User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Dermatofibrosarcoma Protuberans
To the Editor:
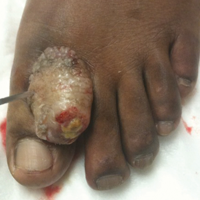
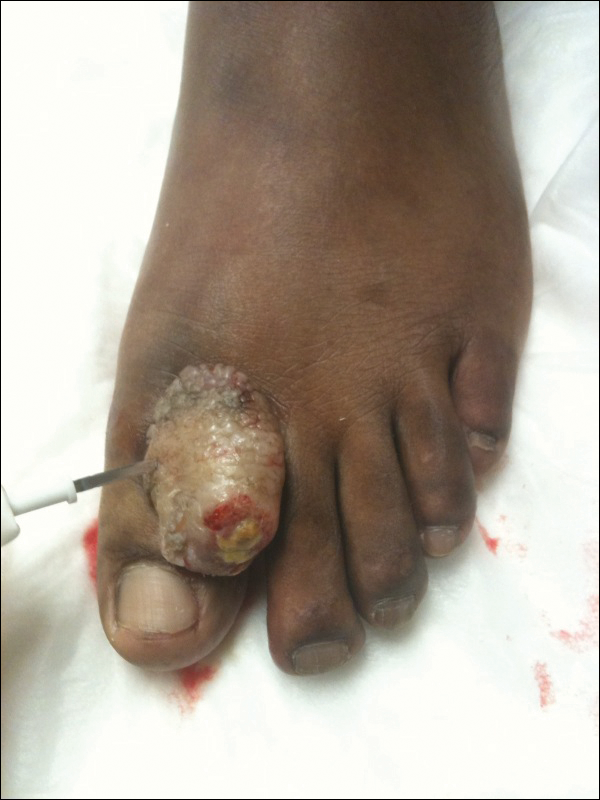
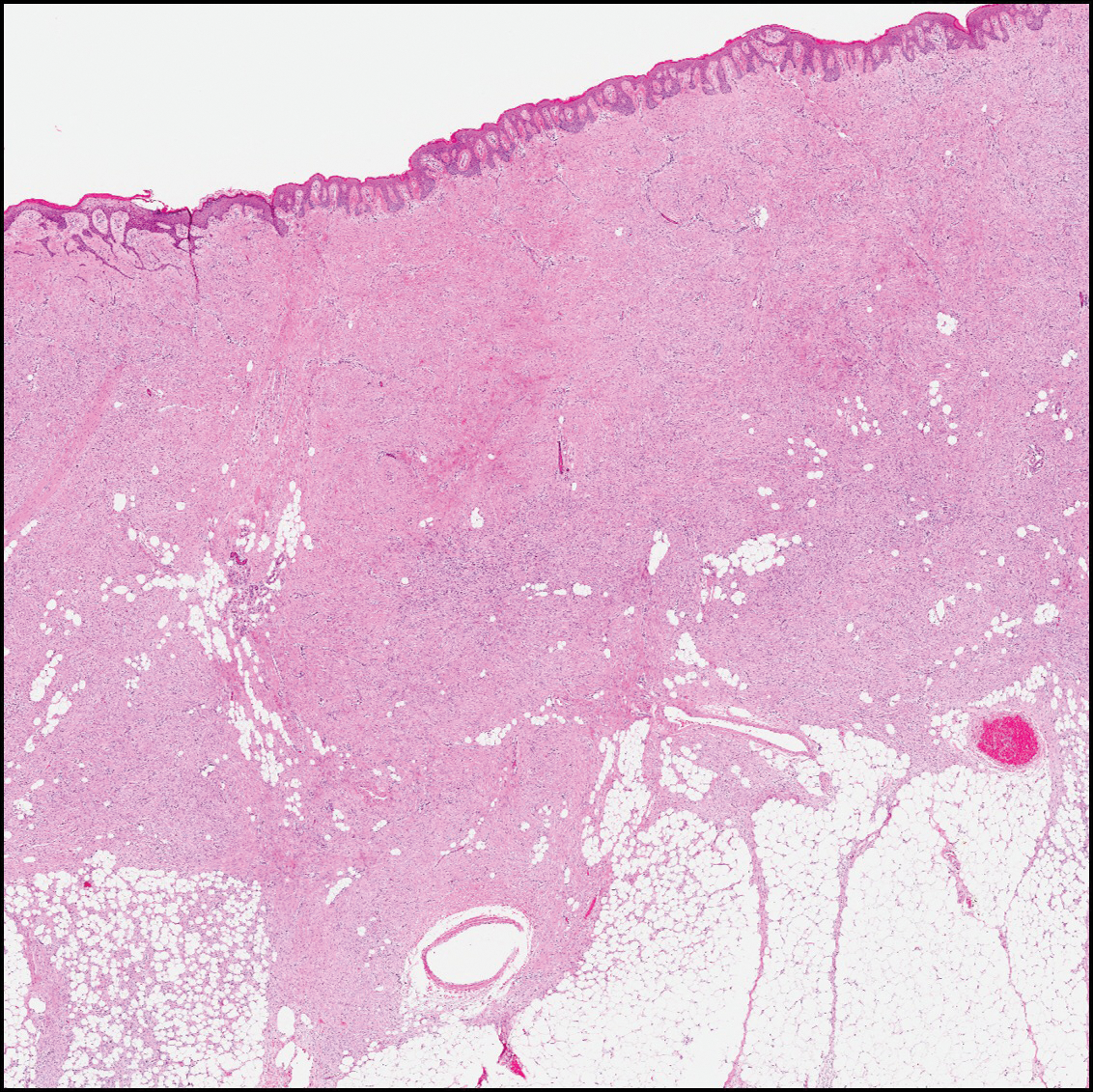
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.
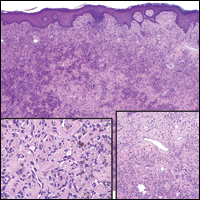
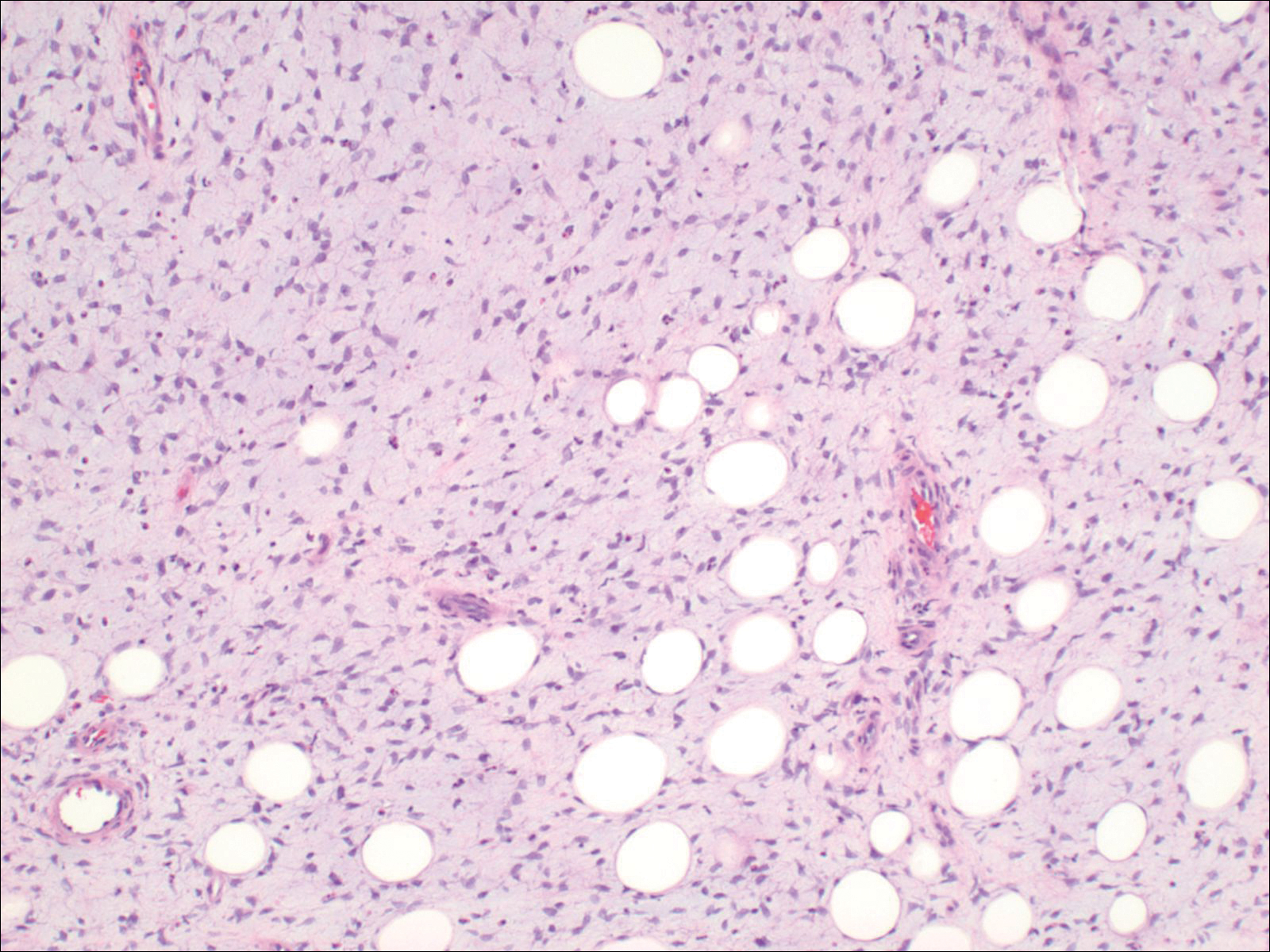
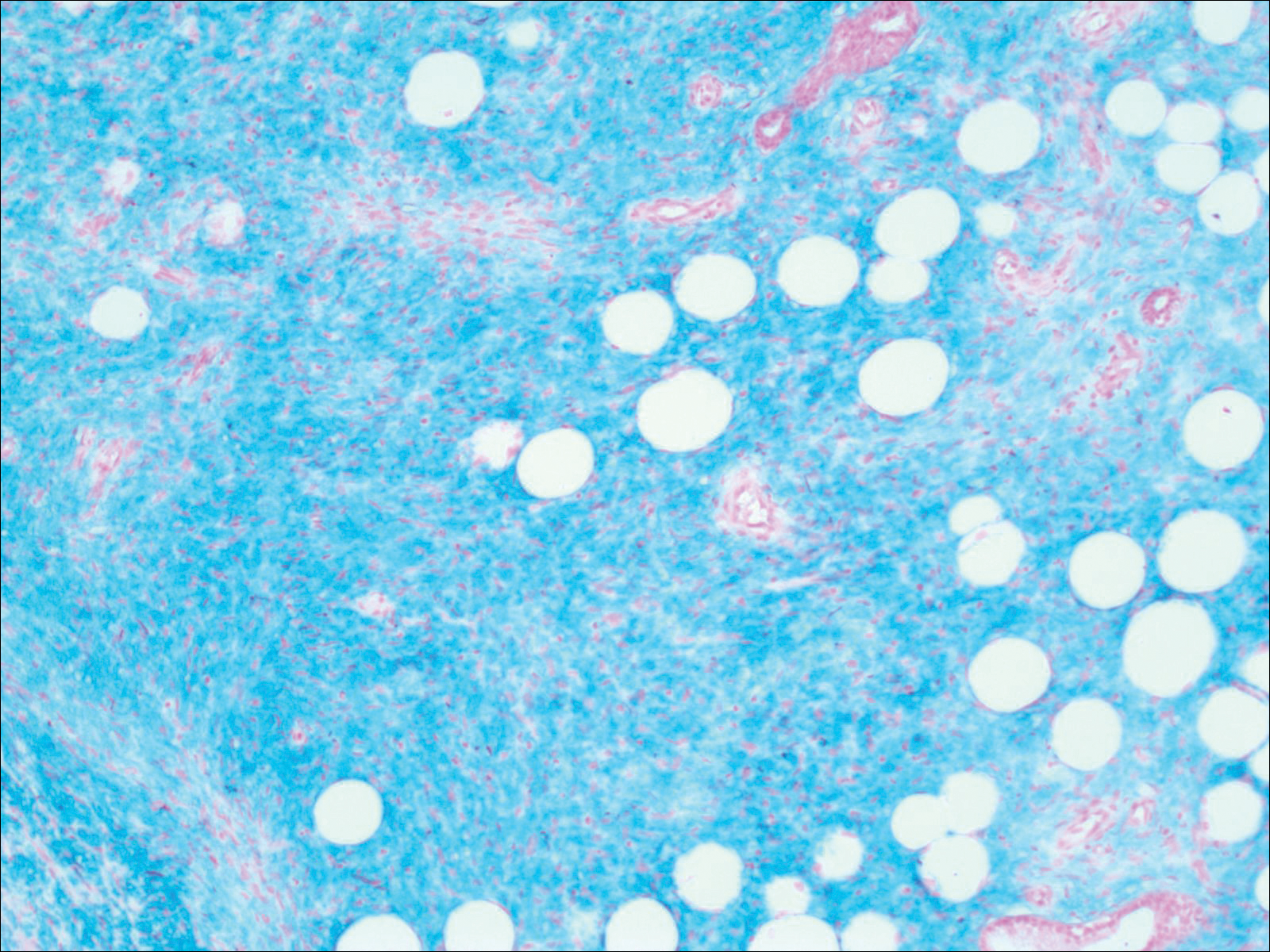
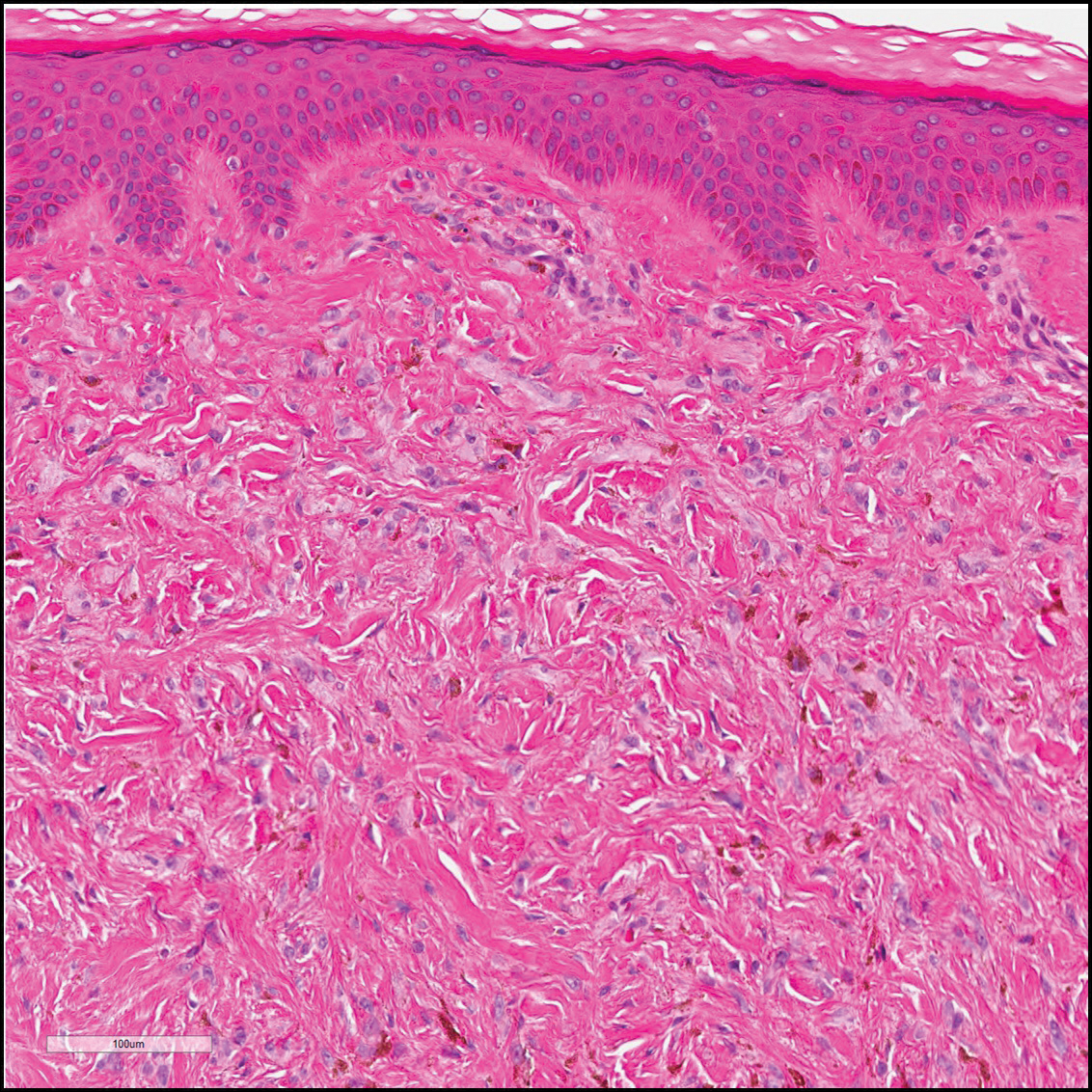
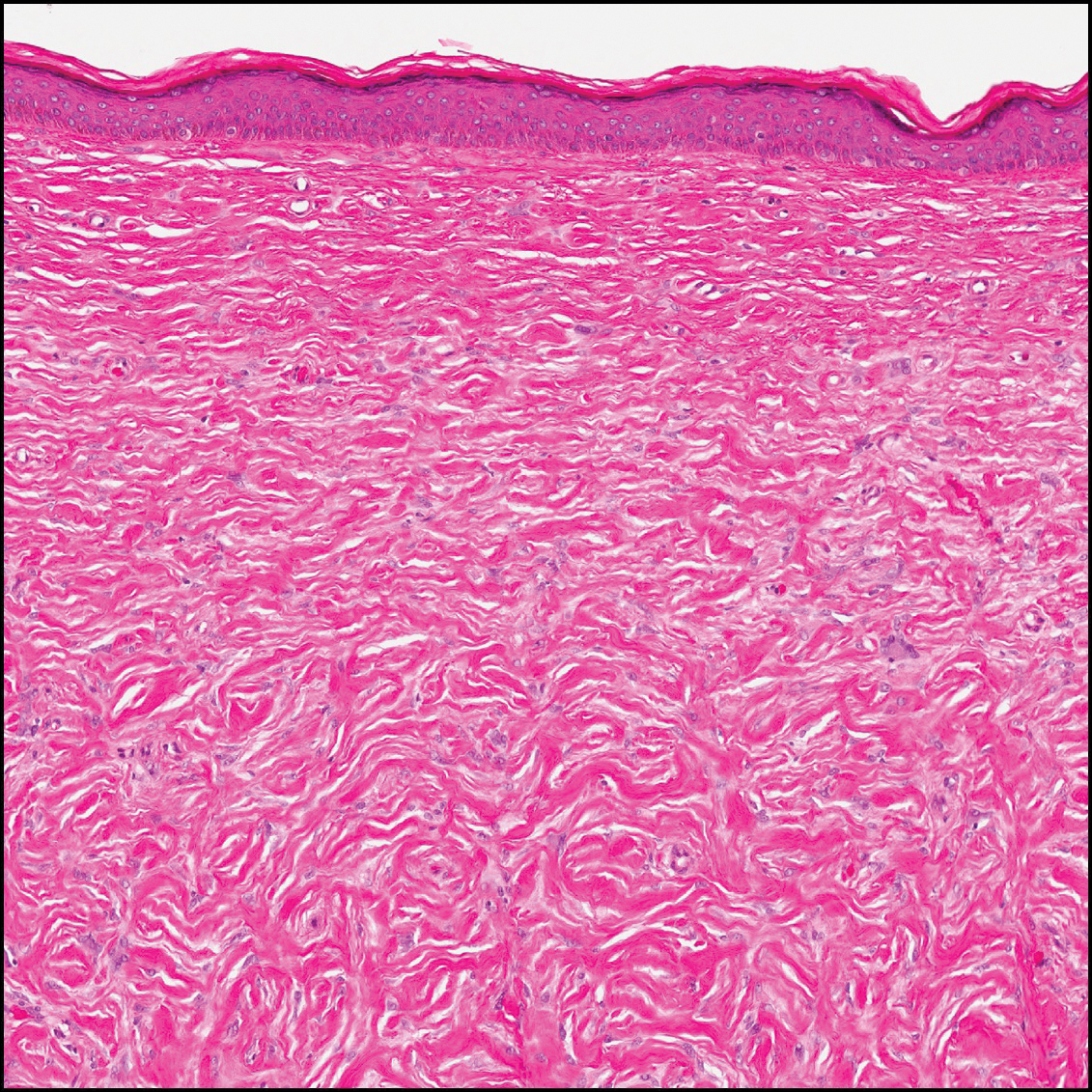
Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.
Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
Practice Points
- Consider dermatofibrosarcoma protuberans for a keloidlike enlarging lesion when there is no history of trauma or prior keloid formation.
- Treatments such as Mohs micrographic surgery or oral imatinib mesylate can provide lower recurrence rates in appropriate patients as stand-alone or adjuvant therapy.
Temporal Triangular Alopecia Acquired in Adulthood
To the Editor:
Temporal triangular alopecia (TTA), a condition first described by Sabouraud1 in 1905, is a circumscribed nonscarring form of alopecia. Also referred to as congenital triangular alopecia, TTA presents as a triangular or lancet-shaped area of hair loss involving the frontotemporal hairline. Temporal triangular alopecia is characterized histologically by a normal number of miniaturized hair follicles without notable inflammation.2 Although the majority of cases arise between birth and 9 years of age,3,4 rare cases of adult-onset TTA also have been reported.5,6 Adult-onset cases can cause notable diagnostic confusion and inappropriate treatment, as reported in our patient.
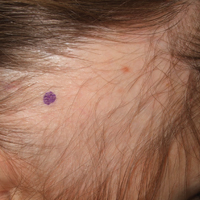
A 25-year-old woman with a history of Hashimoto thyroiditis presented with hair loss affecting the right temporal scalp of 3 years' duration that was first noticed by her husband. The lesion was an asymptomatic, 6×8-cm, roughly lancet-shaped patch of alopecia located on the right temporal scalp, bordering on the frontal hairline (Figure 1). Centrally, the patch appeared almost hairless with a few retained terminal hairs. The frontal hairline was thinned but still present. There was no scaling or erythema, and fine vellus hairs and a few isolated terminal hairs covered the area. The corresponding skin on the contralateral temporal scalp showed normal hair density. The patient insisted that she had normal hair at the affected area until 22 years of age, and she denied a history of trauma or tight hairstyles. Initially diagnosed with alopecia areata by her primary care provider, the patient was treated with topical corticosteroids for 6 months without benefit. She was subsequently referred to a dermatologist who again offered a diagnosis of alopecia areata and treated the lesions with 2 intralesional corticosteroid injections without benefit. No biopsies of the affected area were performed, and the patient was given a trial of topical minoxidil.
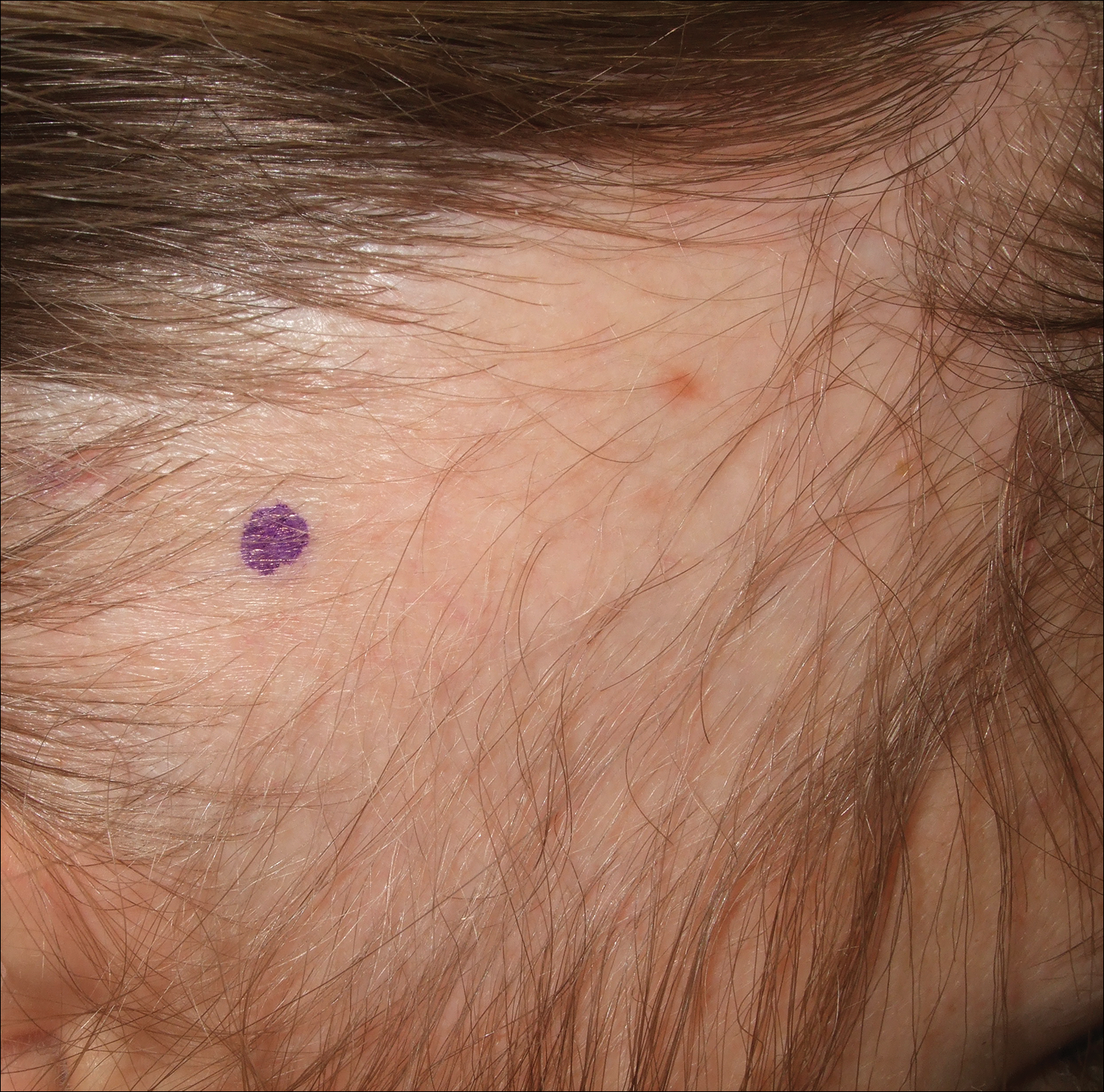
The patient consulted a new primary care provider and was diagnosed with scarring alopecia. She was referred to our dermatology department for further treatment. An initial biopsy at the edge of the affected area was interpreted as normal, but after failing additional intralesional corticosteroid injections, she was referred to our hair clinic where another biopsy was performed in the central portion of the lesion. A 4-mm diameter punch biopsy specimen revealed a normal epidermis and dermis; however, in the lower dermis only a single terminal follicle was seen (Figure 2). Sections through the upper dermis (Figure 3) showed that the total number of hairs was normal or nearly normal with at least 22 follicles, but most were vellus and indeterminate hairs with only a single terminal hair. The dermal architecture was otherwise normal. Given the clinical and histologic findings, a diagnosis of TTA was made. Subsequent to the diagnosis, the patient did not pursue any additional treatment options and preferred to style her hair so that the area of TTA remained covered.
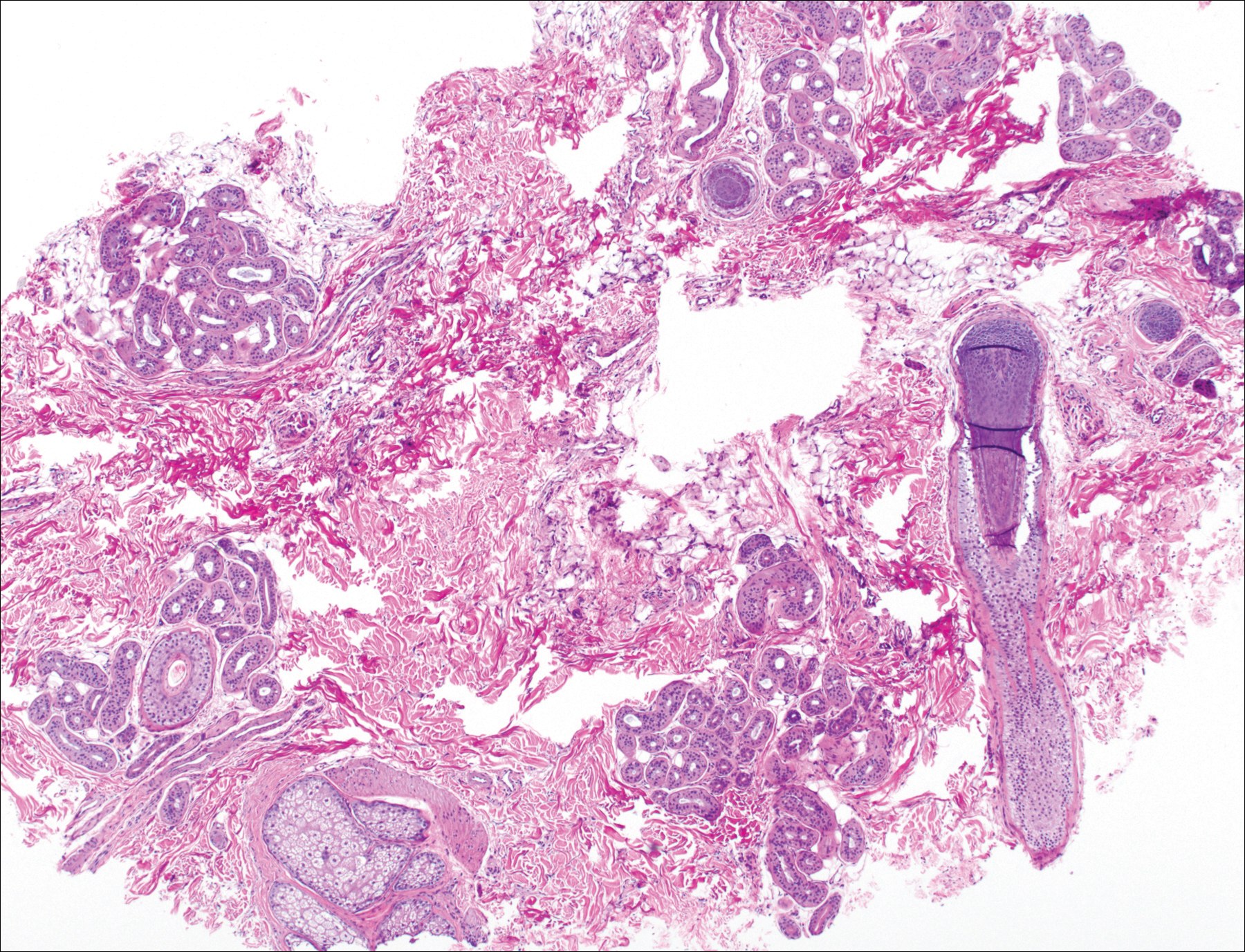
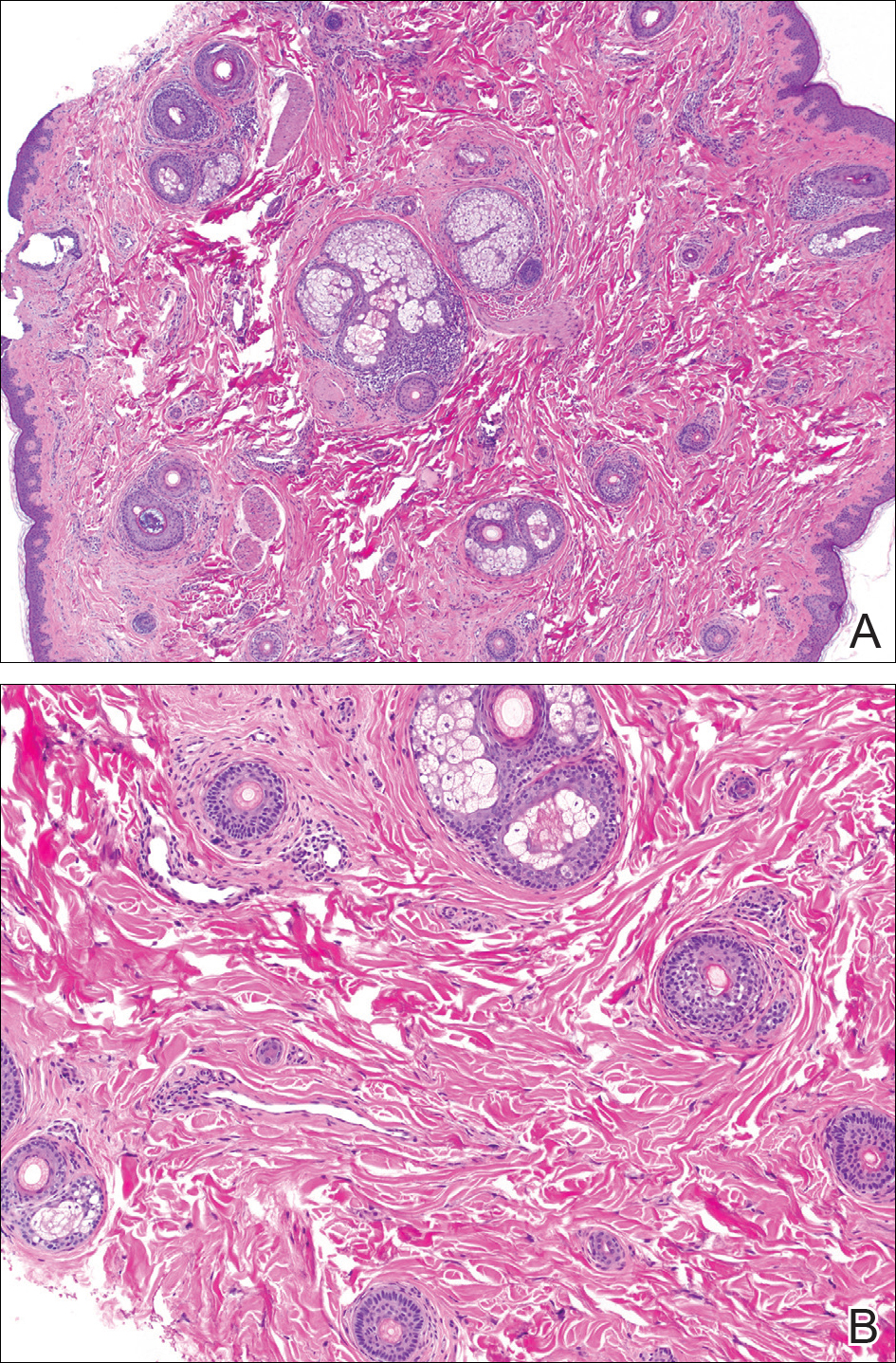
The differential diagnosis in adults presenting with a patch of localized alopecia includes alopecia areata, trichotillomania, pressure-induced alopecia, traction alopecia, lichen planopilaris, discoid lupus erythematosus, and rarely TTA. Temporal triangular alopecia is a fairly common, if underreported, nonscarring form of alopecia that mainly affects young children. A PubMed search of articles indexed for MEDLINE using the terms temporal triangular alopecia or congenital triangular alopecia or triangular alopecia documented only 76 cases of TTA including our own, with the majority of patients diagnosed before 9 years of age. Only 2 cases of adult-onset TTA have been reported,5,6 possibly leading to misdiagnosis of adult patients who present with similar areas of hair loss. As with some prior cases of TTA,5,7 our patient was misdiagnosed with alopecia areata and scarring alopecia, both treated unsuccessfully before a diagnosis of TTA was considered. Clues to the diagnosis included the location, the lack of change in size and shape, the lack of response to intralesional corticosteroids, and the presence of numerous vellus hairs on the surface. A biopsy of the visibly hairless zone was confirmatory. The normal or nearly normal number of miniaturized hairs in specimens of TTA suggest that topical minoxidil therapy (eg, 5% solution twice daily for at least 6 months) might be useful, but the authors have tried it on a few other patients with clinically typical TTA without discernible benefit. When lesions are small, excision provides a fast and permanent solution to the problem, albeit with the usual risks of minor surgery.
- Sabouraud RJA. Manuel Élémentaire de Dermatologie Topographique Régionale. Paris, France: Masson & Cie; 1905:197.
- Trakimas C, Sperling LC, Skelton HG 3rd, et al. Clinical and histologic findings in temporal triangular alopecia. J Am Acad Dermatol. 1994;31:205-209.
- Yamazaki M, Irisawa R, Tsuboi R. Temporal triangular alopecia and a review of 52 past cases. J Dermatol. 2010;37:360-362.
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
- Trakimas CA, Sperling LC. Temporal triangular alopecia acquired in adulthood. J Am Acad Dermatol. 1999;40:842-844.
- Akan IM, Yildirim S, Avci G, et al. Bilateral temporal triangular alopecia acquired in adulthood. Plast Reconstr Surg. 2001;107:1616-1617.
- Gupta LK, Khare AK, Garg A, et al. Congenital triangular alopecia--a close mimicker of alopecia areata. Int J Trichology. 2011;3:40-41.
To the Editor:
Temporal triangular alopecia (TTA), a condition first described by Sabouraud1 in 1905, is a circumscribed nonscarring form of alopecia. Also referred to as congenital triangular alopecia, TTA presents as a triangular or lancet-shaped area of hair loss involving the frontotemporal hairline. Temporal triangular alopecia is characterized histologically by a normal number of miniaturized hair follicles without notable inflammation.2 Although the majority of cases arise between birth and 9 years of age,3,4 rare cases of adult-onset TTA also have been reported.5,6 Adult-onset cases can cause notable diagnostic confusion and inappropriate treatment, as reported in our patient.
A 25-year-old woman with a history of Hashimoto thyroiditis presented with hair loss affecting the right temporal scalp of 3 years' duration that was first noticed by her husband. The lesion was an asymptomatic, 6×8-cm, roughly lancet-shaped patch of alopecia located on the right temporal scalp, bordering on the frontal hairline (Figure 1). Centrally, the patch appeared almost hairless with a few retained terminal hairs. The frontal hairline was thinned but still present. There was no scaling or erythema, and fine vellus hairs and a few isolated terminal hairs covered the area. The corresponding skin on the contralateral temporal scalp showed normal hair density. The patient insisted that she had normal hair at the affected area until 22 years of age, and she denied a history of trauma or tight hairstyles. Initially diagnosed with alopecia areata by her primary care provider, the patient was treated with topical corticosteroids for 6 months without benefit. She was subsequently referred to a dermatologist who again offered a diagnosis of alopecia areata and treated the lesions with 2 intralesional corticosteroid injections without benefit. No biopsies of the affected area were performed, and the patient was given a trial of topical minoxidil.

The patient consulted a new primary care provider and was diagnosed with scarring alopecia. She was referred to our dermatology department for further treatment. An initial biopsy at the edge of the affected area was interpreted as normal, but after failing additional intralesional corticosteroid injections, she was referred to our hair clinic where another biopsy was performed in the central portion of the lesion. A 4-mm diameter punch biopsy specimen revealed a normal epidermis and dermis; however, in the lower dermis only a single terminal follicle was seen (Figure 2). Sections through the upper dermis (Figure 3) showed that the total number of hairs was normal or nearly normal with at least 22 follicles, but most were vellus and indeterminate hairs with only a single terminal hair. The dermal architecture was otherwise normal. Given the clinical and histologic findings, a diagnosis of TTA was made. Subsequent to the diagnosis, the patient did not pursue any additional treatment options and preferred to style her hair so that the area of TTA remained covered.


The differential diagnosis in adults presenting with a patch of localized alopecia includes alopecia areata, trichotillomania, pressure-induced alopecia, traction alopecia, lichen planopilaris, discoid lupus erythematosus, and rarely TTA. Temporal triangular alopecia is a fairly common, if underreported, nonscarring form of alopecia that mainly affects young children. A PubMed search of articles indexed for MEDLINE using the terms temporal triangular alopecia or congenital triangular alopecia or triangular alopecia documented only 76 cases of TTA including our own, with the majority of patients diagnosed before 9 years of age. Only 2 cases of adult-onset TTA have been reported,5,6 possibly leading to misdiagnosis of adult patients who present with similar areas of hair loss. As with some prior cases of TTA,5,7 our patient was misdiagnosed with alopecia areata and scarring alopecia, both treated unsuccessfully before a diagnosis of TTA was considered. Clues to the diagnosis included the location, the lack of change in size and shape, the lack of response to intralesional corticosteroids, and the presence of numerous vellus hairs on the surface. A biopsy of the visibly hairless zone was confirmatory. The normal or nearly normal number of miniaturized hairs in specimens of TTA suggest that topical minoxidil therapy (eg, 5% solution twice daily for at least 6 months) might be useful, but the authors have tried it on a few other patients with clinically typical TTA without discernible benefit. When lesions are small, excision provides a fast and permanent solution to the problem, albeit with the usual risks of minor surgery.
To the Editor:
Temporal triangular alopecia (TTA), a condition first described by Sabouraud1 in 1905, is a circumscribed nonscarring form of alopecia. Also referred to as congenital triangular alopecia, TTA presents as a triangular or lancet-shaped area of hair loss involving the frontotemporal hairline. Temporal triangular alopecia is characterized histologically by a normal number of miniaturized hair follicles without notable inflammation.2 Although the majority of cases arise between birth and 9 years of age,3,4 rare cases of adult-onset TTA also have been reported.5,6 Adult-onset cases can cause notable diagnostic confusion and inappropriate treatment, as reported in our patient.
A 25-year-old woman with a history of Hashimoto thyroiditis presented with hair loss affecting the right temporal scalp of 3 years' duration that was first noticed by her husband. The lesion was an asymptomatic, 6×8-cm, roughly lancet-shaped patch of alopecia located on the right temporal scalp, bordering on the frontal hairline (Figure 1). Centrally, the patch appeared almost hairless with a few retained terminal hairs. The frontal hairline was thinned but still present. There was no scaling or erythema, and fine vellus hairs and a few isolated terminal hairs covered the area. The corresponding skin on the contralateral temporal scalp showed normal hair density. The patient insisted that she had normal hair at the affected area until 22 years of age, and she denied a history of trauma or tight hairstyles. Initially diagnosed with alopecia areata by her primary care provider, the patient was treated with topical corticosteroids for 6 months without benefit. She was subsequently referred to a dermatologist who again offered a diagnosis of alopecia areata and treated the lesions with 2 intralesional corticosteroid injections without benefit. No biopsies of the affected area were performed, and the patient was given a trial of topical minoxidil.

The patient consulted a new primary care provider and was diagnosed with scarring alopecia. She was referred to our dermatology department for further treatment. An initial biopsy at the edge of the affected area was interpreted as normal, but after failing additional intralesional corticosteroid injections, she was referred to our hair clinic where another biopsy was performed in the central portion of the lesion. A 4-mm diameter punch biopsy specimen revealed a normal epidermis and dermis; however, in the lower dermis only a single terminal follicle was seen (Figure 2). Sections through the upper dermis (Figure 3) showed that the total number of hairs was normal or nearly normal with at least 22 follicles, but most were vellus and indeterminate hairs with only a single terminal hair. The dermal architecture was otherwise normal. Given the clinical and histologic findings, a diagnosis of TTA was made. Subsequent to the diagnosis, the patient did not pursue any additional treatment options and preferred to style her hair so that the area of TTA remained covered.


The differential diagnosis in adults presenting with a patch of localized alopecia includes alopecia areata, trichotillomania, pressure-induced alopecia, traction alopecia, lichen planopilaris, discoid lupus erythematosus, and rarely TTA. Temporal triangular alopecia is a fairly common, if underreported, nonscarring form of alopecia that mainly affects young children. A PubMed search of articles indexed for MEDLINE using the terms temporal triangular alopecia or congenital triangular alopecia or triangular alopecia documented only 76 cases of TTA including our own, with the majority of patients diagnosed before 9 years of age. Only 2 cases of adult-onset TTA have been reported,5,6 possibly leading to misdiagnosis of adult patients who present with similar areas of hair loss. As with some prior cases of TTA,5,7 our patient was misdiagnosed with alopecia areata and scarring alopecia, both treated unsuccessfully before a diagnosis of TTA was considered. Clues to the diagnosis included the location, the lack of change in size and shape, the lack of response to intralesional corticosteroids, and the presence of numerous vellus hairs on the surface. A biopsy of the visibly hairless zone was confirmatory. The normal or nearly normal number of miniaturized hairs in specimens of TTA suggest that topical minoxidil therapy (eg, 5% solution twice daily for at least 6 months) might be useful, but the authors have tried it on a few other patients with clinically typical TTA without discernible benefit. When lesions are small, excision provides a fast and permanent solution to the problem, albeit with the usual risks of minor surgery.
- Sabouraud RJA. Manuel Élémentaire de Dermatologie Topographique Régionale. Paris, France: Masson & Cie; 1905:197.
- Trakimas C, Sperling LC, Skelton HG 3rd, et al. Clinical and histologic findings in temporal triangular alopecia. J Am Acad Dermatol. 1994;31:205-209.
- Yamazaki M, Irisawa R, Tsuboi R. Temporal triangular alopecia and a review of 52 past cases. J Dermatol. 2010;37:360-362.
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
- Trakimas CA, Sperling LC. Temporal triangular alopecia acquired in adulthood. J Am Acad Dermatol. 1999;40:842-844.
- Akan IM, Yildirim S, Avci G, et al. Bilateral temporal triangular alopecia acquired in adulthood. Plast Reconstr Surg. 2001;107:1616-1617.
- Gupta LK, Khare AK, Garg A, et al. Congenital triangular alopecia--a close mimicker of alopecia areata. Int J Trichology. 2011;3:40-41.
- Sabouraud RJA. Manuel Élémentaire de Dermatologie Topographique Régionale. Paris, France: Masson & Cie; 1905:197.
- Trakimas C, Sperling LC, Skelton HG 3rd, et al. Clinical and histologic findings in temporal triangular alopecia. J Am Acad Dermatol. 1994;31:205-209.
- Yamazaki M, Irisawa R, Tsuboi R. Temporal triangular alopecia and a review of 52 past cases. J Dermatol. 2010;37:360-362.
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
- Trakimas CA, Sperling LC. Temporal triangular alopecia acquired in adulthood. J Am Acad Dermatol. 1999;40:842-844.
- Akan IM, Yildirim S, Avci G, et al. Bilateral temporal triangular alopecia acquired in adulthood. Plast Reconstr Surg. 2001;107:1616-1617.
- Gupta LK, Khare AK, Garg A, et al. Congenital triangular alopecia--a close mimicker of alopecia areata. Int J Trichology. 2011;3:40-41.
Practice Points
- Temporal triangular alopecia (TTA) in adults often is confused with alopecia areata.
- An acquired, persistent, unchanging, circumscribed hairless spot in an adult that does not respond to intralesional corticosteroids may represent TTA.
- Hair miniaturization without peribulbar inflammation is consistent with a diagnosis of TTA.
Eroded Plaque on the Lower Lip
The Diagnosis: Squamous Cell Carcinoma
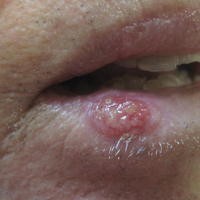
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
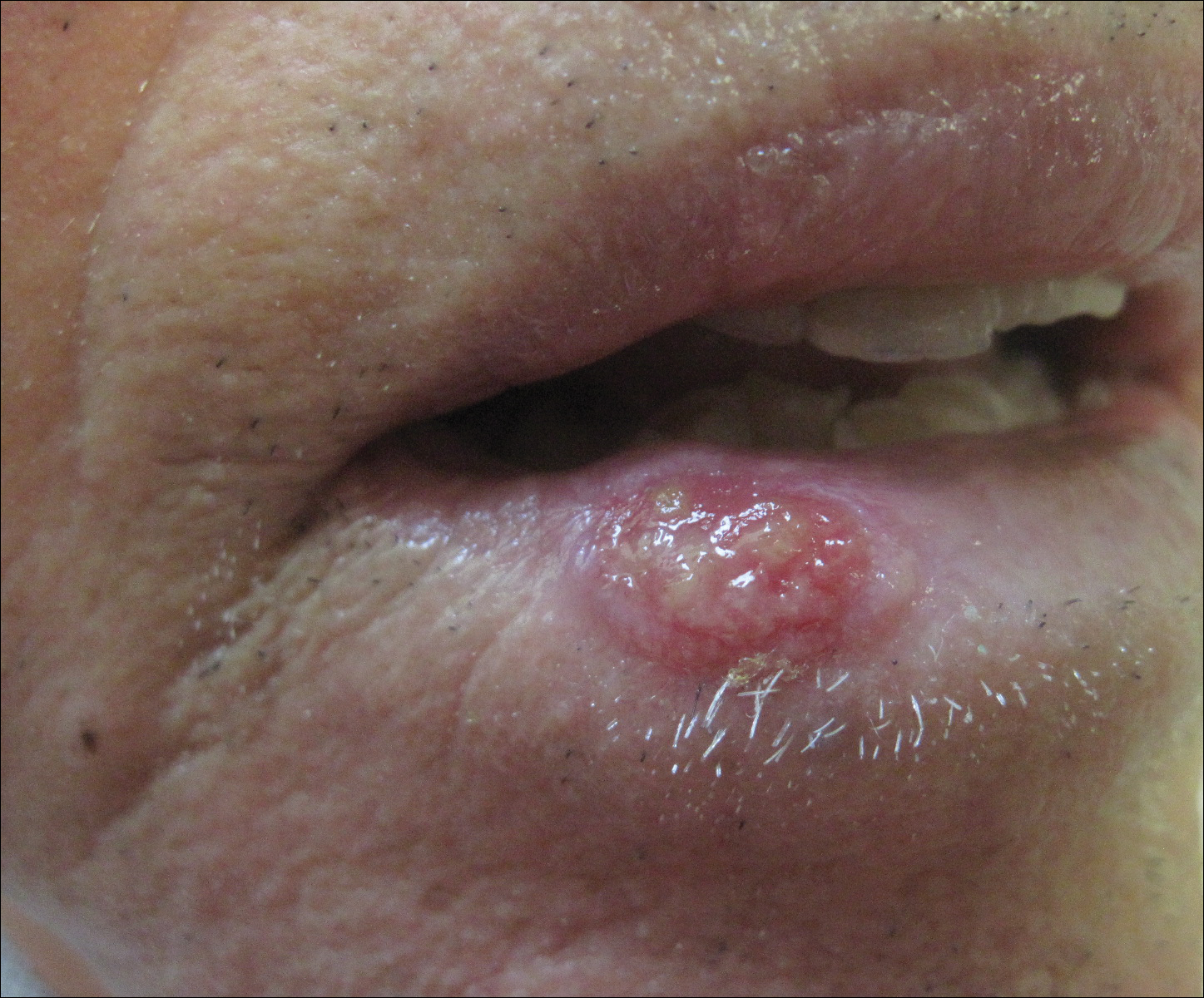
An 83-year-old man presented with a new-onset 1.2-cm eroded plaque on the vermilion border of the right lower lip that reportedly developed 2 weeks prior and was increasing in size. The plaque was moist and was composed of confluent glistening papules. Medical history was notable for the presence of both basal cell and squamous cell carcinomas.
Large Hyperpigmented Nodule on the Leg
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.
Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13
Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16
The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.

Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13

Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16

The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.

Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13

Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16

The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.

A 61-year-old woman presented with a 2.5-cm hyperpigmented exophytic nodule on the anterior aspect of the left shin of approximately 2 years' duration. The patient initially noticed a small lesion following a bee sting, but it subsequently grew over the ensuing 2 years. A shave biopsy was obtained.
Black Adherence Nodules on the Scalp Hair Shaft
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).
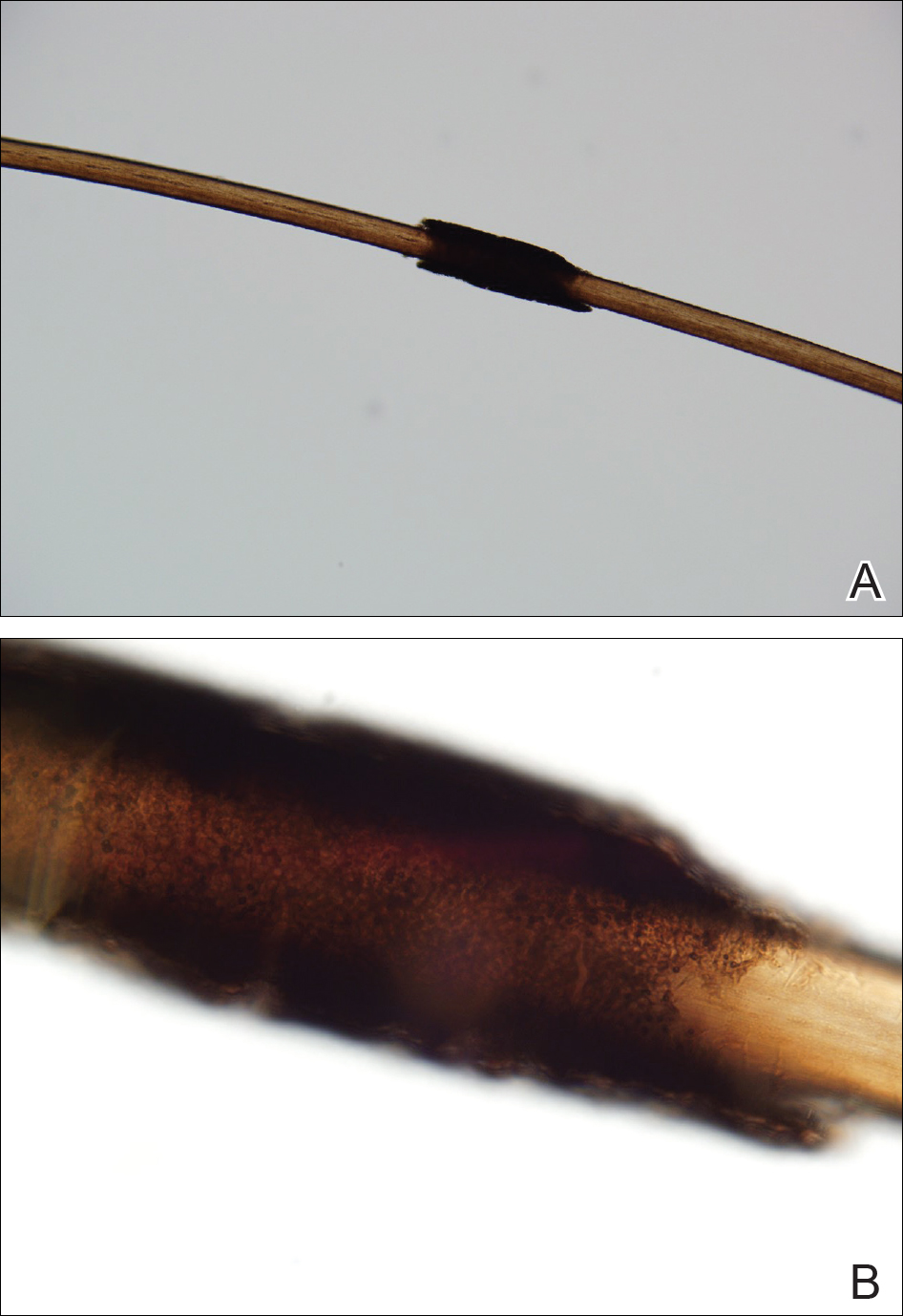
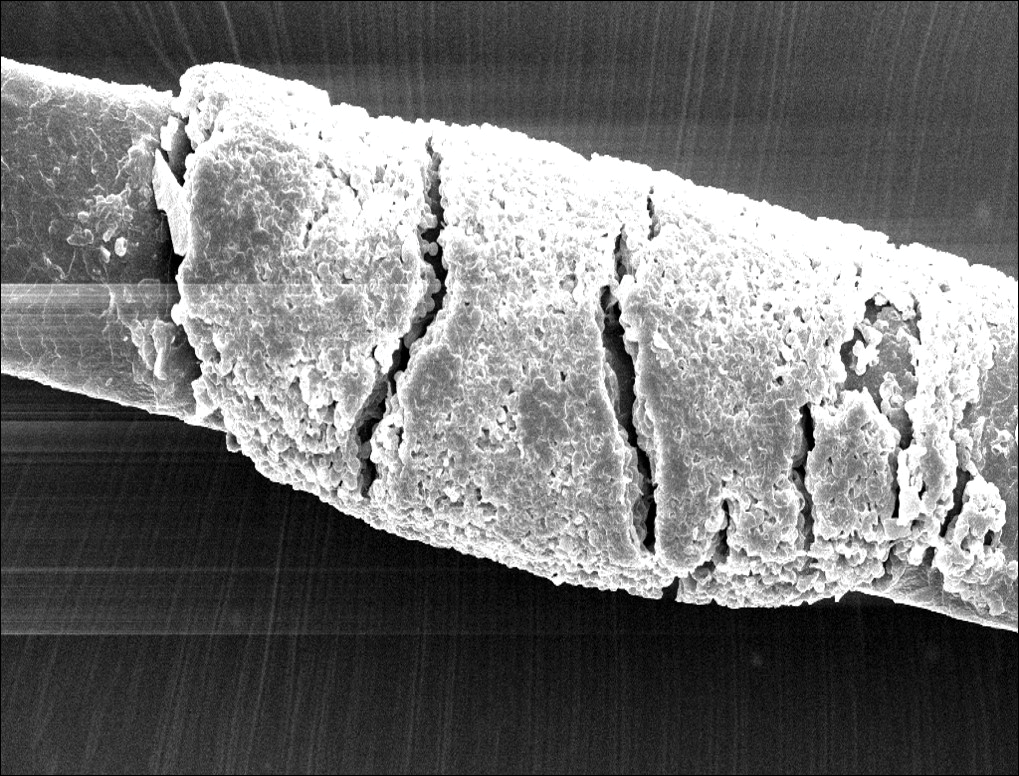
Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
The Diagnosis: Piedra
Microscopic examination of the hair shafts revealed brown to black, firmly adherent concretions (Figure 1). Scanning electron microscopy of the nodules was performed, which allowed for greater definition of the constituent hyphae and arthrospores (Figure 2).


Fungal cultures grew Trichosporon inkin along with other dematiaceous molds. The patient initially was treated with a combination of ketoconazole shampoo and weekly application of topical terbinafine. She trimmed 15.2 cm of the hair of her own volition. At 2-month follow-up the nodules were still present, though smaller and less numerous. Repeat cultures were obtained, which again grew T inkin. She then began taking oral terbinafine 250 mg daily for 6 weeks.
This case of piedra is unique in that our patient presented with black nodules clinically, but cultures grew only the causative agent of white piedra, T inkin. A search of PubMed articles indexed for MEDLINE using the terms black piedra, white piedra, or piedra, and mixed infection or coinfection yielded one other similar case.1 Kanitakis et al1 speculated that perhaps there was coinfection of black and white piedra and that Piedraia hortae, the causative agent of black piedra, was unable to flourish in culture facing competition from other fungi. This scenario also could apply to our patient. However, the original culture taken from our patient also grew other dematiaceous molds including Cladosporium and Exophiala species. It also is possible that these other fungi could have contributed pigment to the nodules, giving it the appearance of black piedra when only T inkin was present as the true pathogen.
White piedra is a rare fungal infection of the hair shaft caused by organisms of the genus Trichosporon, with Trichosporon ovoides most likely to infect the scalp.2 Black piedra is a similar fungal infection caused by P hortae. Piedra means stone in Spanish, reflecting the appearance of these organisms on the hair shaft. It is common in tropical regions of the world such as Southeast Asia and South America, flourishing in the high temperatures and humidity.2 Both infectious agents are found in the soil or in standing water.3 White piedra most commonly is found in facial, axilla, or pubic hair, while black piedra most often is found in the hair of the scalp.2,4 Local cultural practices may contribute to transfer of Trichosporon or P hortae to the scalp, including the use of Brazilian plant oils in the hair or tying a veil or hijab to wet hair. Interestingly, some groups intentionally introduce the fungus to their hair for cosmetic reasons in endemic areas.2,3,5
Patients with white or black piedra generally are asymptomatic.4 Some may notice a rough texture to the hair or hear a characteristic metallic rattling sound as the nodules make contact with brush bristles.2,3 On inspection of the scalp, white piedra will appear to be white to light brown nodules, while black piedra presents as brown to black in color. The nodules are often firm on palpation.2,3 The nodules of white piedra generally are easy to remove in contrast to black piedra, which involves nodules that securely attach to the hair shaft but can be removed with pressure.3,5 Piedra has natural keratolytic activities and with prolonged infection can penetrate the hair cuticle, causing weakness and eventual breakage of the hair. This invasion into the hair cortex also can complicate treatment regimens, contributing to the chronic course of these infections.6
Diagnosis is based on clinical and microscopic findings. Nodules on hair shafts can be prepared with potassium hydroxide and placed on glass slides for examination.4 Dyes such as toluidine blue or chlorazol black E stain can be used to assist in identifying fungal structures.2 Sabouraud agar with cycloheximide may be the best choice for culture medium.2 Black piedra slowly grows into small dome-shaped colonies. White piedra will grow more quickly into cream-colored colonies with wrinkles and sometimes mucinous characteristics.3
The best treatment of black or white piedra is to cut the hair, thereby eliminating the fungi,7 which is not an easy option for many patients, such as ours, because of the aesthetic implications. Alternative treatments include azole shampoos such as ketoconazole.2,4 Treatment with oral terbinafine 250 mg daily for 6 weeks has been successfully used for black piedra.7 Patients must be careful to thoroughly clean or discard hairbrushes, as they can serve as reservoirs of fungi to reinfect patients or spread to others.5,7
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.
- Kanitakis J, Persat F, Piens MA, et al. Black piedra: report of a French case associated with Trichosporon asahii. Int J Dermatol. 2006;45:1258-1260.
- Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
- Khatu SS, Poojary SA, Nagpur NG. Nodules on the hair: a rare case of mixed piedra. Int J Trichology. 2013;5:220-223.
- Elewski BE, Hughey LC, Sobera JO, et al. Fungal diseases. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences; 2012:1251-1284.
- Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2013;53:1008-1011.
- Figueras M, Guarro J, Zaror L. New findings in black piedra infection. Br J Dermatol. 1996;135:157-158.
- Gip L. Black piedra: the first case treated with terbinafine (Lamisil). Br J Dermatol. 1994;130(suppl 43):26-28.

A 21-year-old woman presented to the dermatology clinic with what she described as small black dots in her hair that she first noted 3 months prior to presentation. The black nodules were asymptomatic, but the patient noted that they seemed to be moving up the hair shaft. They were firmly attached and great effort was required to remove them. The patient's sister recently developed similar nodules. The patient and her sister work as missionaries and had spent time in India, Southeast Asia, and Central America within the last few years. Physical examination revealed firmly adherent black nodules involving the mid to distal portions of the hair shafts on the scalp. There were no nail or skin findings. Cultures were obtained, and microscopic examination was performed.
Cosmeceuticals and Alternative Therapies for Rosacea
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
Debunking Acne Myths: Is Itching a Symptom of Acne?
Myth: Itching is not a symptom of acne
Acne vulgaris typically is not considered to be a pruritic disease; however, many patients experience itching, which leads them to scratch their acne lesions, causing secondary bacterial infections and subsequent scarring, hypopigmentation, or hyperpigmentation of the involved skin. Although itching rarely is mentioned as a clinical feature of acne, pruritus can be an important contributory factor to the burden of disability and impaired quality of life in acne patients of all ages, and acne itching may be an important target for therapy.
In a descriptive study of 120 consecutive acne patients in Singapore, itch was found to be a common (70% of patients) and debilitating symptom of acne. The majority of patients (83%) reported itch at noon with severity that was comparable to a mosquito bite, and the most common physical descriptor was tickling (68%). Common aggravating factors included sweat (71%), heat (62%), and stress (31%). Fifty-five percent of patients said itching had a negative impact on their mood, and 52% reported that they had scratched or rubbed the affected area.
A study of 108 adolescents with acne limited to the face yielded half who reported itching within acne lesions. The presence of itching was unrelated to age, gender, where they lived, positive family history, or acne severity. In most patients, pruritus appeared relatively infrequently and for a short period of time: 7.4% reported itching every day, 24.1% on a weekly basis, 29.6% at least once a month, and 37.7% even less frequently. Itch episodes lasted less than 1 minute in most participants. However, 31.5% of participants sought medical treatment to reduce itching. The most important factors aggravating the intensity of itching were sweat, stress, physical effort, heat, fatigue, and dry air, respectively.
Regarding the impact of acne itching on quality of life, 29.6% of participants felt depressed and 1.8% were anxious because of their itching. Some participants also noted that itching caused difficulties in falling asleep and awakening from itching.
The pathogenesis of localized itching in acne could be connected with the change in pH of the microenvironment of the acne follicle, providing an optimal environment for the production of histamine or histaminelike products by Propionibacterium acnes. Pruritus also may be a complication of certain acne therapies. Increased awareness among patients of this potential side effect may be helpful in preventing the unnecessary discontinuation of an otherwise effective acne therapy. Understanding factors that may aggravate itching in acne lesions also may be helpful to patients.
Lim YL, Chan YH, Yosipovitch G, et al. Pruritus is a common and significant symptom of acne [published online July 8, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1332-1336.
Reich A, Trybucka K, Tracinska A, et al. Acne itch: do acne patients suffer from itching? Acta Derm Venereol. 2008;88:38-42.
Myth: Itching is not a symptom of acne
Acne vulgaris typically is not considered to be a pruritic disease; however, many patients experience itching, which leads them to scratch their acne lesions, causing secondary bacterial infections and subsequent scarring, hypopigmentation, or hyperpigmentation of the involved skin. Although itching rarely is mentioned as a clinical feature of acne, pruritus can be an important contributory factor to the burden of disability and impaired quality of life in acne patients of all ages, and acne itching may be an important target for therapy.
In a descriptive study of 120 consecutive acne patients in Singapore, itch was found to be a common (70% of patients) and debilitating symptom of acne. The majority of patients (83%) reported itch at noon with severity that was comparable to a mosquito bite, and the most common physical descriptor was tickling (68%). Common aggravating factors included sweat (71%), heat (62%), and stress (31%). Fifty-five percent of patients said itching had a negative impact on their mood, and 52% reported that they had scratched or rubbed the affected area.
A study of 108 adolescents with acne limited to the face yielded half who reported itching within acne lesions. The presence of itching was unrelated to age, gender, where they lived, positive family history, or acne severity. In most patients, pruritus appeared relatively infrequently and for a short period of time: 7.4% reported itching every day, 24.1% on a weekly basis, 29.6% at least once a month, and 37.7% even less frequently. Itch episodes lasted less than 1 minute in most participants. However, 31.5% of participants sought medical treatment to reduce itching. The most important factors aggravating the intensity of itching were sweat, stress, physical effort, heat, fatigue, and dry air, respectively.
Regarding the impact of acne itching on quality of life, 29.6% of participants felt depressed and 1.8% were anxious because of their itching. Some participants also noted that itching caused difficulties in falling asleep and awakening from itching.
The pathogenesis of localized itching in acne could be connected with the change in pH of the microenvironment of the acne follicle, providing an optimal environment for the production of histamine or histaminelike products by Propionibacterium acnes. Pruritus also may be a complication of certain acne therapies. Increased awareness among patients of this potential side effect may be helpful in preventing the unnecessary discontinuation of an otherwise effective acne therapy. Understanding factors that may aggravate itching in acne lesions also may be helpful to patients.
Myth: Itching is not a symptom of acne
Acne vulgaris typically is not considered to be a pruritic disease; however, many patients experience itching, which leads them to scratch their acne lesions, causing secondary bacterial infections and subsequent scarring, hypopigmentation, or hyperpigmentation of the involved skin. Although itching rarely is mentioned as a clinical feature of acne, pruritus can be an important contributory factor to the burden of disability and impaired quality of life in acne patients of all ages, and acne itching may be an important target for therapy.
In a descriptive study of 120 consecutive acne patients in Singapore, itch was found to be a common (70% of patients) and debilitating symptom of acne. The majority of patients (83%) reported itch at noon with severity that was comparable to a mosquito bite, and the most common physical descriptor was tickling (68%). Common aggravating factors included sweat (71%), heat (62%), and stress (31%). Fifty-five percent of patients said itching had a negative impact on their mood, and 52% reported that they had scratched or rubbed the affected area.
A study of 108 adolescents with acne limited to the face yielded half who reported itching within acne lesions. The presence of itching was unrelated to age, gender, where they lived, positive family history, or acne severity. In most patients, pruritus appeared relatively infrequently and for a short period of time: 7.4% reported itching every day, 24.1% on a weekly basis, 29.6% at least once a month, and 37.7% even less frequently. Itch episodes lasted less than 1 minute in most participants. However, 31.5% of participants sought medical treatment to reduce itching. The most important factors aggravating the intensity of itching were sweat, stress, physical effort, heat, fatigue, and dry air, respectively.
Regarding the impact of acne itching on quality of life, 29.6% of participants felt depressed and 1.8% were anxious because of their itching. Some participants also noted that itching caused difficulties in falling asleep and awakening from itching.
The pathogenesis of localized itching in acne could be connected with the change in pH of the microenvironment of the acne follicle, providing an optimal environment for the production of histamine or histaminelike products by Propionibacterium acnes. Pruritus also may be a complication of certain acne therapies. Increased awareness among patients of this potential side effect may be helpful in preventing the unnecessary discontinuation of an otherwise effective acne therapy. Understanding factors that may aggravate itching in acne lesions also may be helpful to patients.
Lim YL, Chan YH, Yosipovitch G, et al. Pruritus is a common and significant symptom of acne [published online July 8, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1332-1336.
Reich A, Trybucka K, Tracinska A, et al. Acne itch: do acne patients suffer from itching? Acta Derm Venereol. 2008;88:38-42.
Lim YL, Chan YH, Yosipovitch G, et al. Pruritus is a common and significant symptom of acne [published online July 8, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1332-1336.
Reich A, Trybucka K, Tracinska A, et al. Acne itch: do acne patients suffer from itching? Acta Derm Venereol. 2008;88:38-42.
What's Eating You? Sticktight Flea Revisited
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).
Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
Identifying Characteristics
The sticktight flea (Echidnophaga gallinacea) earns its name by embedding its head in the host's skin using broad and serrated laciniae and can feed at one site for up to 19 days.1 It differs in morphology from dog (Ctenocephalides canis) and cat (Ctenocephalides felis) fleas, lacking genal (mustache area) and promotal (back of the head) ctenidia (combs), and is half the size of the cat flea. It has 2 pairs of setae (hairs) behind the antennae with an anteriorly flattened head (Figure).

Disease Transmission
Although its primary host is poultry and it also is known as the stickfast or chicken flea, the sticktight flea has been found in many species of birds and mammals, including humans. It is becoming more common in dogs in many parts of the world, including the United States,2-5 and has been found to be the most common flea on dogs in areas of South Africa.6 Other noted hosts of E gallinacea are rodents, cottontail rabbits, cats, ground squirrels, and pigs.7-14 Human infestation occurs from exposure to affected animals.15 As blood feeders, fleas have long been known to serve as vectors for many diseases, including bubonic plague, typhus, and tularemia, as well as an intermediate host of the dog tapeworm (Dipylidium caninum).5 Rickettsia felis, belonging to the spotted fever group, is an emerging infectious disease in humans commonly found in the cat flea (C felis) but also has been detected in E gallinacea.7 Echidnophaga gallinacea is found worldwide in the tropics, subtropics, and temperate zones, and it is the only representative of the genus found in the United States.1 Given the wide range of wild and domestic animal hosts and wide geographic distribution for E gallinacea, it represents an increasing risk for humans.
Echidnophaga gallinacea favors feeding from fleshy areas without thick fur or plumage. In birds, the area around the eyes, comb, and wattles is included; in dogs, it can be the eyes, in between the toes, and in the genital area.1 Flea bites cause irritation and itching for hosts including humans, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.15 Severe bites also may lead to bullous lesions. In birds, symptoms can be extreme, with infestation around the eyes leading to swelling and blindness, a decline in egg production, weight loss, and death in young birds.1 Similar to other fleas, E gallinacea is wingless and depends on jumping onto a host for transmission, which can be from the ground, carpeting and flooring, furniture, or another host. Fleas are champion jumpers (relative to body size) and can jump 100 times their length.16
Management
Treating sticktight fleas can be tricky, as they embed tightly into the host's skin. Animals should be treated by a qualified veterinarian. Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin. If the infestation is considerable, malathion 5% liquid or gel can be applied. Patients can treat itching with topical steroids and antipruritic creams, and oral antihistamines can be used to relieve symptoms and reduce the likelihood of damaged skin as well as the potential for secondary infection. The flea-infested environment should be treated with insecticides. For treatment of hard surfaces, dichlorvos and propetamphos are effective. Organophosphates work well on fabric and carpeting. Domestic pets and livestock may be treated by a veterinarian with agents such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, and pyriproxyfen.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596.
- Kalkofen UP, Greenberg J. Echidnophaga gallinacea infestation in dogs. J Am Vet Med Assoc. 1974;165:447-448.
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140.
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948.
- Durden LA, Judy TN, Martin JE, et al. Fleas parasitizing domestic dogs in Georgia, USA: species composition and seasonal abundance. Vet Parasitol. 2005;130:157-162.
- Rautenbach GH, Boomker J, de Villiers IL. A descriptive study of the canine population in a rural town in southern Africa. J S Afr Vet Assoc. 1991;62:158-162.
- Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
- Guernier V, Lagadec E, LeMinter G, et al. Fleas of small mammals on Reunion Island: diversity, distribution and epidemiological consequences. PLoS Negl Trop Dis. 2014;8:e3129.
- Cantó GJ, Guerrero RI, Olvera-Ramírez AM, et al. Prevalence of fleas and gastrointestinal parasites in free-roaming cats in central Mexico [published online April 3, 2013]. PLoS One. 2013;8:e60744.
- Akucewich LH, Philman K, Clark A, et al. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet Parasitol. 2002;109:129-139.
- Linardi PM, Gomes AF, Botelho JR, et al. Some ectoparasites of commensal rodents from Huambo, Angola. J Med Entomol. 1994;31:754-756.
- Pfaffenberger GS, Valencia VB. Ectoparasites of sympatric cottontails (Sylvilagus audubonii Nelson) and jack rabbits (Lepus californicus Mearns) from the high plains of eastern New Mexico. J Parasitol. 1988;74:842-846.
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123.
- Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241-244.
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4.
- Rothschild M, Schlein Y, Parker K, et al. The flying leap of the flea. Scientific American. 1973;229:92.
Practice Points
- Although the primary host of the sticktight flea is poultry, it has been found in many species of birds and mammals, including humans.
- Flea bites cause irritation and itching for hosts, typically resulting in clusters of firm, pruritic, erythematous papules with a central punctum.
- Removal of attached fleas in humans requires grasping the flea firmly with tweezers and pulling from the skin.
Phototherapy Coding and Documentation in the Time of Biologics
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
Lack of Significant Anti-inflammatory Activity With Clindamycin in the Treatment of Rosacea: Results of 2 Randomized, Vehicle-Controlled Trials
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.
Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).
At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).
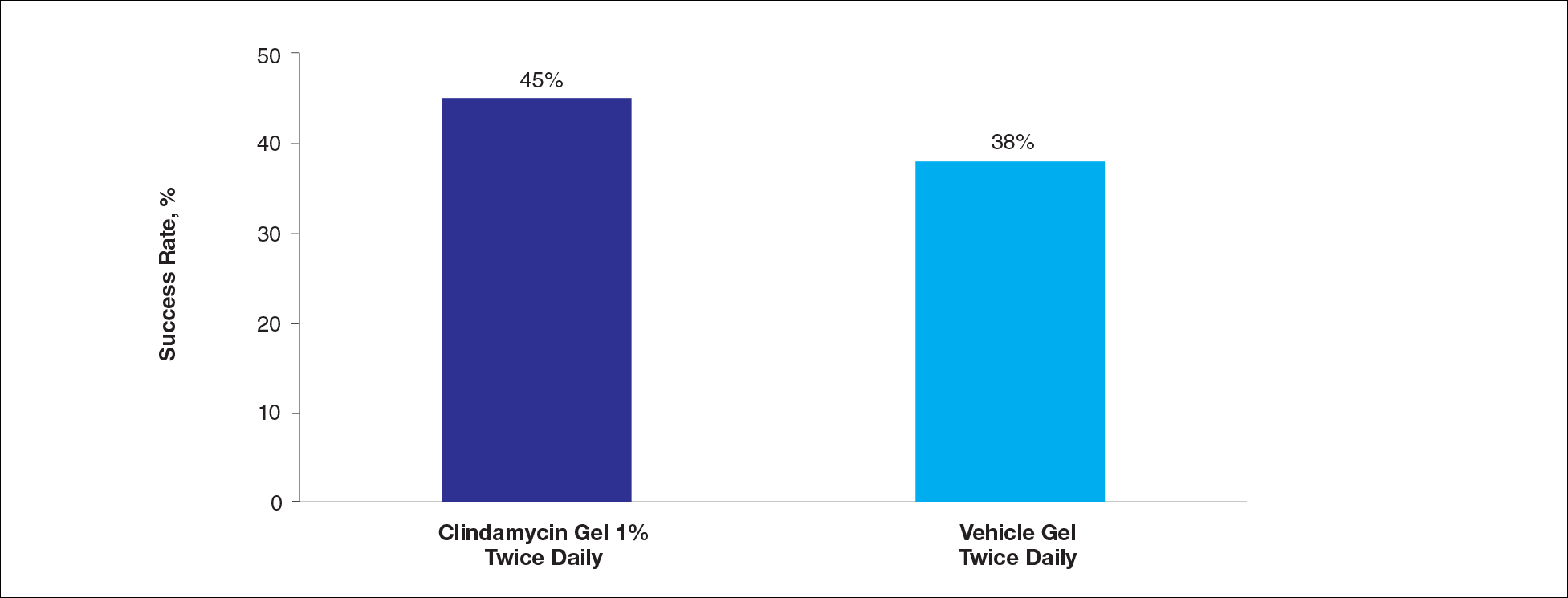
For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).
There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.

Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).

For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.

Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).

For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Practice Points
- Clindamycin cream 0.3% and 1% and clindamycin gel 1% were no more effective than their respective vehicles in the treatment of moderate to severe rosacea.
- Clindamycin may have no intrinsic anti-inflammatory activity in rosacea.