver enzymes (Tollefson et al). Children with psoriasis have greater central obesity and adiposity, often accompanied by a family history of obesity. Interventions in this direction may be needed for long-term disease control and general health (Mercy and Paller). One target population is hospitalized children with psoriasis, particularly black and Hispanic children aged 0 to 9 years. This population has been identified to have a greater risk for obesity, diabetes mellitus, hypertension, arrhythmia, and valvular heart disease (Kwa et al). Therefore, it can be said that dermatologists can help to improve the overall health and lifestyle long-term in children with psoriasis.
Early-onset disease also is associated with greater risk for lifetime quality-of-life impairments including poor lifetime dermatology life quality index scores, depression and psoriasis-induced depression, social discrimination, sleep problems, and recreational drug usage (Kim et al).
How does psoriasis in children differ from adults?
Children have a variety of features that differ from adult disease. First, they are more likely to have an infectious trigger and therefore may have an identifiable treatable source. Second, they are more likely to have a family history of disease, with one-third having a relative with psoriasis, therefore, identifying the child at risk for long-standing disease. Third, children have far more visible head and neck disease, especially facial involvement including eyelids (Raychaudhuri and Gross), which increases the risk of bullying, social stigma, and negative effects on self-image. Of course, site is affected by age, and in infancy diaper dermatitis and inverse disease with maceration and overlying candidal diaper dermatitis can occur. Although children have less joint disease, it can be dramatic and crippling to the developing child.
What treatments are available for children?
In childhood, identification of precipitating infections such as streptococcal infection is ideal with appropriate intervention thereafter. Topical therapies are appropriate for limited disease with minimal disability; however, phototherapy and systemic agents can be used in pediatric psoriasis in extensive cases. Topical therapies can include corticosteroids, calcineurin inhibitors often used in sensitive skin such as the face and intertriginous areas, and calcipotriene (Eichenfield et al). Additional agents such as tar and salicylic acid can be used, with limitations on the latter due to risk for absorption in smaller children. Systemic interventions often are introduced after years of disease. A recent study identified practitioners with special interest in pediatric psoriasis and determined that systemic interventions were on average introduced 3 years after psoriasis was diagnosed and most commonly included methotrexate followed by etanercept, the latter having fewer gastrointestinal tract side effects. The panel found that usage of folic acid 6 days weekly minimized gastrointestinal tract side effects with methotrexate. Acitretin and cyclosporine were alternatives (Bronckers et al; Psoriasis Investigator Group [PsIG] of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis [EWGPP]).
Recently, dermatologists have become aware of the dramatic benefits of immune response modifiers and some biologics on pediatric psoriasis. In the setting of joint and skin involvement, I allow the rheumatologist to make the choice of agents for the child's best outcome. However, for pediatric and adolescent psoriasis, we now have 2 US Food and Drug Administration-approved agents and more rapid and thorough testing of adult-approved agents in children, with a hope of greater ability to modify disease course at a younger age, both now and in the future.
Which biologics are approved for the pediatric patient population?
Currently, in the United States 2 biologics have been approved: (1) etanercept, a fusion protein of tumor necrosis factor receptor extracellular domain linked to the Fc portion of human IgG, for moderate to severe plaque psoriasis in patients 4 years and older, and (2) ustekinumab, a human IgG1κ monoclonal antibody against the shared p40 subunit of the IL-12 and IL-23 cytokines, for moderate to severe plaque psoriasis in patients 12 years and older based on the encouraging data of the CADMUS trial (Kellen et al; Landells et al). In Europe, adalimumab has been approved as a first-line therapy in pediatric psoriasis (age ≥4 years), and etanercept (age ≥6 years) and ustekinumab (age ≥12 years) have been approved as second-line agents, all with grade A evidence, according to a recent Italian panel (Fortina et al). (A thorough review of the guidelines on screening, administration, and vaccination is available from Eichenfield et al.)
What treatments are in the pipeline?
In the United States we have clinical trials ongoing of adult-approved topical and immune response-modifying agents such as apremilast. These agents, as they become available and the data are gathered, will be added to what I refer to as our "pharmamentarium" of agents we can use to combat a difficult and disabling illness.
What gaps are there in the pediatric psoriasis research?
Currently, there is poor awareness that there is research for pediatric psoriasis, and there is a need for pediatric groups and the National Psoriasis Foundation to allow children, adolescents, and their families to know that clinical trials are available looking into newer, more targeted, and less immunosuppressive agents. There is new hope on the horizon!
Suggested Readings
Bronckers IMGJ, Seyger MMB, West DP, et al; Psoriasis Investigator Group (PsIG) of the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis (EWGPP). Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147-1157.
Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives [published online January 4, 2018]. Pediatr Dermatol. doi:10.1111/pde.13382.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group [published online August 23, 2017]. Eur J Pediatr. 2017;176:1339-1354.
Kellen R, Silverberg NB, Lebwohl M. Efficacy and safety of ustekinumab in adolescents. Pediatric Health Med Ther. 2016;7:109-120.
Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis [published online December 29, 2014]. Pediatr Dermatol. 2015;32:656-662.
Kwa L, Kwa MC, Silverberg JI. Cardiovascular comorbidities of pediatric psoriasis among hospitalized children in the United States. J Am Acad Dermatol. 2017;77:1023-1029.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study [published online August 7, 2015]. J Am Acad Dermatol. 2015;73:594-603.
Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17:174-178.
Tollefson MM, Van Houten HK, Asante D, et al. Association of psoriasis with comorbidity development in children with psoriasis [published online January 10, 2018]. JAMA Dermatol. doi:10.1001/jamadermatol.2017.5417.
Biologics and Systemic Therapies for Psoriasis: Treat the Patient, Not the Disease
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
Current Guidelines for Psoriasis Treatment: A Work in Progress
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
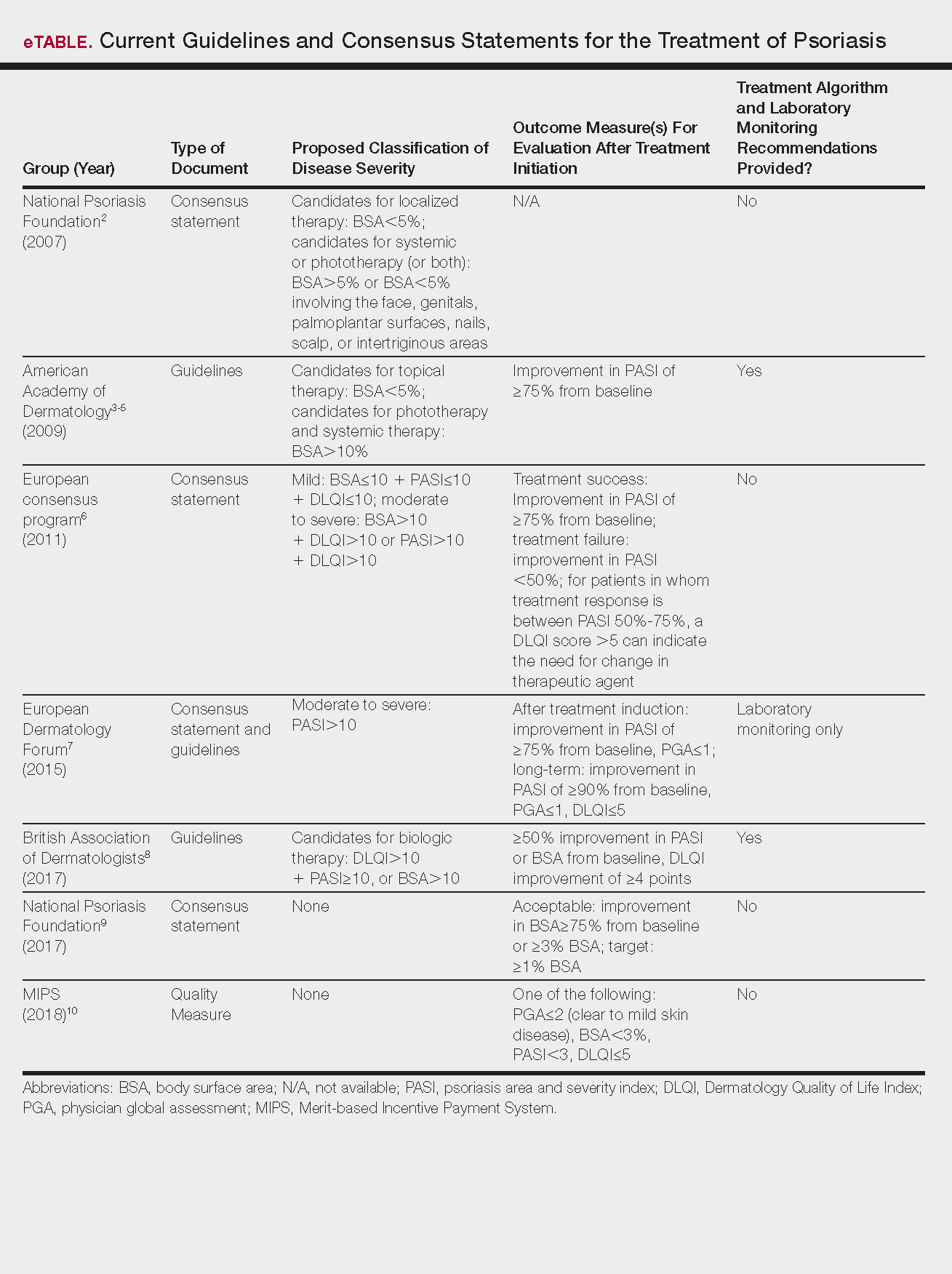
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Practice Points
- Guidelines and consensus statements for psoriasis treatment are generally but not always consistent.
- As guidelines evolve, individual patient preferences, disease severity, and comorbid conditions remain important considerations when selecting treatment agents for psoriasis.
- More frequent updates to psoriasis treatment guidelines are becoming increasingly important given the rapid changes in the field.
Emerging Therapies In Psoriasis: A Systematic Review
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.
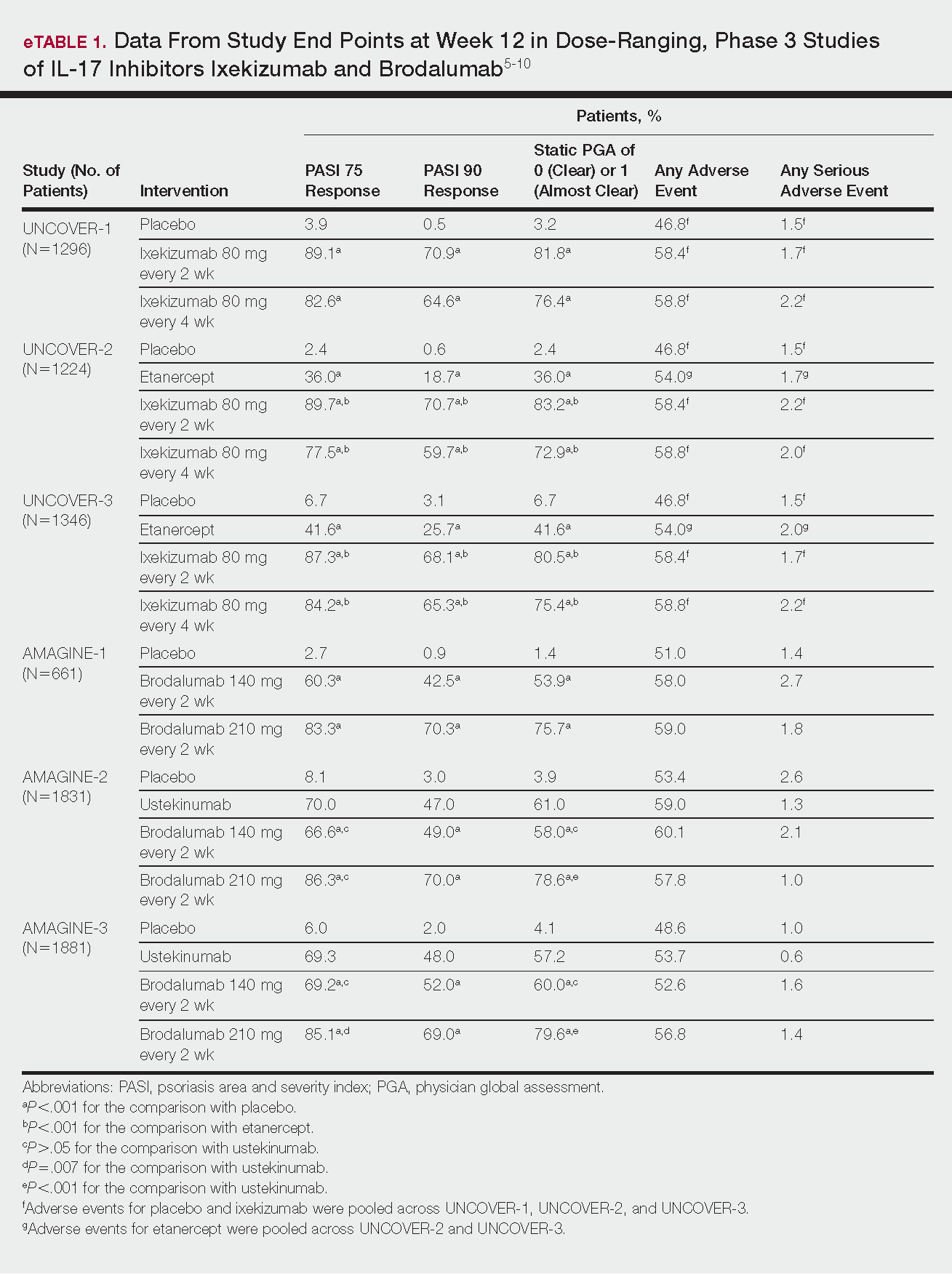
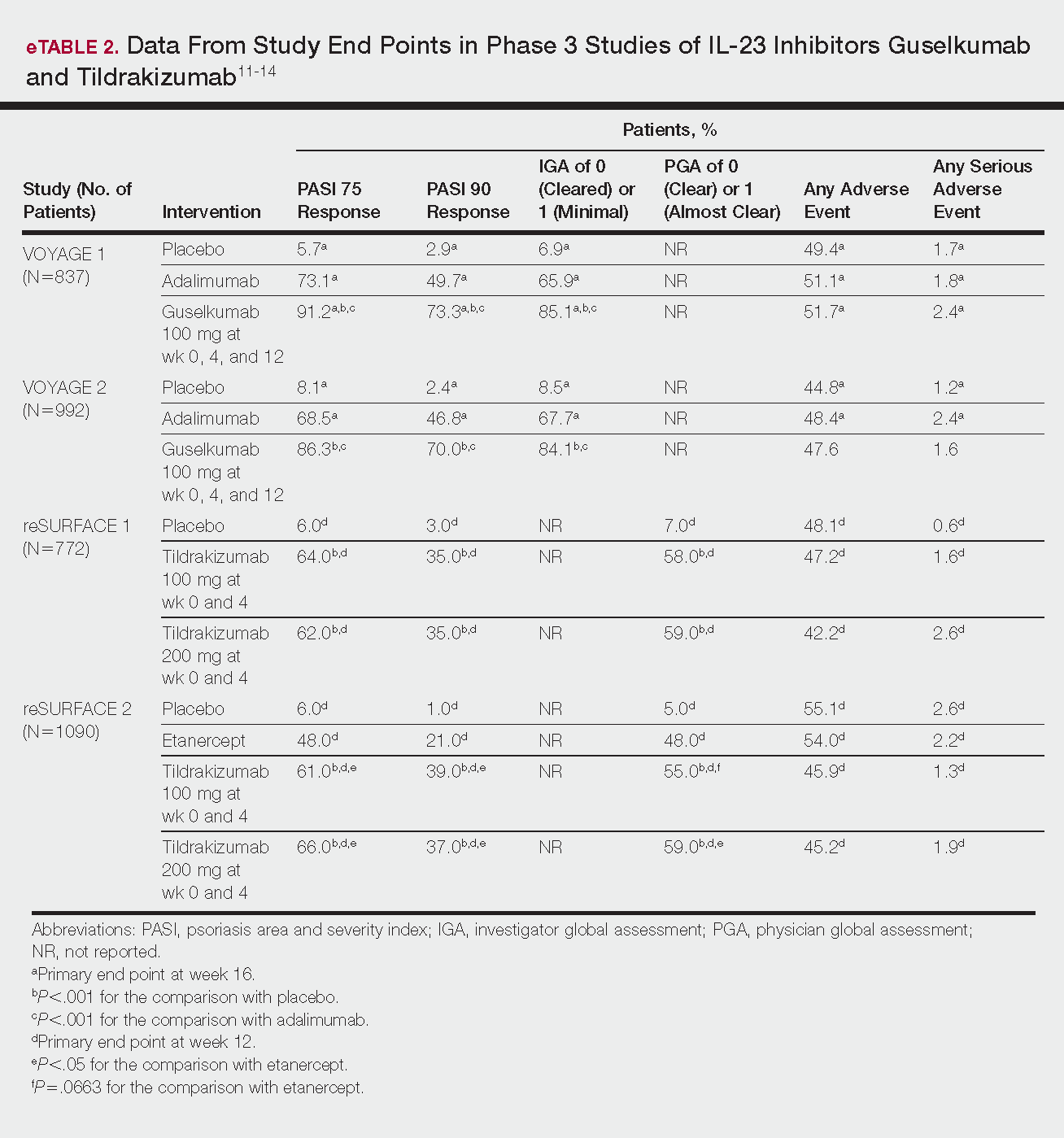
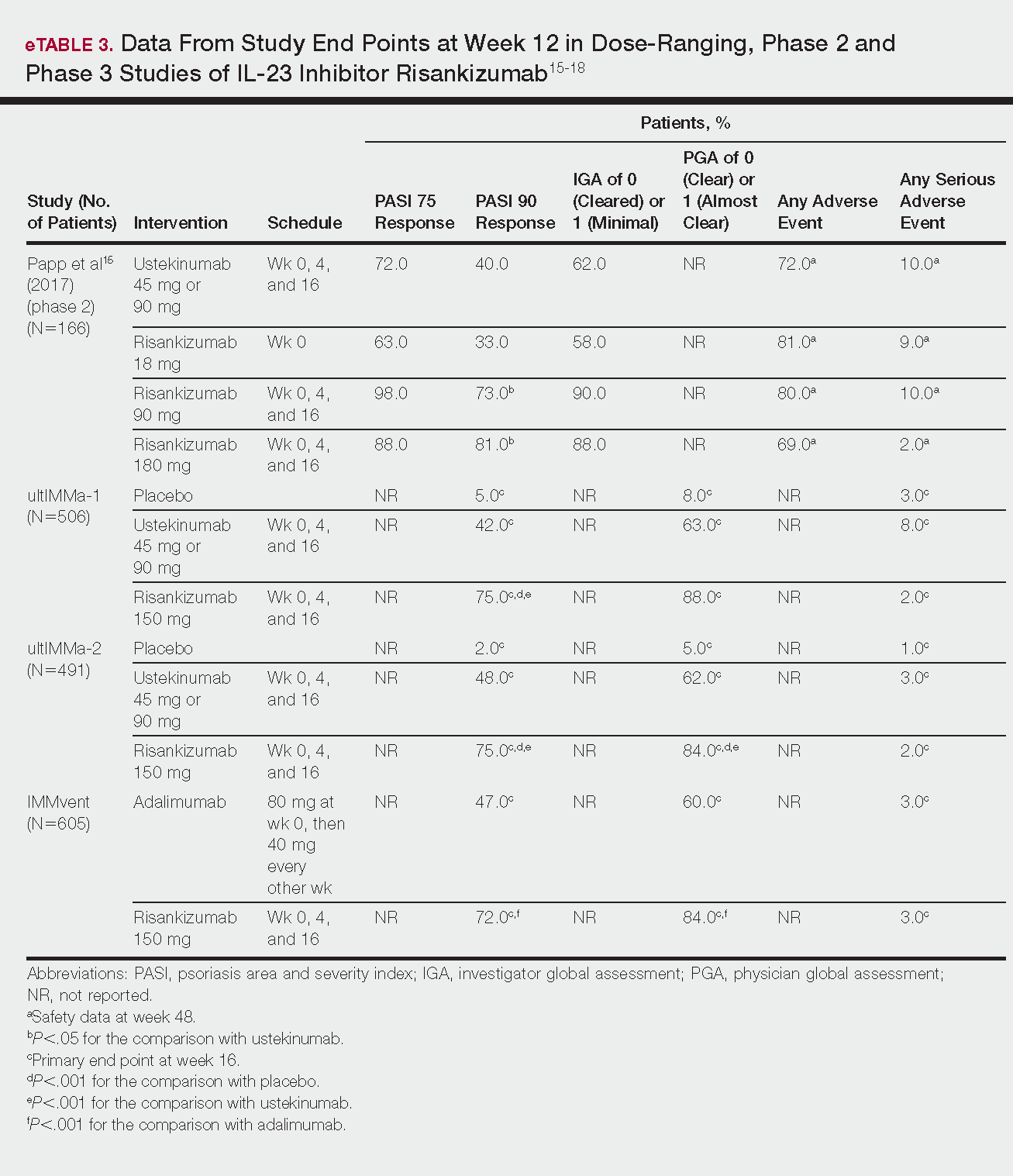
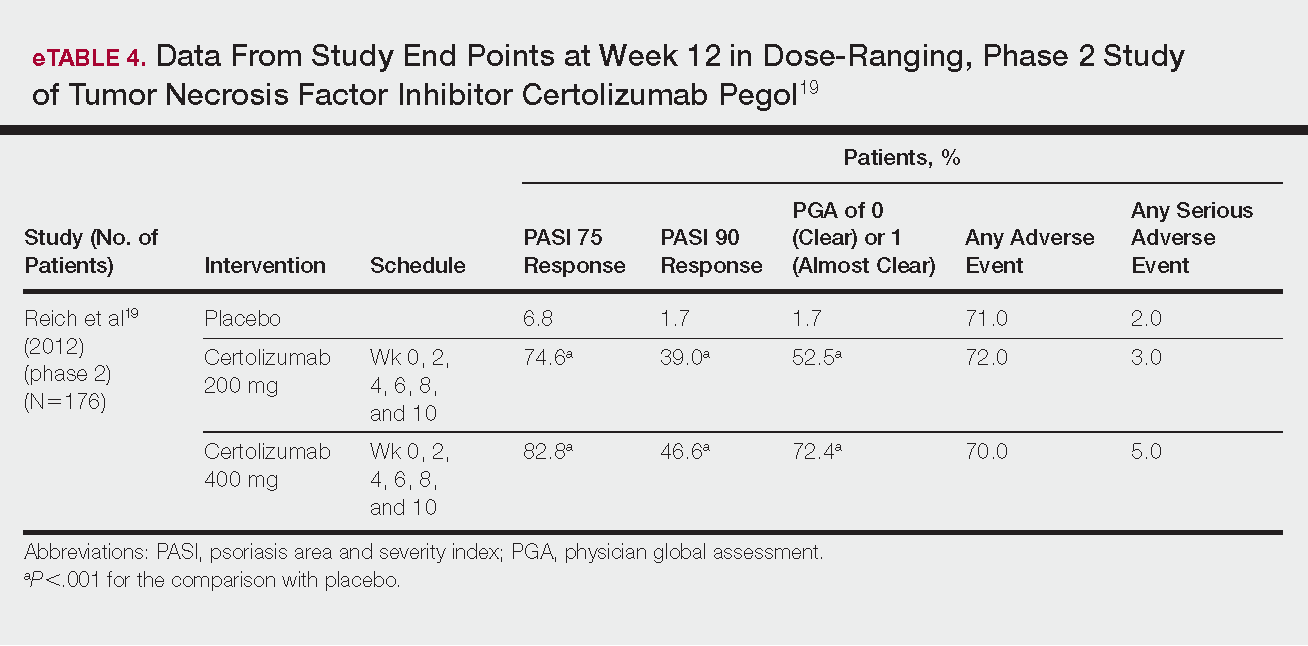
IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Practice Points
- Tumor necrosis factor α, IL-23, and IL-17A are key targets for psoriasis therapy based on an understanding of the key role that these cytokines play in the pathophysiology of disease.
- The biologic agents secukinumab and ixekizumab are approved for use in the management of psoriasis. Other biologics—brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol—have been (and some continue to be) the focus of phase 2 and phase 3 clinical trials.
- Findings of several of those trials support the idea that therapies targeting IL-23, specifically its p19 subunit, but that spare IL-12 are effective against psoriasis.
- Longer-term studies are needed to determine whether the agents reviewed here, including those approved for clinical use, are suitable for prolonged administration.