User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
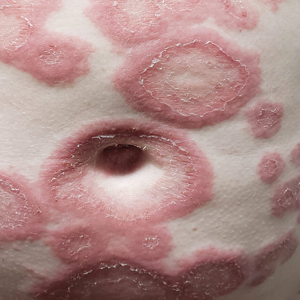
Diffuse Urticarial Rash in a Pregnant Patient
The Diagnosis: Pemphigoid Gestationis
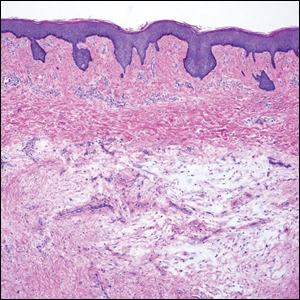
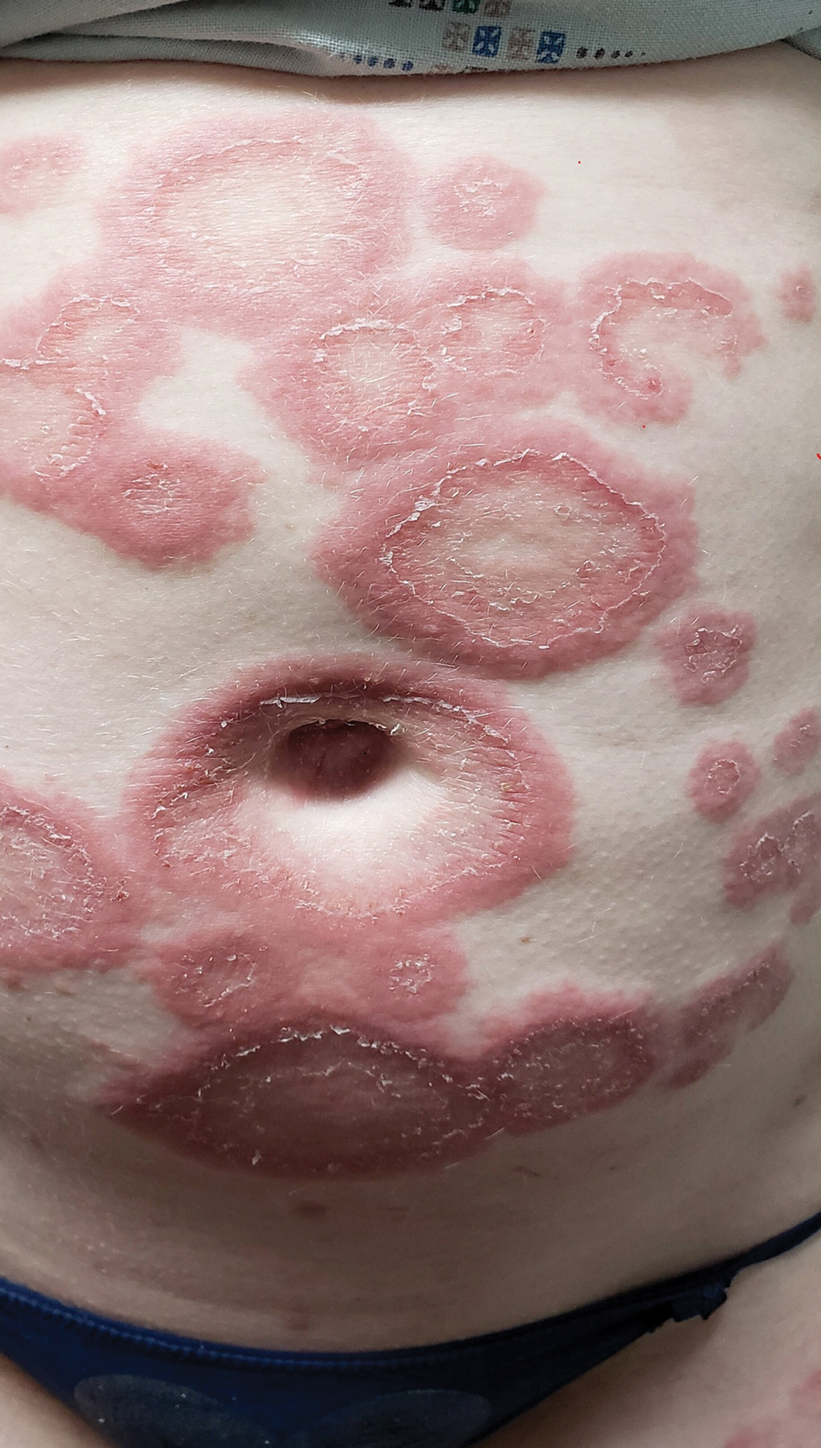
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
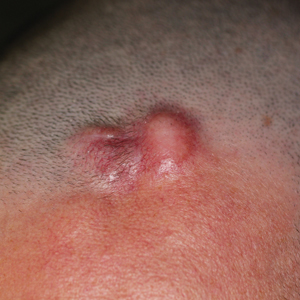
A 29-year-old pregnant woman at 18 weeks and 5 days of gestation presented with a diffuse, pruritic, blistering rash of 5 weeks’ duration that started on the forearms and generalized to affect the trunk, legs, palms, and soles. Physical examination showed diffuse urticarial papules and plaques with small tense vesicles with an annular configuration on the abdomen and marked periumbilical involvement.
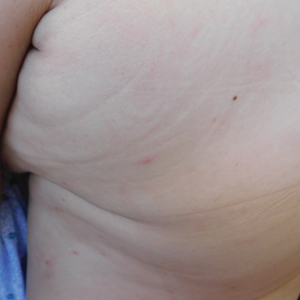
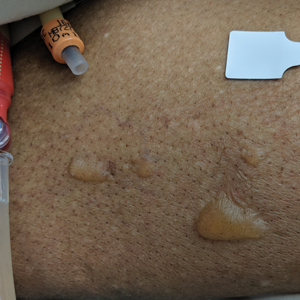
Pruritic Papules on the Trunk, Extremities, and Face
The Diagnosis: Gamasoidosis
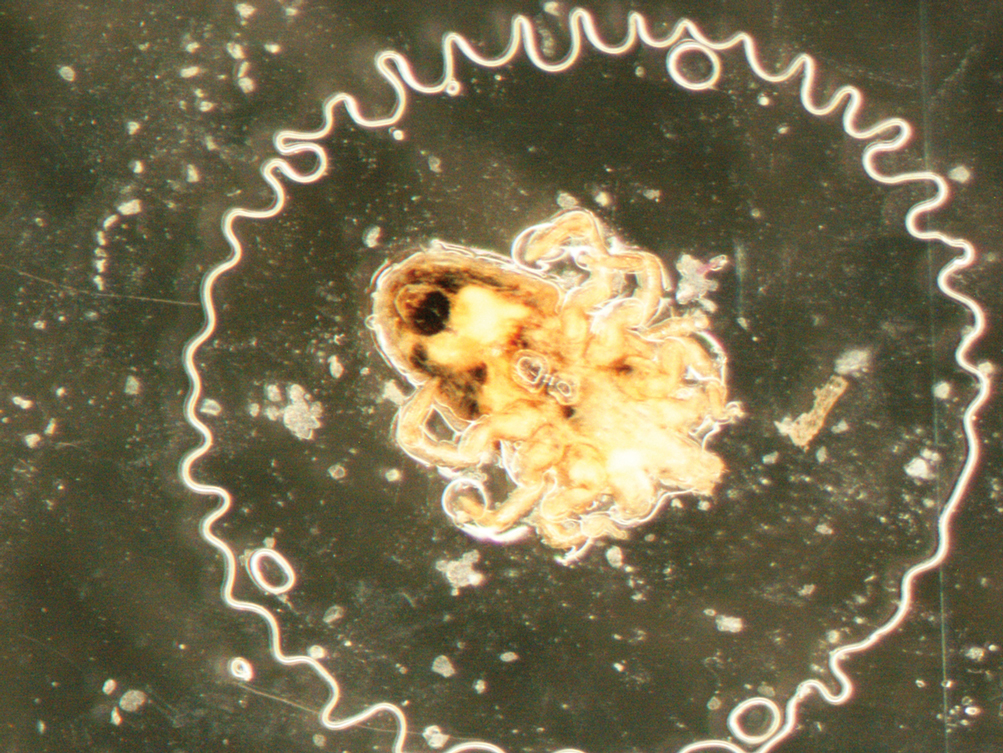
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
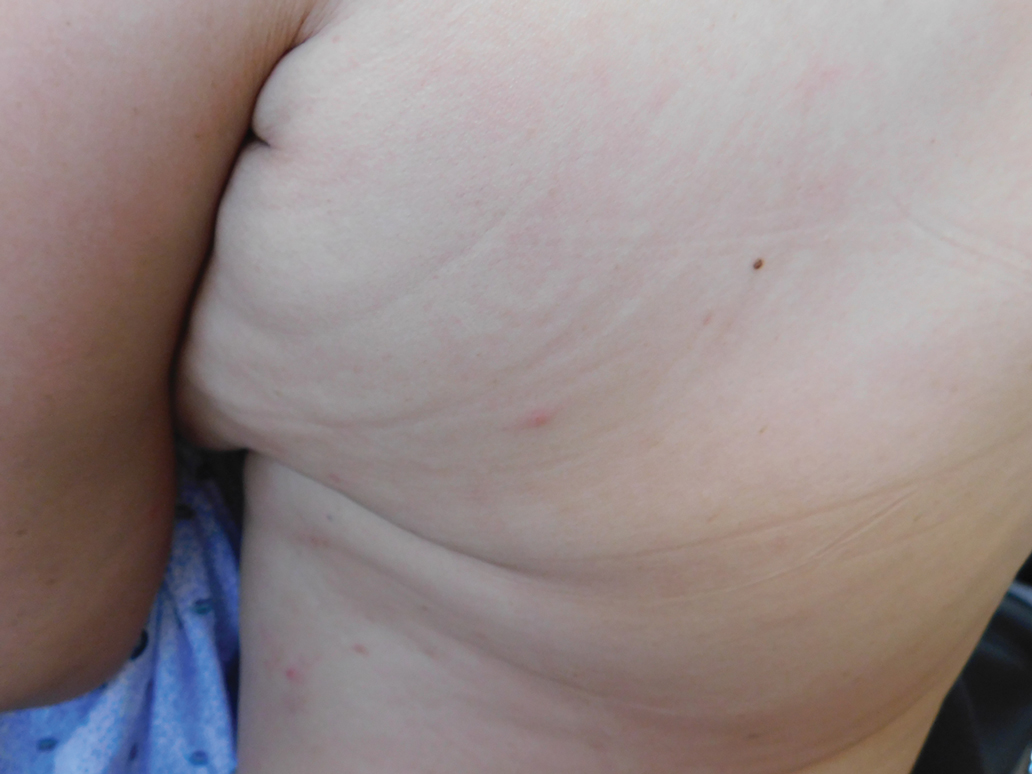
A 69-year-old woman presented in early summer in southeastern Michigan with several itchy bumps (top) of 4 to 5 weeks’ duration that erupted and remitted over the trunk, extremities, and face. She had taken no new medications. She had an asymptomatic cat and no exposure to anyone else who had been itching. Physical examination revealed approximately a dozen 2- to 5-mm edematous papules on the trunk, arms, shins, thighs, and left cheek, as well as one 3-mm vesicle on the forearm. No burrows could be identified on physical examination. Lesions treated with betamethasone dipropionate cream 0.05% improved, but new lesions continued to arise. An exterminator was contacted but found no signs of bedbugs or other infestations. Later, the patient reported seeing 3 tiny black dots crawl across the screen of her cell phone as she read in bed. She was able to capture them on tape and bring them to her appointment. The specimens were approximately 1 mm in length (bottom).
What’s in a White Coat? The Changing Trends in Physician Attire and What it Means for Dermatology
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
The White Coat Ceremony is an enduring memory from my medical school years. Amidst the tumult of memories of seemingly endless sleepless nights spent in libraries and cramming for clerkship examinations between surgical cases, I recall a sunny spring day in 2016 where I gathered with my classmates, family, and friends in the medical school campus courtyard. There were several short, mostly forgotten speeches after which proud fathers and mothers, partners, or siblings slipped the all-important white coat onto the shoulders of the physicians-to-be. At that moment, I felt the weight of tradition centuries in the making resting on my shoulders. Of course, the pomp of the ceremony might have felt a tad overblown had I known that the whole thing had fewer years under its belt than the movie Die Hard.
That’s right, the first White Coat Ceremony was held 5 years after the release of that Bruce Willis classic. Dr. Arnold Gold, a pediatric neurologist on faculty at Columbia University, conceived the ceremony in 1993, and it spread rapidly to medical schools—and later nursing schools—across the United States.1 Although the values highlighted by the White Coat Ceremony—humanism and compassion in medicine—are timeless, the ceremony itself is a more modern undertaking. What, then, of the white coat itself? Is it the timeless symbol of doctoring—of medicine—that we all presume it to be? Or is it a symbol of modern marketing, just a trend that caught on? And is it encountering its twilight—as trends often do—in the face of changing fashion and, more fundamentally, in changes to who our physicians are and to their roles in our society?
The Cleanliness of the White Coat
Until the end of the 19th century, physicians in the Western world most frequently dressed in black formal wear. The rationale behind this attire seems to have been twofold. First, society as a whole perceived the physician’s work as a serious and formal matter, and any medical encounter had to reflect the gravity of the occasion. Additionally, physicians’ visits often were a portent of impending demise, as physicians in the era prior to antibiotics and antisepsis frequently had little to offer their patients outside of—at best—anecdotal treatments and—at worst—sheer quackery.2 Black may have seemed a respectful choice for patients who likely faced dire outcomes regardless of the treatment afforded.3
With the turn of the century came a new understanding of the concepts of antisepsis and disease transmission. While Joseph Lister first published on the use of antisepsis in 1867, his practices did not become commonplace until the early 1900s.4 Around the same time came the Flexner report,5 the publication of William Osler’s Principles and Practice of Medicine,6 and the establishment of the modern medical residency, all of which contributed to the shift from the patient’s own bedside and to the hospital as the house of medicine, with cleanliness and antisepsis as part of its core principles.7 The white coat arose as a symbol of purity and freedom from disease. Throughout the 20th century and into the 21st, it has remained the predominant symbol of cleanliness and professionalism for the medical practitioner.
Patient Preference of Physician Attire
Although the white coat may serve as a professional symbol and is well respected medicine, it also plays an important role in the layperson’s perception of their health care providers.8 There is little denying that patients prefer their physicians, almost uniformly, to wear a white coat. A systematic review of physician attire that included 30 studies mainly from North America, Europe, and the United Kingdom found that patient preference for formal attire and white coats is near universal.9 Patients routinely rate physicians wearing a white coat as more intelligent and trustworthy and feel more confident in the care they will receive.10-13 They also freely admit that a physician’s appearance influences their satisfaction with their care.14 The recent adoption of the fleece, or softshell, jacket has not yet pervaded patients’ perceptions of what is considered appropriate physician attire. A 500-respondent survey found that patients were more likely to rate a model wearing a white coat as more professional and experienced compared to the same model wearing a fleece or softshell jacket or other formal attire sans white coat.15
Closer examination of the same data, however, reveals results reproduced with startling consistency across several studies, which suggest those of us adopting other attire need not dig those white coats out of the closet just yet. First, while many studies point to patient preference for white coats, this preference is uniformly strongest in older patients, beginning around age 40 years and becoming an entrenched preference in those older than 65 years.9,14,16-18 On the other hand, younger patient populations display little to no such preference, and some studies indicate that younger patients actually prefer scrubs over formal attire in specific settings such as surgical offices, procedural spaces, or the emergency department.12,14,19 This suggests that bias in favor of traditional physician garb may be more linked to age demographics and may continue to shift as the overall population ages. Additionally, although patients might profess a strong preference for physician attire in theory, it often does not translate into any impact on the patient’s perception of the physician following a clinic visit. The large systematic review on the topic noted that only 25% of studies that surveyed patients about a clinical visit following the encounter reported that physician attire influenced their satisfaction with that visit, suggesting that attire may be less likely to influence patients in the real-world context of receiving care.9 In fact, a prospective study of patient perception of medical staff and interactions found that staff style of dress not only had no bearing on the perception of staff or visit satisfaction but that patients often failed to even accurately recall physician attire when surveyed.20 Another survey study echoed these conclusions, finding that physician attire had no effect on the perception of a proposed treatment plan.21
What do we know about patient perception of physician attire in the dermatology setting specifically, where visits can be unique in their tendency to transition from medical to procedural in the span of a 15-minute encounter depending on the patient’s chief concern? A survey study of dermatology patients at the general, surgical, and wound care dermatology clinics of an academic medical center (Miami, Florida) found that professional attire with a white coat was strongly preferred across a litany of scenarios assessing many aspects of dermatologic care.21 Similarly, a study of patients visiting a single institution’s dermatology and pediatric dermatology clinics surveyed patients and parents regarding attire prior to an appointment and specifically asked if a white coat should be worn.13 Fifty-four percent of the adult patients (n=176) surveyed professed a preference for physicians in white coats, with a stronger preference for white coats reported by those 50 years and older (55%; n=113). Parents or guardians presenting to the pediatric dermatology clinic, on the other hand, favored less formal attire.13 A recent, real-world study performed at an outpatient dermatology clinic examined the influence of changing physician attire on a patient’s perceptions of care received during clinic encounters. They found no substantial difference in patient satisfaction scores before and following the adoption of a new clinic uniform that transitioned from formal attire to fitted scrubs.22
Racial and Gender Bias Affecting Attire Preference
With any study of preference, there is the underlying concern over respondent bias. Many of the studies discussed here have found secondarily that a patient’s implicit bias does not end at the clothes their physician is wearing. The survey study of dermatology patients from the academic medical center in Miami, Florida, found that patients preferred that Black physicians of either sex be garbed in professional attire at all times but generally were more accepting of White physicians in less formal attire.21 Adamson et al23 published a response to the study’s findings urging dermatologists to recognize that a physician’s race and gender influence patients’ perceptions in much the same way that physician attire seems to and encouraged the development of a more diverse dermatologic workforce to help combat this prejudice. The issue of bias is not limited to the specialty of dermatology; the recent survey study by Xun et al15 found that respondents consistently rated female models garbed in physician attire as less professional than male model counterparts. Additionally, female models wearing white coats were mistakenly identified as medical technicians, physician assistants, or nurses with substantially more frequency than males, despite being clothed in the traditional physician garb. Several other publications on the subject have uncovered implicit bias, though it is rarely, if ever, the principle focus of the study.10,24,25 As is unfortunately true in many professions, female physicians and physicians from ethnic minorities face barriers to being perceived as fully competent physicians.
Impact of the COVID-19 Pandemic
Finally, of course, there is the ever-present question of the effect of the pandemic. Although the exact role of the white coat as a fomite for infection—and especially for the spread of viral illness—remains controversial, the perception nonetheless has helped catalyze the movement to alternatives such as short-sleeved white coats, technical jackets, and more recently, fitted scrubs.26-29 As with much in this realm, facts seem less important than perceptions; Zahrina et al30 found that when patients were presented with information regarding the risk for microbial contamination associated with white coats, preference for physicians in professional garb plummeted from 72% to only 22%. To date no articles have examined patient perceptions of the white coat in the context of microbial transmission in the age of COVID-19, but future articles on this topic are likely and may serve to further the demise of the white coat.
Final Thoughts
From my vantage point, it seems the white coat will be claimed by the outgoing tide. During this most recent residency interview season, I do not recall a single medical student wearing a short white coat. The closest I came was a quick glimpse of a crumpled white jacket slung over an arm or stuffed in a shoulder bag. Rotating interns and residents from other services on rotation in our department present in softshell or fleece jackets. Fitted scrubs in the newest trendy colors speckle a previously all-white canvas. I, for one, have not donned my own white coat in at least a year, and perhaps it is all for the best. Physician attire is one small aspect of the practice of medicine and likely bears little, if any, relation to the wearer’s qualifications. Our focus should be on building rapport with our patients, providing high-quality care, reducing the risk for nosocomial infection, and developing a health care system that is fair and equitable for patients and health care workers alike, not on who is wearing what. Perhaps the introduction of new physician attire is a small part of the disruption we need to help address persistent gender and racial biases in our field and help shepherd our patients and colleagues to a worldview that is more open and accepting of physicians of diverse backgrounds.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
- White Coat Ceremony. Gold Foundation website. Accessed December 26, 2021. https://www.gold-foundation.org/programs/white-coat-ceremony/
- Shryock RH. The Development of Modern Medicine. University of Pennsylvania Press; 2017.
- Hochberg MS. The doctor’s white coat—an historical perspective. Virtual Mentor. 2007;9:310-314.
- Lister J. On the antiseptic principle in the practice of surgery. Lancet. 1867;90:353-356.
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching. Carnegie Foundation for the Advancement of Teaching; 1910.
- Osler W. Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine. D. Appleton & Company; 1892.
- Blumhagen DW. The doctor’s white coat: the image of the physician in modern America. Ann Intern Med. 1979;91:111-116.
- Verghese BG, Kashinath SK, Jadhav N, et al. Physician attire: physicians’ perspectives on attire in a community hospital setting among non-surgical specialties. J Community Hosp Intern Med Perspect. 2020;10:1-5.
- Petrilli CM, Mack M, Petrilli JJ, et al. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5:E006678.
- Rehman SU, Nietert PJ, Cope DW, et al. What to wear today? effect of doctor’s attire on the trust and confidence of patients. Am J Med. 2005;118:1279-1286.
- Jennings JD, Ciaravino SG, Ramsey FV, et al. Physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474:1908-1918.
- Gherardi G, Cameron J, West A, et al. Are we dressed to impress? a descriptive survey assessing patients preference of doctors’ attire in the hospital setting. Clin Med (Lond). 2009;9:519-524.
- Thomas MW, Burkhart CN, Lugo-Somolinos A, et al. Patients’ perceptions of physician attire in dermatology clinics. Arch Dermatol. 2011;147:505-506.
- Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8:E021239.
- Xun H, Chen J, Sun AH, et al. Public perceptions of physician attire and professionalism in the US. JAMA Network Open. 2021;4:E2117779.
- Kamata K, Kuriyama A, Chopra V, et al. Patient preferences for physician attire: a multicenter study in Japan [published online February 11, 2020]. J Hosp Med. 2020;15:204-210.
- Budny AM, Rogers LC, Mandracchia VJ, et al. The physician’s attire and its influence on patient confidence. J Am Podiatr Assoc. 2006;96:132-138.
- Lill MM, Wilkinson TJ. Judging a book by its cover: descriptive survey of patients’ preferences for doctors’ appearance and mode of address. Br Med J. 2005;331:1524-1527.
- Hossler EW, Shipp D, Palmer M, et al. Impact of provider attire on patient satisfaction in an outpatient dermatology clinic. Cutis. 2018;102:127-129.
- Boon D, Wardrope J. What should doctors wear in the accident and emergency department? patients’ perception. J Accid Emerg Med. 1994;11:175-177.
- Fox JD, Prado G, Baquerizo Nole KL, et al. Patient preference in dermatologist attire in the medical, surgical, and wound care settings. JAMA Dermatol. 2016;152:913-919.
- Bray JK, Porter C, Feldman SR. The effect of physician appearance on patient perceptions of treatment plans. Dermatol Online J. 2021;27. doi:10.5070/D327553611
- Adamson AS, Wright SW, Pandya AG. A missed opportunity to discuss racial and gender bias in dermatology. JAMA Dermatol. 2017;153:110-111.
- Hartmans C, Heremans S, Lagrain M, et al. The doctor’s new clothes: professional or fashionable? Primary Health Care. 2013;3:135.
- Kurihara H, Maeno T, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13:2.
- Treakle AM, Thom KA, Furuno JP, et al. Bacterial contamination of health care workers’ white coats. Am J Infect Control. 2009;37:101-105.
- Banu A, Anand M, Nagi N, et al. White coats as a vehicle for bacterial dissemination. J Clin Diagn Res. 2012;6:1381-1384.
- Haun N, Hooper-Lane C, Safdar N. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol. 2016;37:1367-1373.
- Tse G, Withey S, Yeo JM, et al. Bare below the elbows: was the target the white coat? J Hosp Infect. 2015;91:299-301.
- Zahrina AZ, Haymond P, Rosanna P, et al. Does the attire of a primary care physician affect patients’ perceptions and their levels of trust in the doctor? Malays Fam Physician. 2018;13:3-11.
Resident Pearls
- Until the end of the 19th century, Western physicians most commonly wore black formal wear. The rise of the physician’s white coat occurred in conjunction with the shift to hospital medicine.
- Patient surveys repeatedly have demonstrated a preference for physicians to wear white coats; whether or not this has any bearing on patient satisfaction in real-world scenarios is less clear.
- The impact of the COVID-19 pandemic on trends in white coat wear has not yet been elucidated.
Blisters in a Comatose Elderly Woman
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
An 82-year-old woman presented to the emergency department after her daughter found her unconscious in the bathroom laying on her right side. Her medical history was notable for hypertension and asthma for which she was on losartan, furosemide, diltiazem, and albuterol. She recently had been prescribed lorazepam for insomnia and had started taking the medication 2 days prior. She underwent intubation and was noted to have flaccid, fluid-filled bullae on the right thigh (top) along with large areas of desquamation on the right lateral arm (bottom) with minimal surrounding erythema. There was no mucous membrane involvement. Urine toxicology was positive for benzodiazepines and negative for all other drugs, including barbiturates.
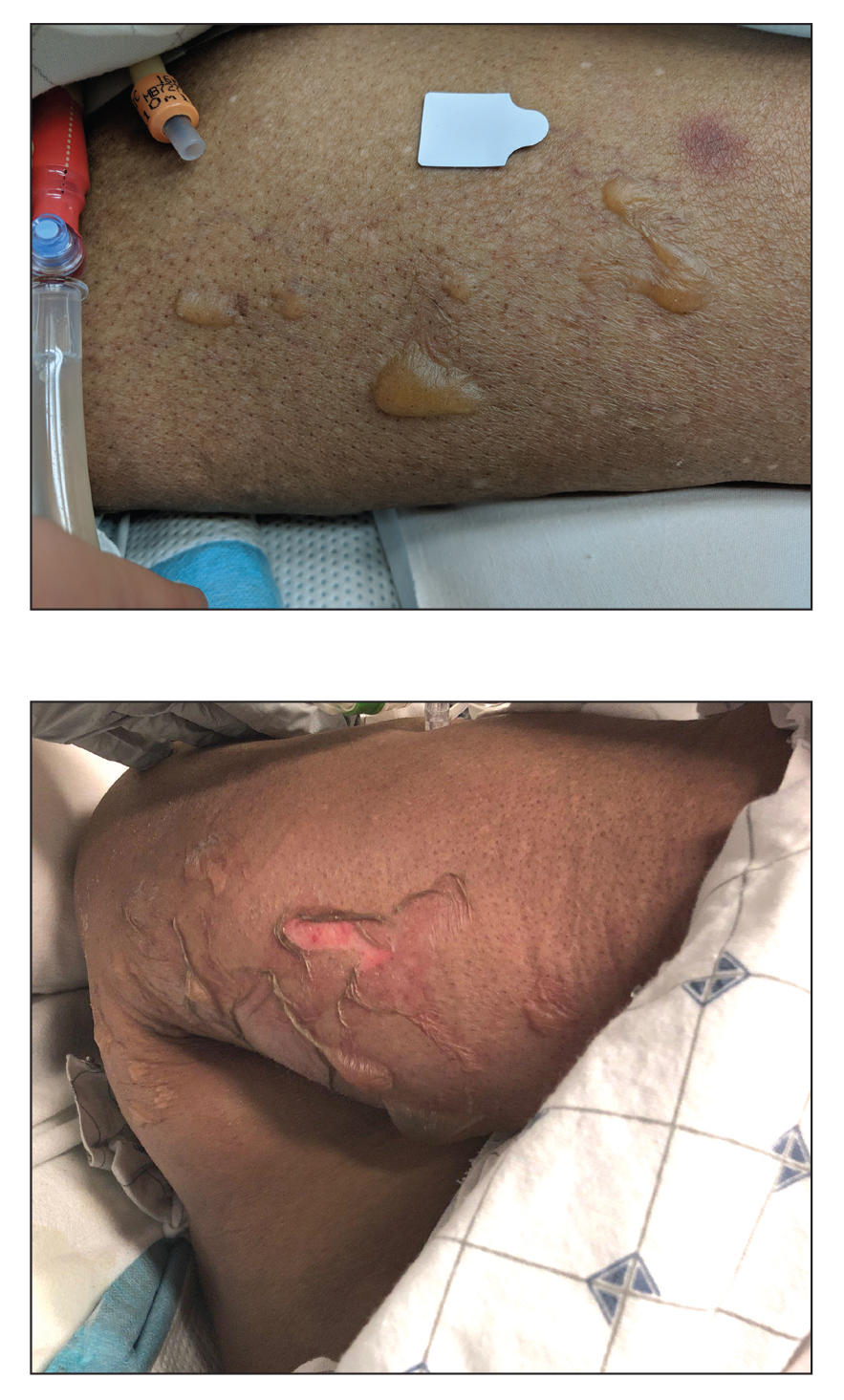
Enlarging Nodule on the Back
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.
Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7
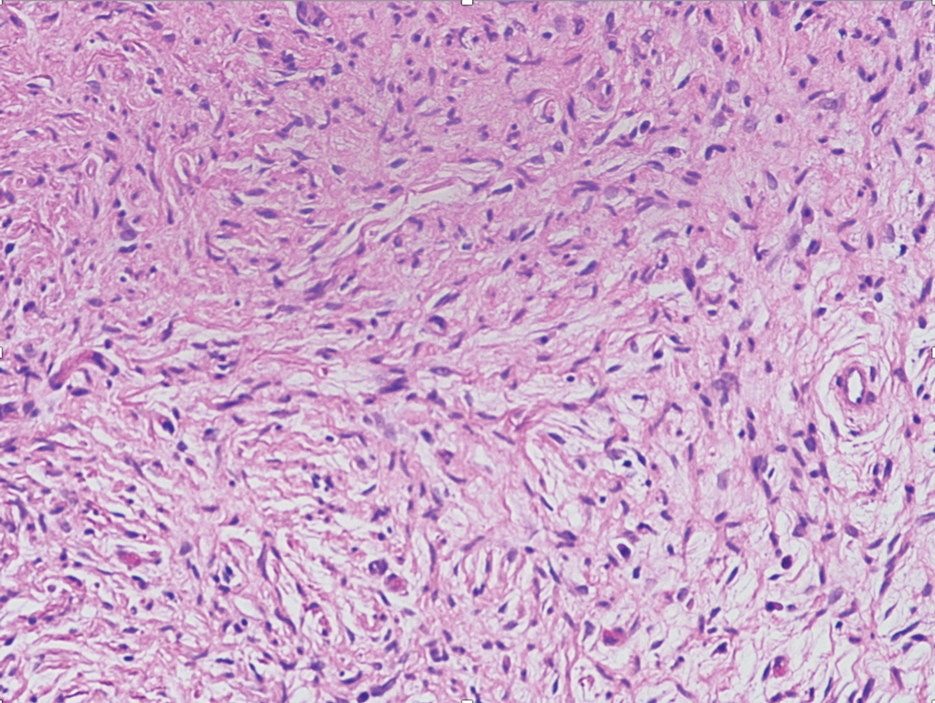
Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8
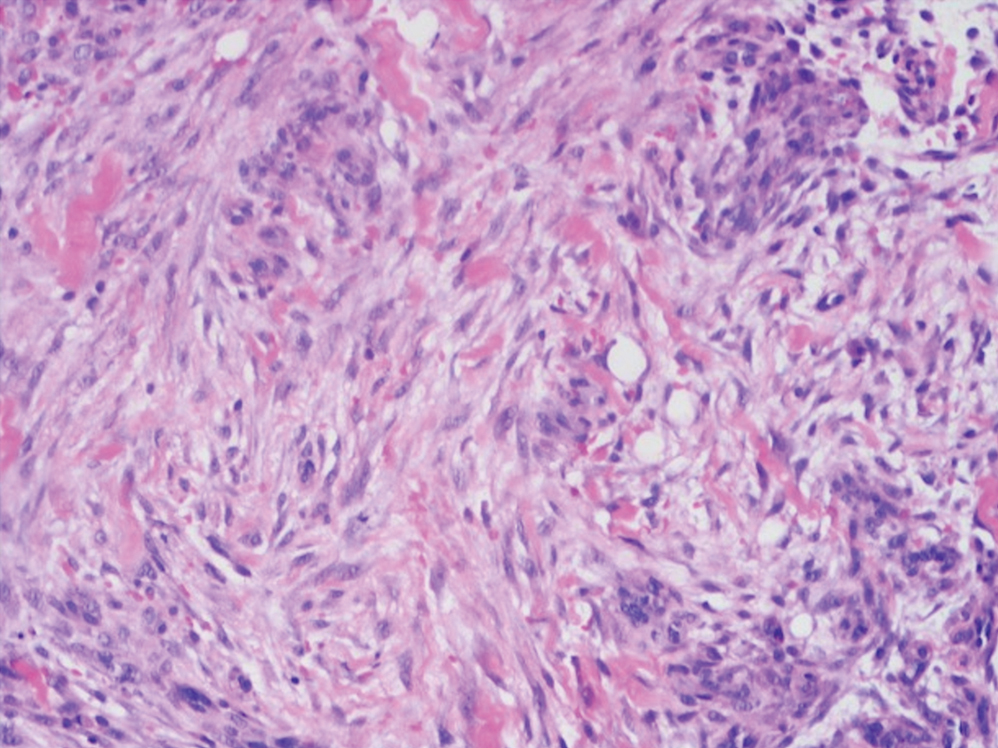
Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4
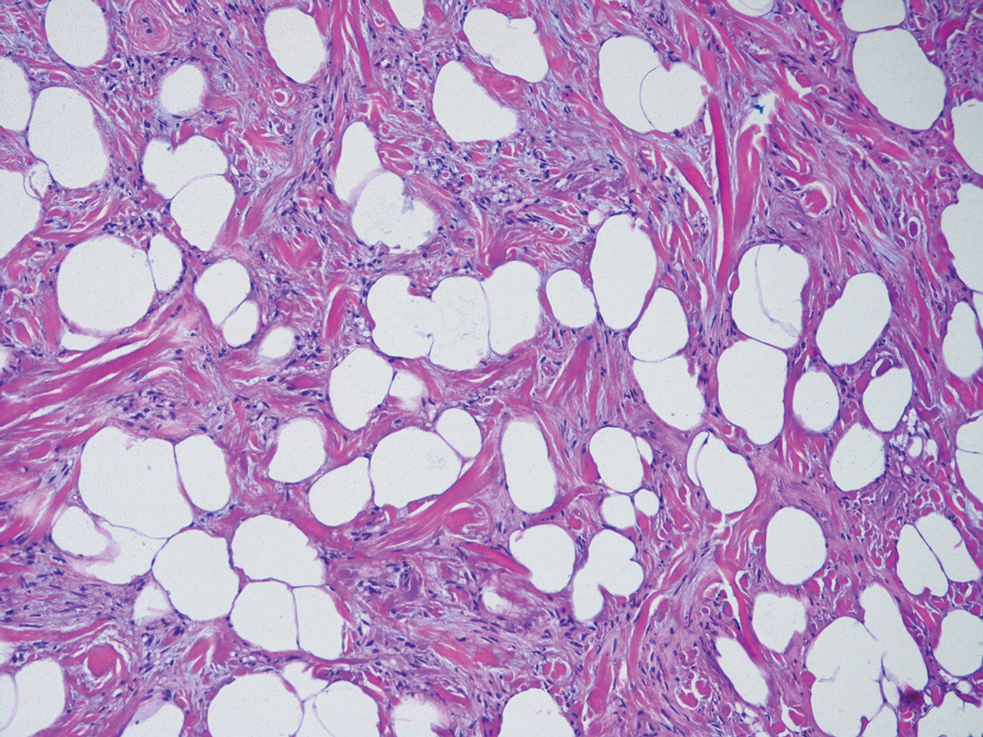
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.

Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7

Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8

Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4

The Diagnosis: Cutaneous Myxoma
Microscopic analysis showed features of cutaneous myxoma (quiz images). The epidermis was essentially unremarkable. Stellate to spindle cells with bland nuclear chromatin were present in the dermis with abundant pools of myxoid stroma. Colloidal iron staining highlighted the markedly increased dermal mucin.
Cutaneous myxomas (also referred to as superficial angiomyxomas) are rare, well-demarcated tumors of the dermis and subcutis.1,2 They can present as solitary, fleshcolored nodules on the trunk, lower extremities, head, or neck, and they often measure between 1 and 5 cm.2,3 Histologically, cutaneous myxomas are hypocellular with some stellate fibroblasts, occasional epithelial structures, and an abundant myxoid stroma, with notable thinwalled small blood vessels.2,4 These lesions contain pools of mucin and are positive for mesenchymal mucin stains such as colloidal iron and Alcian blue.1 Moreover, perivascular neutrophils are a distinguishing characteristic of cutaneous myxomas.4
Multiple cutaneous myxomas should raise concern for Carney complex,1,5 a genodermatologic syndrome that arises due to a mutation in the protein kinase CAMP-dependent type I regulatory subunit alpha gene, PRKAR1A, on chromosome 2.1,5 Additional cutaneous manifestations include blue nevi, lentigines, and café-aulait macules.5 Carney complex also is known for endocrine overactivity and cardiac myxomas, which can cause serious embolic complications.1
Recommended management is complete excision with close follow-up, as these lesions may recur in up to one-third of cases. Although there is a potential for recurrence, metastases are uncommon.3 Even without recurrence in the presenting location, follow-up should include screening for manifestations of Carney complex.1,3
The clinical and histological differential for cutaneous myxoma may include nerve sheath myxoma or neurofibroma. A nerve sheath myxoma is a dermal tumor that manifests as a solitary, flesh-colored nodule, measuring less than 2 cm. These lesions commonly present on the head, neck, and upper body.6 Cutaneous myxomas can grow larger than 2 cm, but these two lesions have a great deal of overlap in their other features.3,6 Thus, histology can be used to distinguish them.

Nerve sheath myxomas are circumscribed nonencapsulated tumors of the dermis composed of multilobular aggregates of spindle to epithelioid cells in a mucinous matrix (Figure 1). Clefts often are present around the cell aggregates. Despite previously being termed myxoid neurothekeomas, nerve sheath myxomas are S-100 positive, whereas cellular neurothekeomas are S-100 negative and likely not of neural origin. Cutaneous myxomas, in contrast to nerve sheath myxomas, are S-100 negative. Nerve sheath myxomas are more cellular and lack the characteristic mucin pools compared with cutaneous myxomas.1,2,6 Neurofibromas frequently are flesh colored and pedunculated, as was the lesion in our patient, yet they are vastly different microscopically. The stroma of neurofibromas can vary, but cellularity typically is greater than a cutaneous myxoma and consists of increased numbers of bland spindle cells with wavy nuclei (Schwann cells) and fibrillar cytoplasm as well as mast cells and fibroblasts (Figure 2). Neurofibromas stain positively for S-100 and SOX-10 (Sry-related HMg-box 10).2,7 In addition to café-au-lait macules, axillary freckling, optic gliomas, and positive family history, neurofibromas are associated with neurofibromatosis type 1, which is linked to a defect in a tumor suppressor gene that codes for neurofibromin.7

Nodular fasciitis is a self-limited myofibroblastic neoplasm that contains fusion genes, with the most common being myosin-9–ubiquitin specific peptidase 6, MYH9-USP6, which leads to overexpression of USP6. Nodular fasciitis presents as a solitary, rapidly enlarging nodule affecting the subcutaneous tissue, muscles, or fascia.8,9 It usually presents in the third or fourth decades of life.8 The arms are the most common location in adults, while the most commonly affected site in children is the head or neck. Histopathology reveals a characteristic tissue culture pattern with a proliferation of plump spindle and stellate fibroblasts as well as myofibroblasts (Figure 3). Early lesions have haphazard spindle cells with a proliferation of small blood vessels and extravasated erythrocytes. Despite increased mitotic figures, cellular atypia is rare. The fibroblasts and myofibroblasts react positively for vimentin and muscle-specific actin.8 This lesion is highly cellular comparatively and notably lacks the perivascular neutrophils and epithelial structures that would be expected in a cutaneous myxoma.4,8

Spindle cell lipomas, solitary subcutaneous masses commonly presenting on the upper back in middle-aged men, also can mimic cutaneous myxomas.4 Histologically, these lesions may contain short bundles of spindle cells arranged in a school of fish–like pattern, mature adipocytes, or myxoid stroma and characteristic CD34 positivity (Figure 4). Spindle cell lipomas often will present with ropey collagen, which can easily distinguish them from cutaneous myxomas.4

- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
- Lanjewar DN, Bhatia VO, Lanjewar SD, et al. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460-462.
- Choi HJ, Kim YJ, Yim JH, et al. Unusual presentation of solitary cutaneous myxoma. J Eur Acad Dermatol Venereol. 2007;21:403-404. doi:10.1111/j.1468-3083.2006.01881.x
- Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59:1-3. doi:10.4103/0019-5154.139893
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918.
- Sarfo A, Helm K, Flamm A. Cutaneous myxomas and a psammomatous melanotic schwannoma in a patient with Carney complex. J Cutan Pathol. 2019;46:93-96. doi:10.1111/cup.13385
- Gill P, Abi Daoud MS. Multiple cellular neurothekeomas in a middleaged woman including the lower extremity: a case report and review of the current literature. J Cutan Pathol. 2019;46:67-73. doi:10.1111/ cup.13366
- Ohgaki H, Kim Y, Steinbach JP. Nervous system tumors associated with familial tumor syndromes. Curr Opin Neurol. 2010;23:583-591. doi:10.1097/WCO.0b013e3283405b5f
- Luna A, Molinari L, Bollea Garlatti LA, et al. Nodular fasciitis, a forgotten entity. Int J Dermatol. 2019;58:190-193. doi:10.1111/ijd.14219
- Patel N, Chrisinger J, Demicco E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol. 2017; 30:1577-1588.
A 43-year-old man with an unremarkable medical history presented to our clinic with an enlarging painful nodule on the upper back that was present for years without bleeding or ulceration. He denied prior treatment or any similar lesions. Physical examination was notable for a 2×1.5-cm, pedunculated, flesh-colored nodule on the left upper back. A shave excision of the lesion was performed.
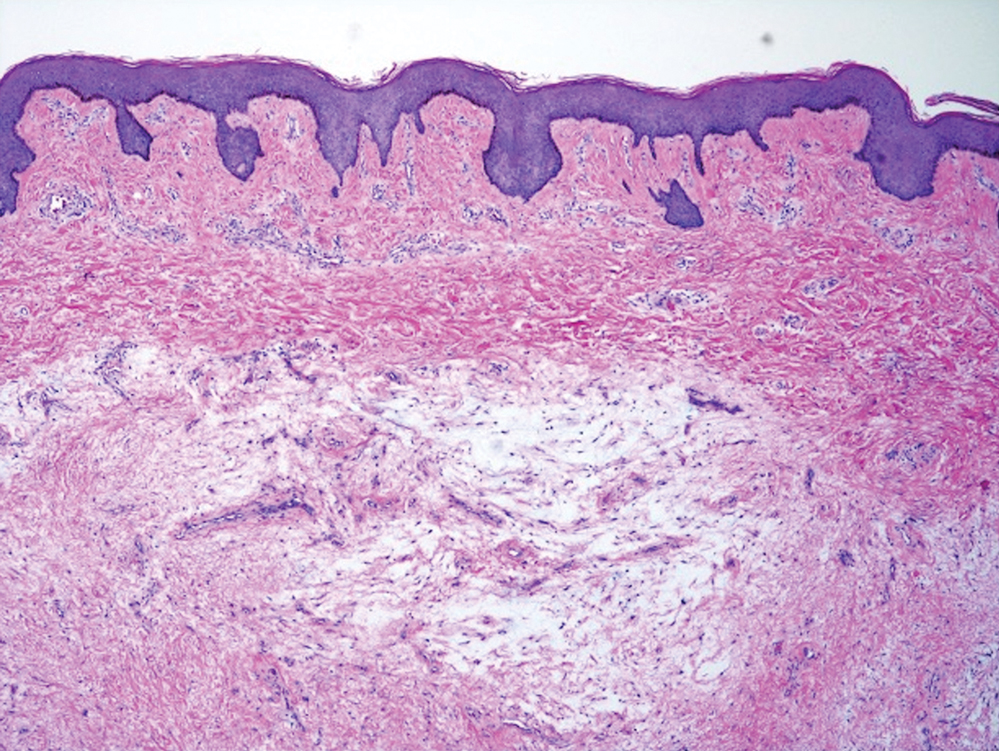
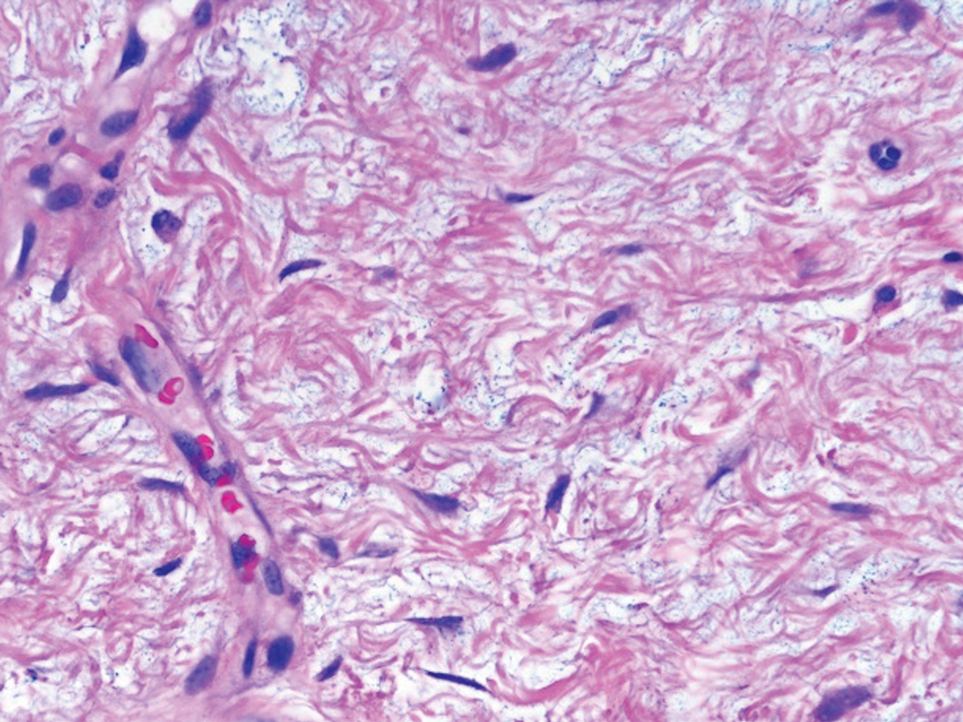
Erythematous Indurated Nodule on the Forehead
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
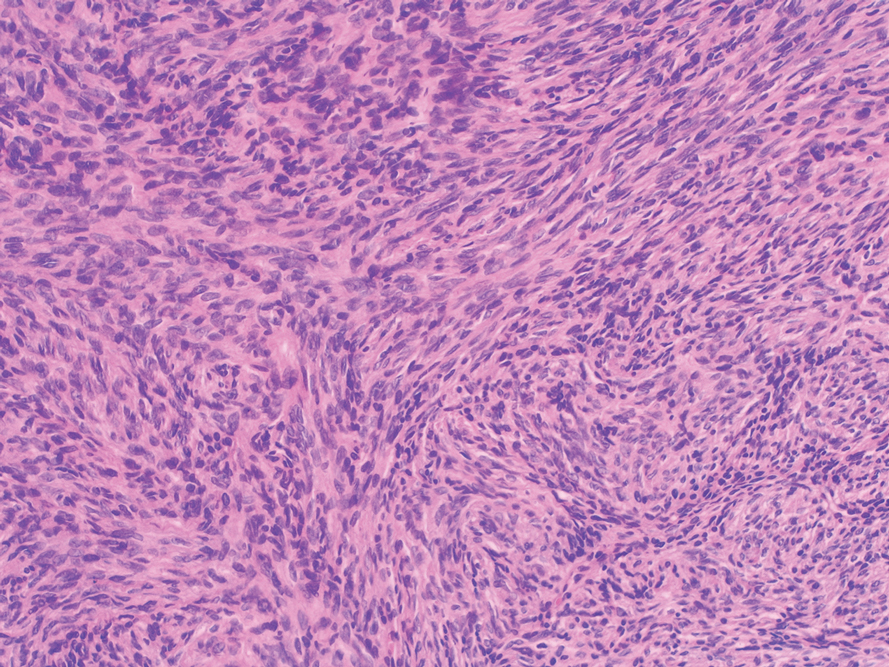
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
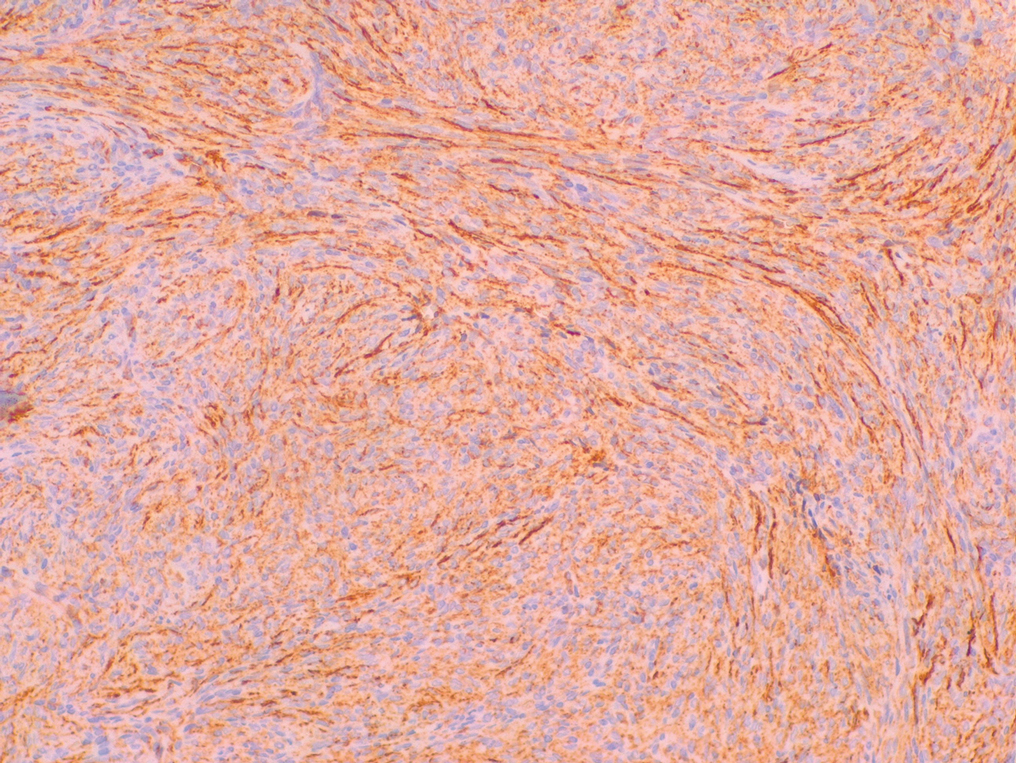
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
A 39-year-old man presented with an enlarging asymptomatic nodule on the forehead of more than 3 years’ duration. Physical examination revealed a 3.4×2.3-cm, indurated, firm, erythematous nodule on the frontotemporal scalp. The patient denied any history of trauma to the area.

Sarcoidosis

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20

THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous. In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjusting for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
- Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
- Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66:121.e1-121.e14.
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/oxfordjournals.aje.a009096
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis if the features in 170 patients. Respir Med. 2003;97:978-982.
- Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
- Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
- Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
- James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
- Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
- Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
- Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
Contact Allergy to Topical Medicaments, Part 2: Steroids, Immunomodulators, and Anesthetics, Oh My!
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
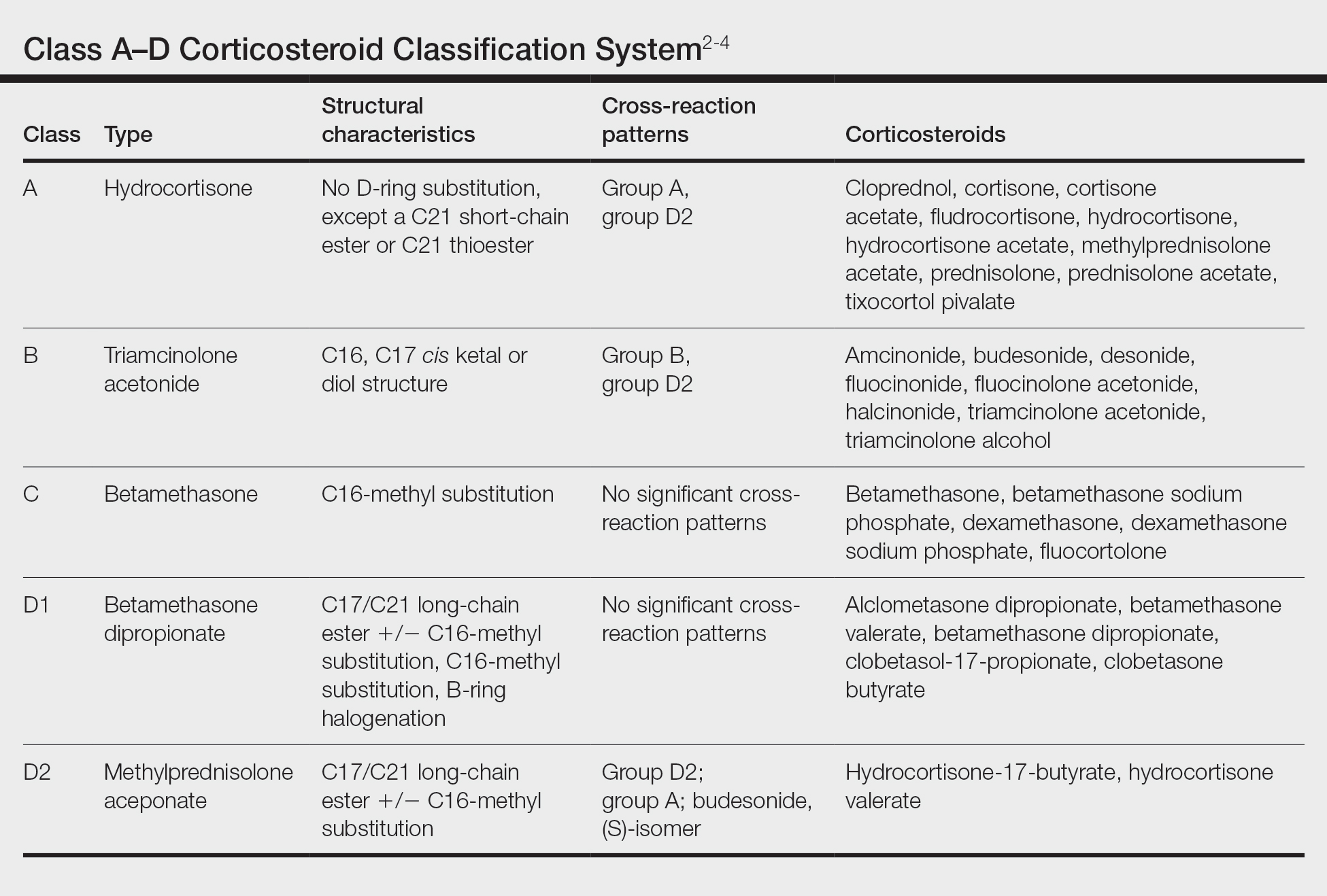
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
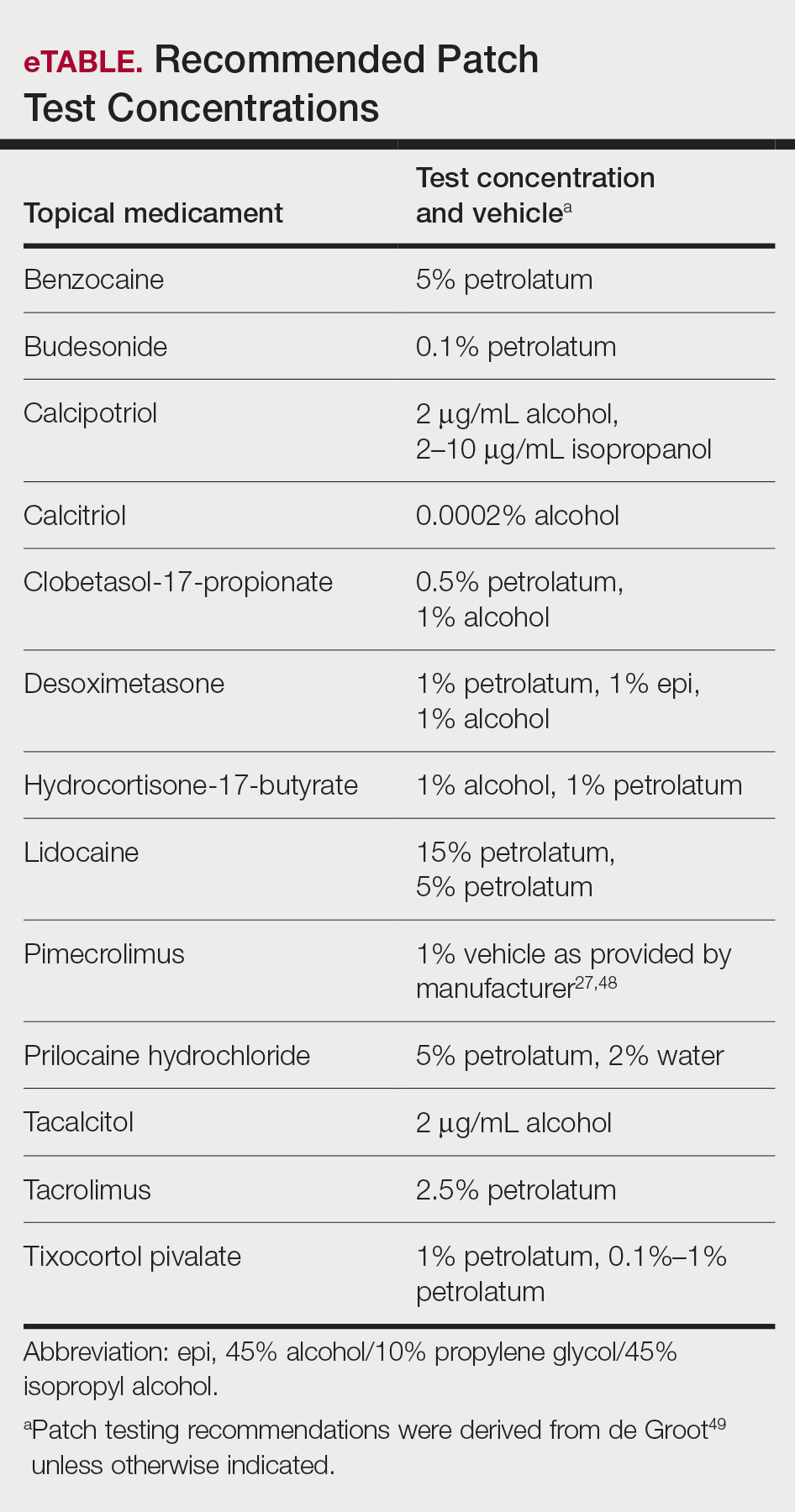
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5

Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.

Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
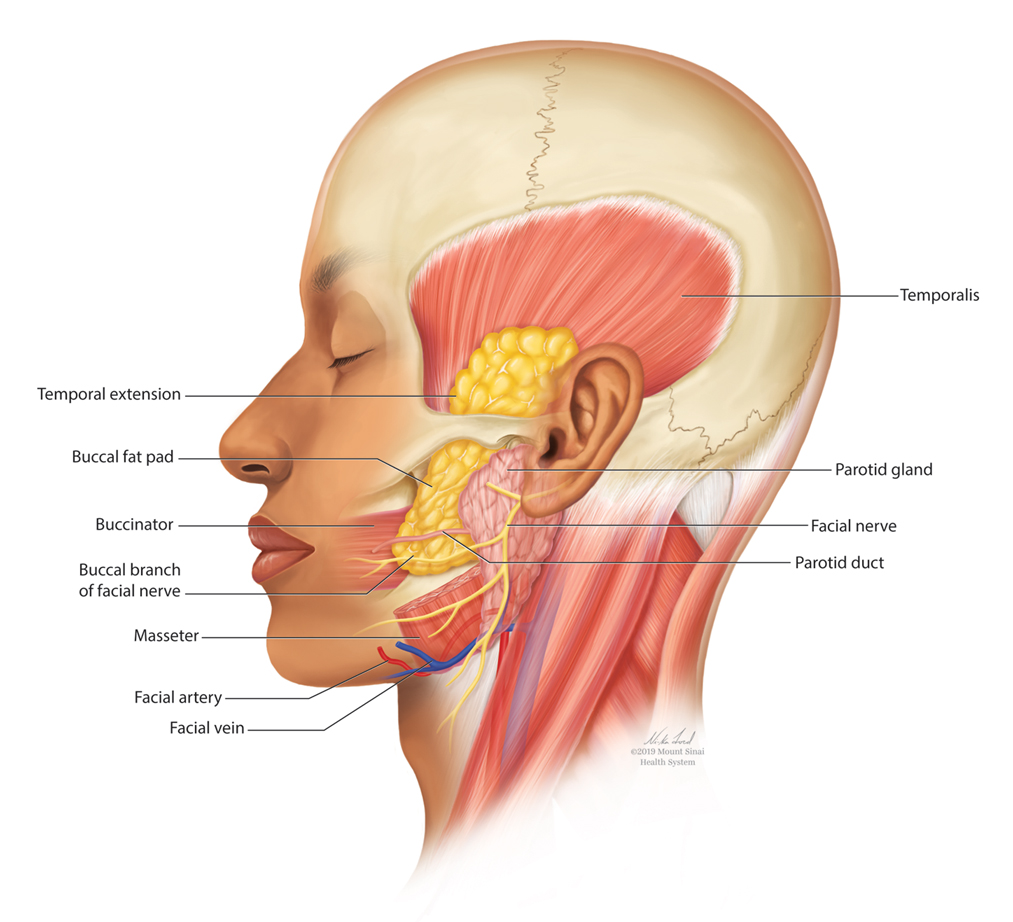
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
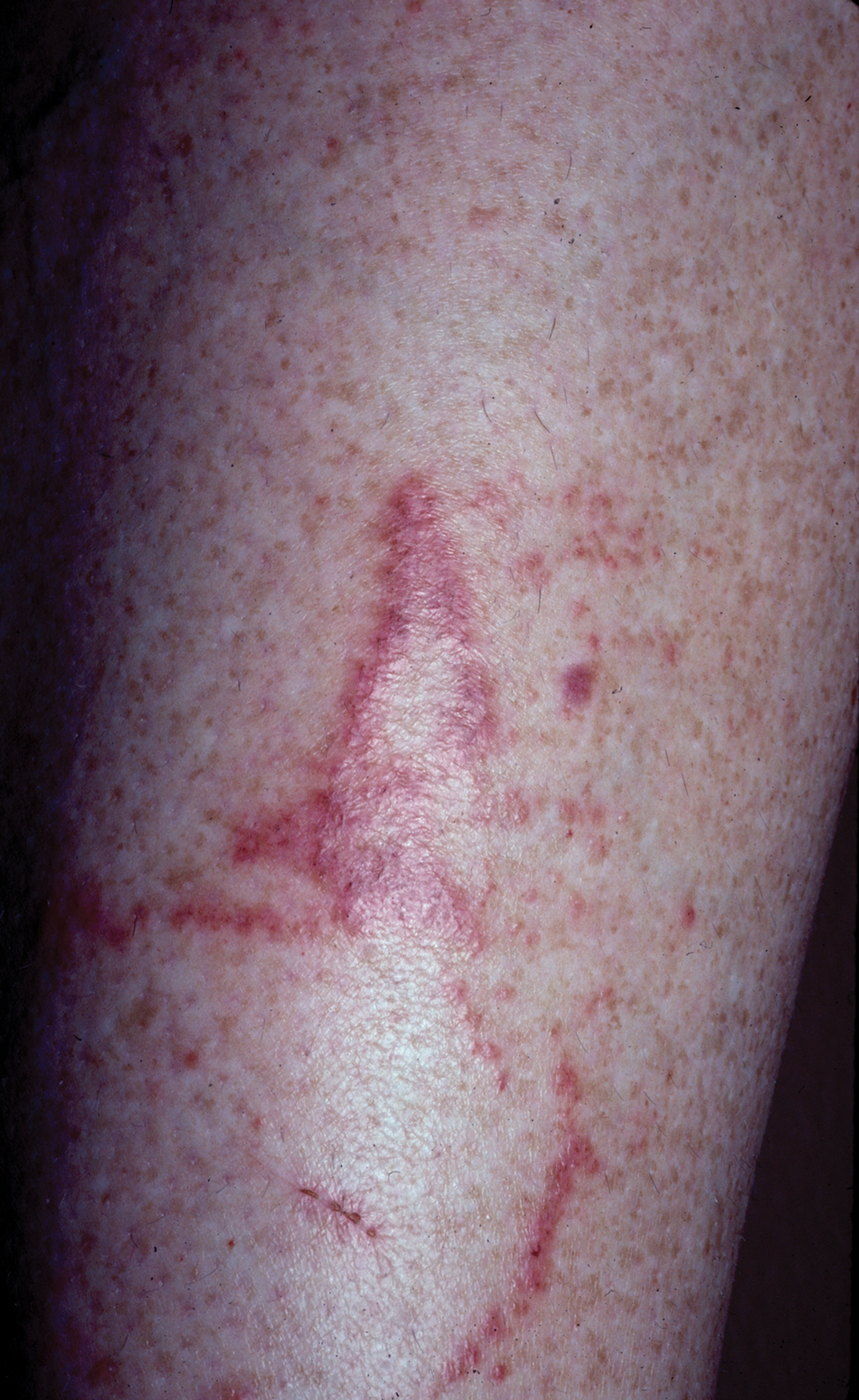
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.