User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Sporotrichoid Pattern of Mycobacterium chelonae-abscessus Infection
To the Editor:
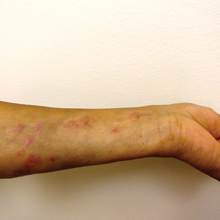
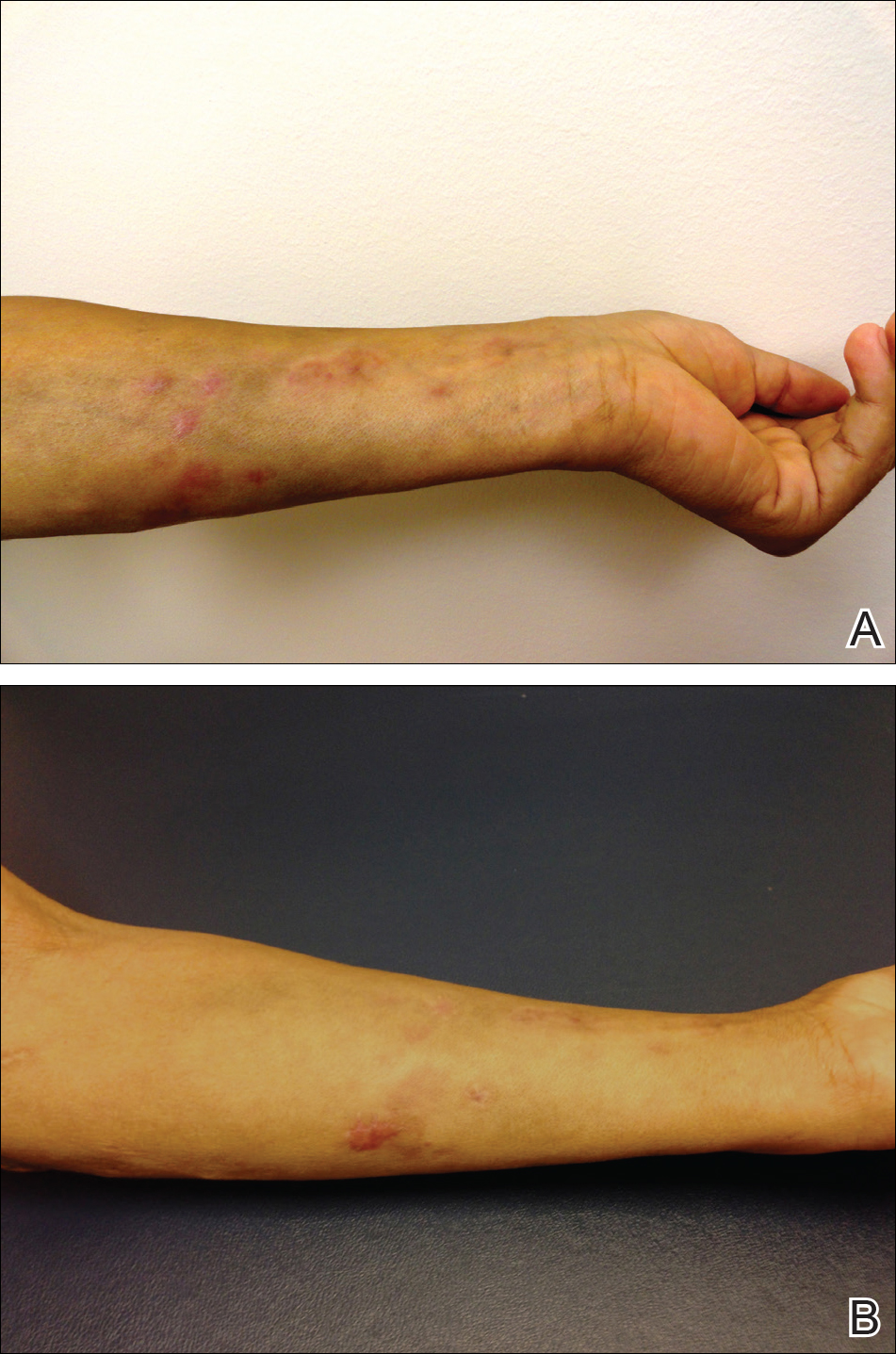
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).
The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2 Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
To the Editor:
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).

The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2 Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
To the Editor:
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).

The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2 Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
Practice Points
- Dermatologists should consider atypical mycobacterial infections, including rapidly growing mycobacteria, in the differential diagnosis for lesions with sporotrichoid-pattern spread.
- Multidrug therapy often is required for treatment of infection caused by Mycobacteria chelonae-abscessus complex.
Microneedling With Stem Cells
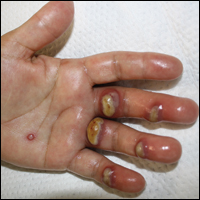
Tender Edematous Nodules on the Hand
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
The Diagnosis: Ecthyma Contagiosum (Orf)
Orf, or ecthyma contagiosum, is a zoonotic cutaneous infection caused by the orf DNA virus of the genus Parapoxvirus of the family Poxviridae. It is transmitted to humans through direct contact with infected animals, namely sheep and goats, and as such is most commonly seen in patients with occupational exposure to these animals such as butchers, farmers, veterinarians, and shepherds.1,2 Human-to-human transmission is exceedingly rare in immunocompetent patients.2,3 In affected animals, lesions usually are found around the mouth, muzzle, and eyes. In humans, hands are the most commonly affected site, and lesions occur 3 to 10 days after contact. Clinically, the lesions are nonspecific, and our patient presented with tender, erythematous, edematous nodules on the left hand. The differential diagnosis is broad and includes a milker's nodule, pyogenic granuloma, tularemia, anthrax, atypical mycobacterial infection, and sporotrichosis.1,4,5
The diagnosis usually is made with a thorough history and examination, but in cases of uncertainty, routine pathology with hematoxylin and eosin staining, electron microscopy, or real-time polymerase chain reaction may be used.2-4 Histopathologically, lesions demonstrate intraepidermal vesicles, vacuolization of keratinocytes of the upper epidermis with characteristic cytoplasmic inclusion bodies, rete ridge elongation, and dilated vessels in the intervening dermal papillae. Central necrosis may occur in well-developed lesions.2,6 Interestingly, our patient's biopsy exhibited all of these findings (Figure). Immunostains for cytomegalovirus and herpes simplex virus were negative, and Grocott-Gomori methenamine-silver and acid-fast bacillus stains also were negative.

Our patient also developed lymphangitic streaking suggestive of a bacterial superinfection and was treated with a course of intravenous antibiotics. She eventually was discharged with reassurance, wound care instructions, and outpatient antibiotics. She returned to an outside institution's emergency department for further evaluation, and she was admitted for workup. A lesional swab was sent for real-time polymerase chain reaction, which confirmed the diagnosis as orf. When the patient was contacted for follow-up 1 week after biopsy, the hand lesions had notably improved.
Orf is self-limited and typically resolves within 4 to 8 weeks after undergoing evolution through 5 described stages. The maculopapular stage is denoted by enlarging erythematous macule. The targetoid stage is described by a red center within a white halo surrounded by a broader red halo. The nodular stage is self-descriptive. The regenerative and regression stages describe the progressively improving, drier, and crusted nodules.3
Because orf is self-limited, no treatment is required, and patients should be counseled that their lesions should resolve within weeks. Complications include lymphangitis, secondary bacterial infection, and erythema multiforme.1,2,4,5 Immunocompromised patients may develop recalcitrant, giant, or multiple lesions that may be treated with topical imiquimod, topical cidofovir, intralesional interferon alfa, or surgical excision.1,2,4,7
We present a case of orf to remind practitioners of this rare entity. Although the disease is endemic worldwide, it likely is underreported due to its self-limited nature.2,4 A careful history may reveal the diagnosis, and overtreatment with antibiotics, many of which have their own significant side-effect profile, can then be avoided.
Acknowledgment
We thank Eric Behling, MD (Camden, New Jersey), for his contributions in obtaining the histologic images.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
- Veraldi S, Nazzaro G, Vaira F, et al. Presentation of orf (ecthyma contagiosum) after sheep slaughtering for religious feasts. Infection. 2014;42:767-769.
- Al-Salam S, Nowotny N, Sohail MR, et al. Ecthyma contagiosum (orf)--report of a human case from the United Arab Emirates and review of the literature. J Cutan Pathol. 2008;35:603-607.
- Thurman RJ, Fitch RW. Images in clinical medicine. contagious ecthyma. N Engl J Med. 2015;372:E12.
- Meier R, Sommacal A, Stahel A, et al. Orf--an orphan disease? JRSM Open. 2015;6:2054270415593718.
- Joseph RH, Haddad FA, Matthews AL, et al. Erythema multiforme after orf virus infection: a report of two cases and literature review. Epidemiol Infect. 2015;143:385-390.
- Xu X, Yun SJ, Erikson L, et al. Diseases caused by viruses. In: Elder DE, Elenitsas R, Rosenbach M, eds. Lever's Histopathology of the Skin. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:781-815.
- Koufakis T, Katsaitis P, Gabranis I. Orf disease: a report of a case. Braz J Infect Dis. 2014;18:568-569.
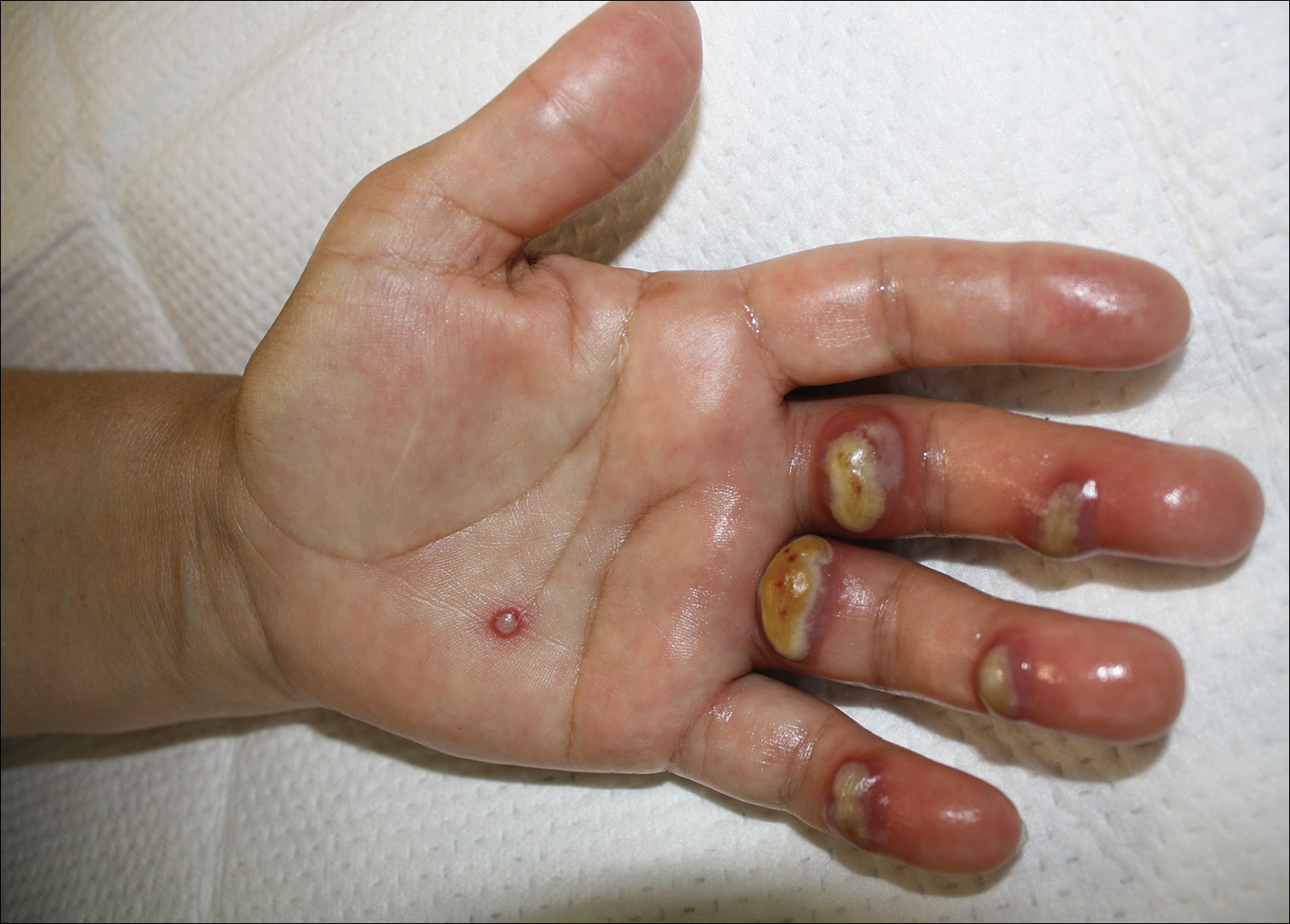
A 57-year-old woman presented to the emergency department (ED) for evaluation of a rash on the left hand of 2 weeks' duration. She described pinpoint red lesions on the left palm, as well as the third, fourth, and fifth fingers, which gradually enlarged and became painful. She denied any specific trauma but recalled cutting her hand on a piece of metal in the ground prior to the onset of the rash. She worked on a farm and bottle-fed sheep and chickens. Physical examination revealed tender edematous nodules with central gray pustules, and the left axillary lymph node was enlarged and tender. Ulceration was not appreciated. Various antibiotics including cephalexin, trimethoprim-sulfamethoxazole, and clindamycin were prescribed during prior ED visits, but she reported no improvement with these medications. She remained afebrile throughout the course of the hand rash, and laboratory workup was consistently unremarkable. Two sets of herpes simplex virus cultures from the ED visits showed no growth, and a hand radiograph also was normal. Medical history included coronary artery disease, myocardial infarction, mitral regurgitation, and hyperlipidemia.
Making Practice Perfect Download
Please click either of the links below to download your free eBook
Onecount Call To Arms
Orange Nodules on the Scalp
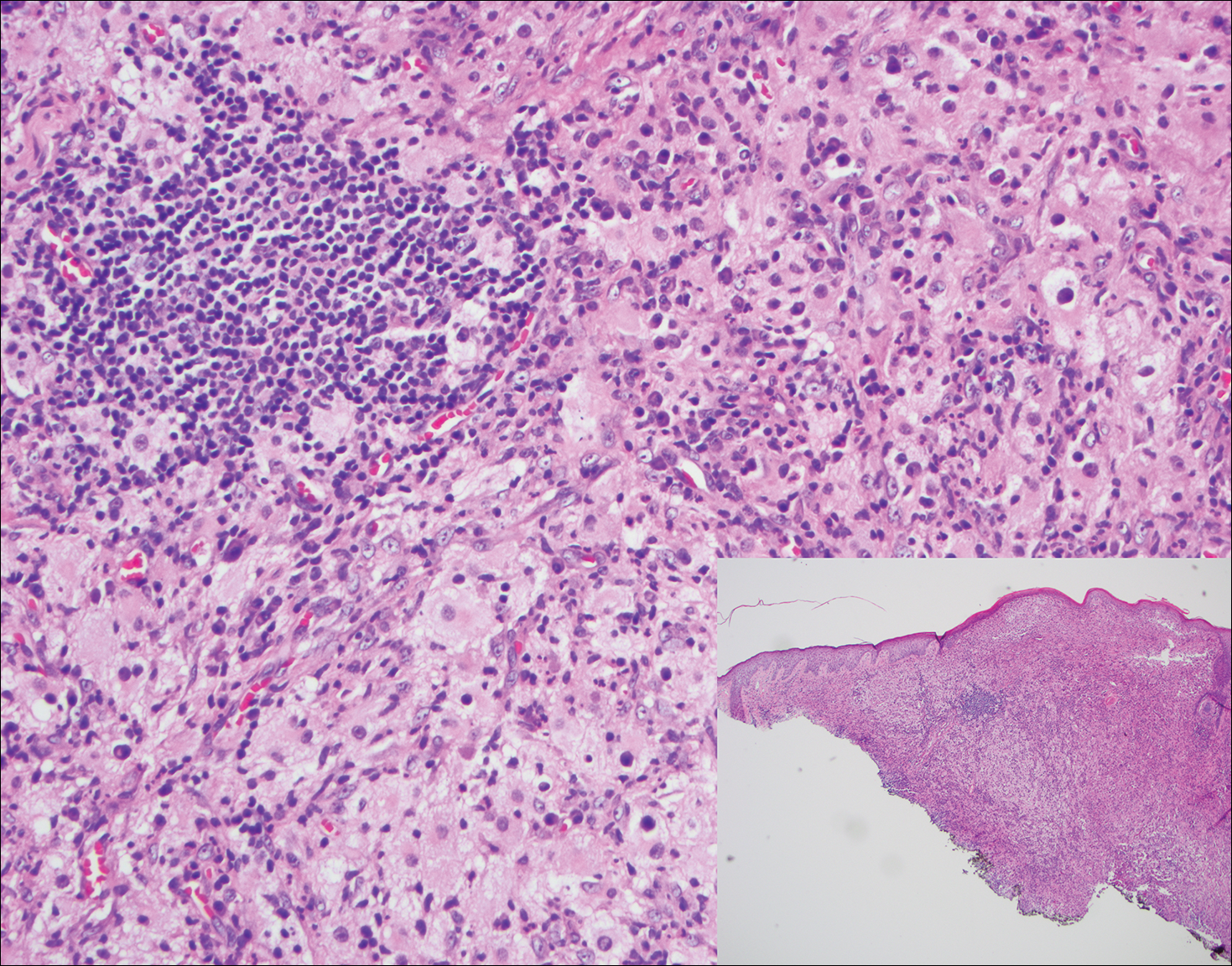
The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
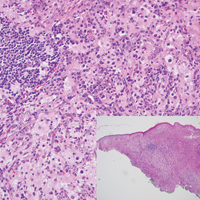
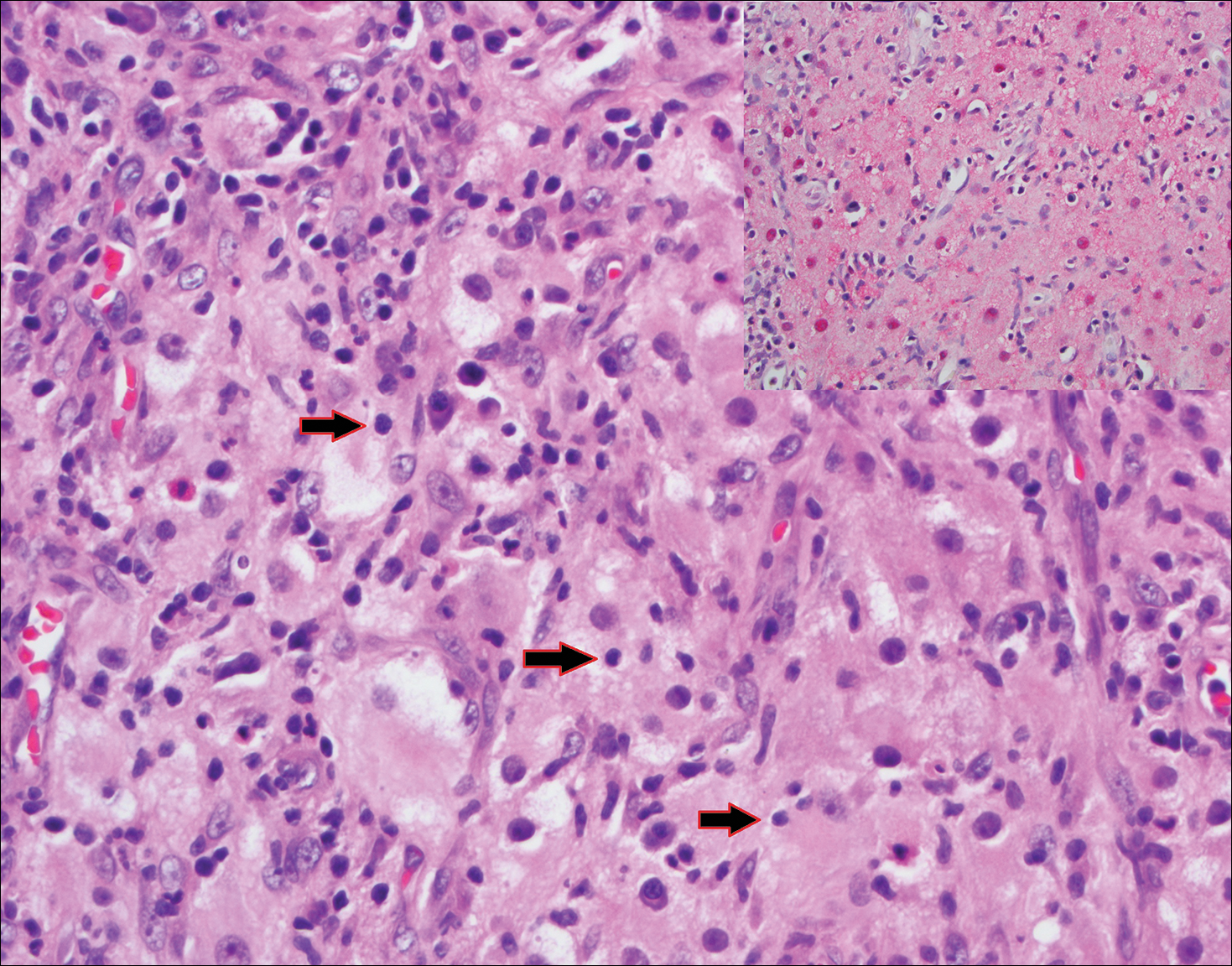
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.
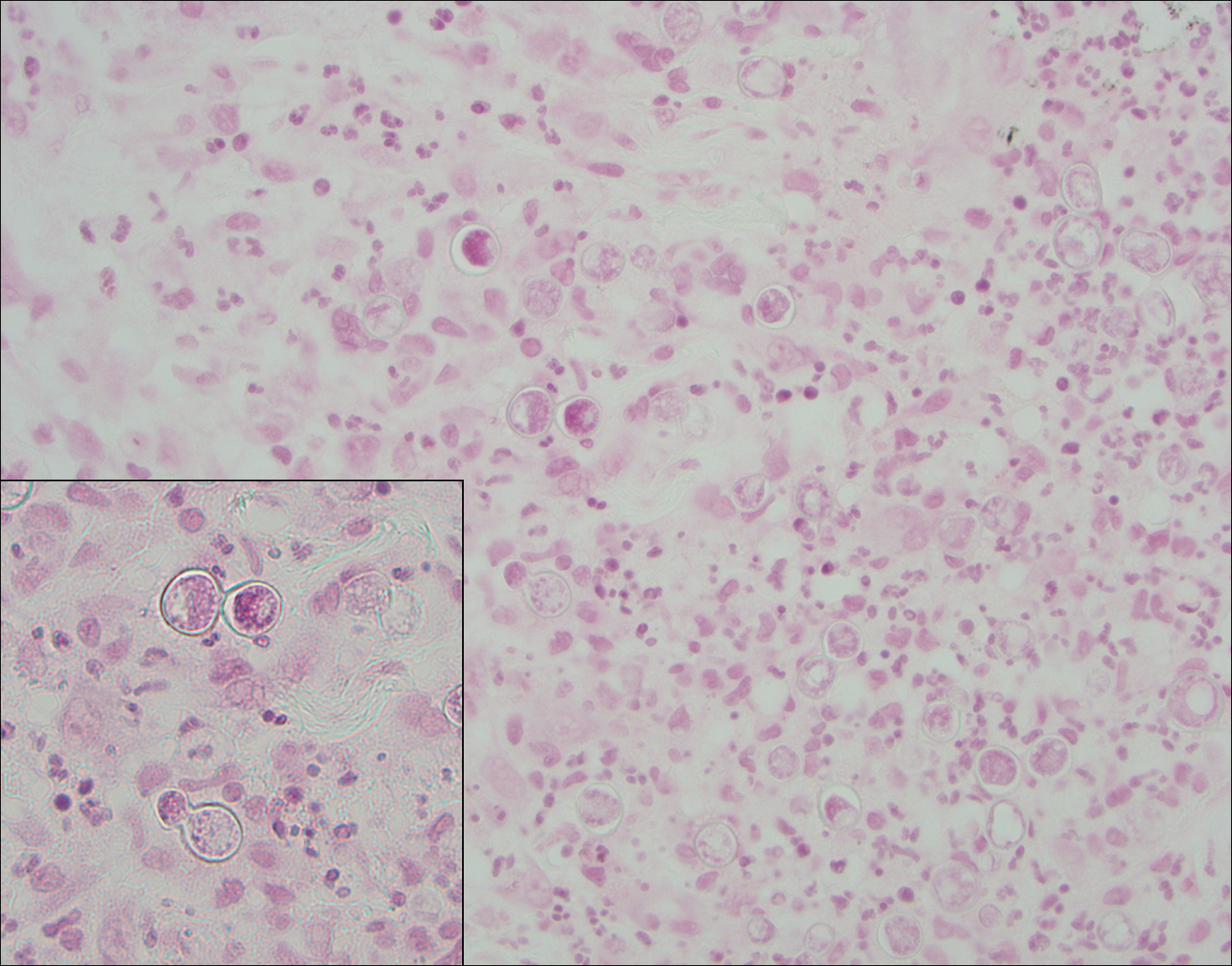
Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3
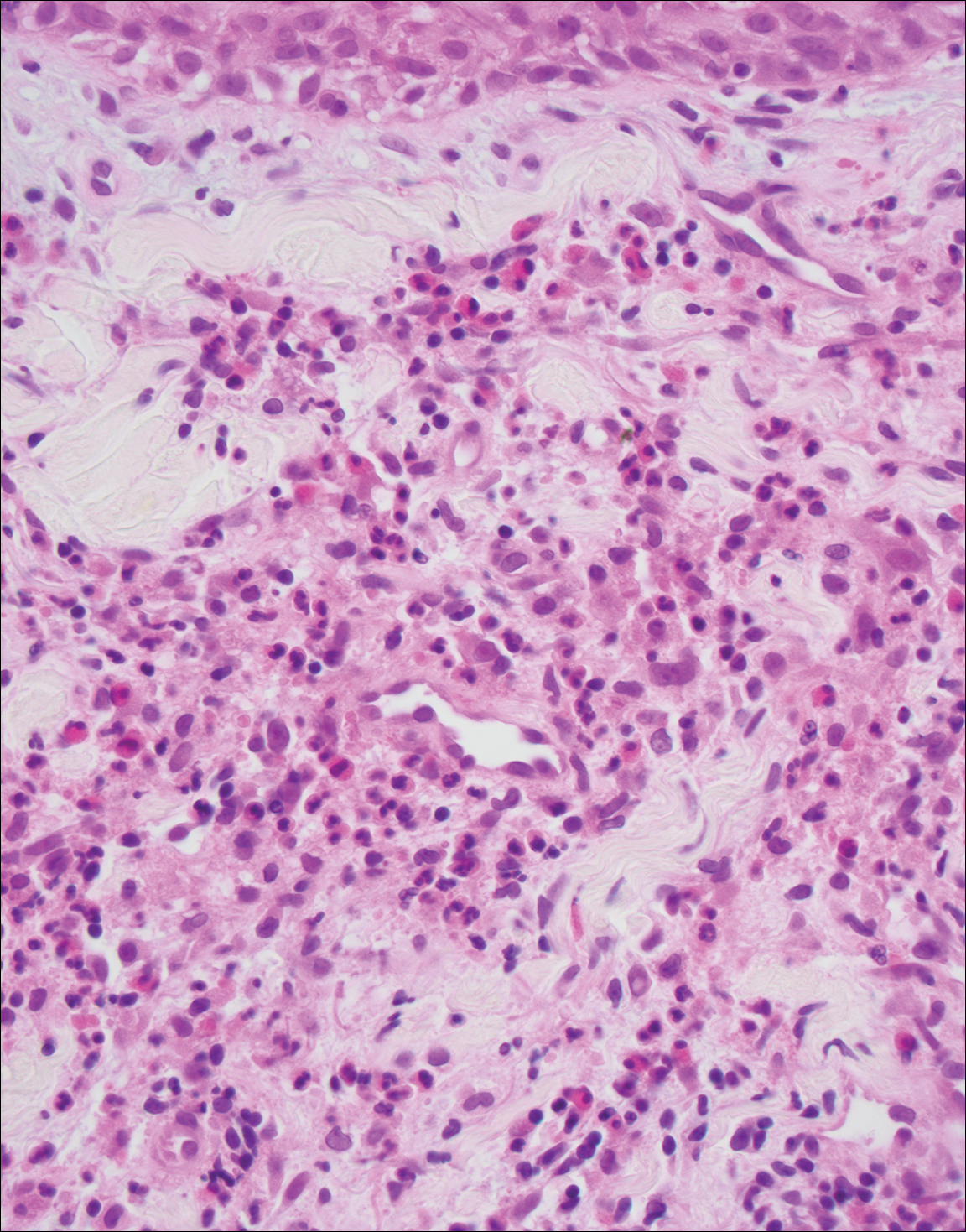
Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.
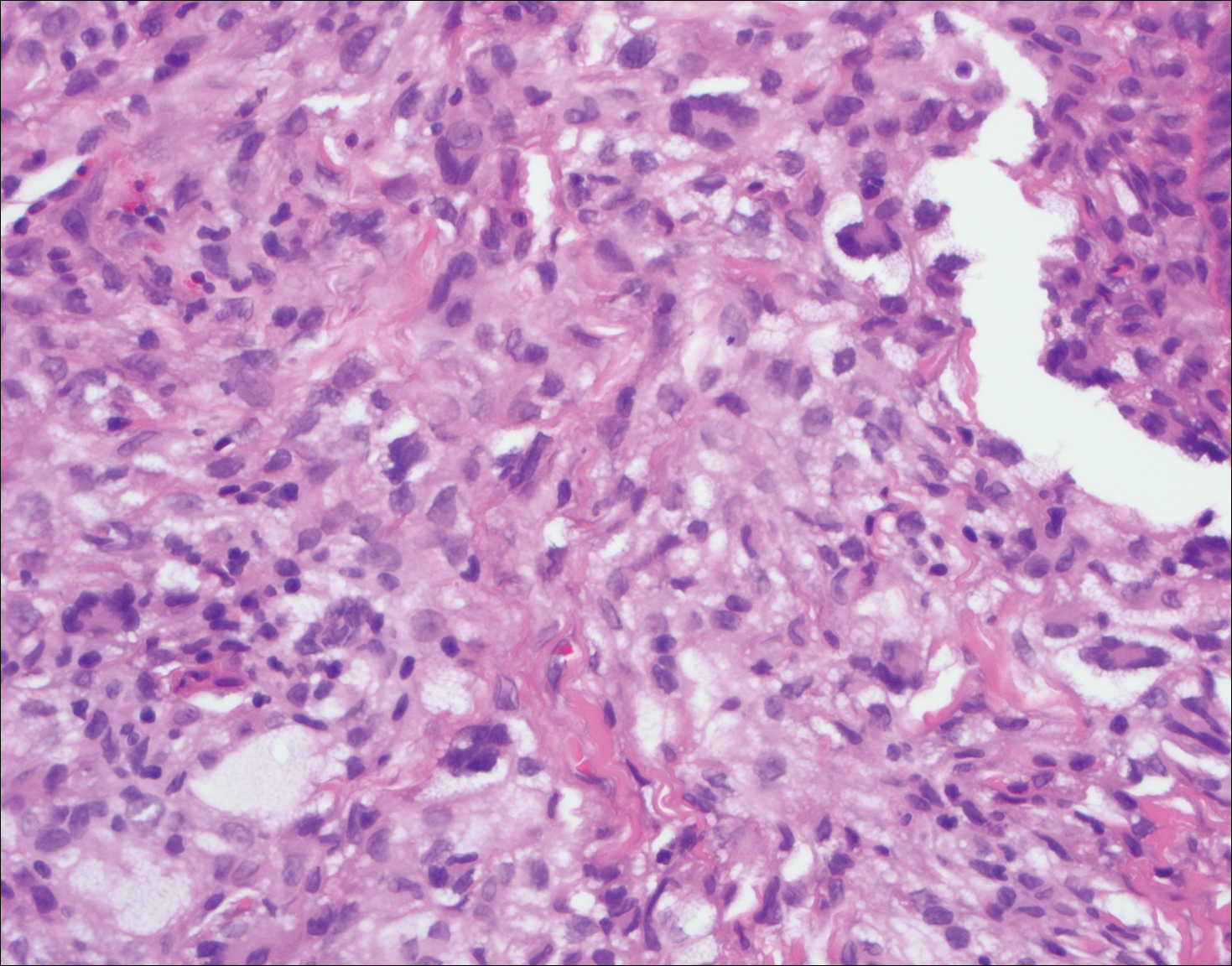
Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.
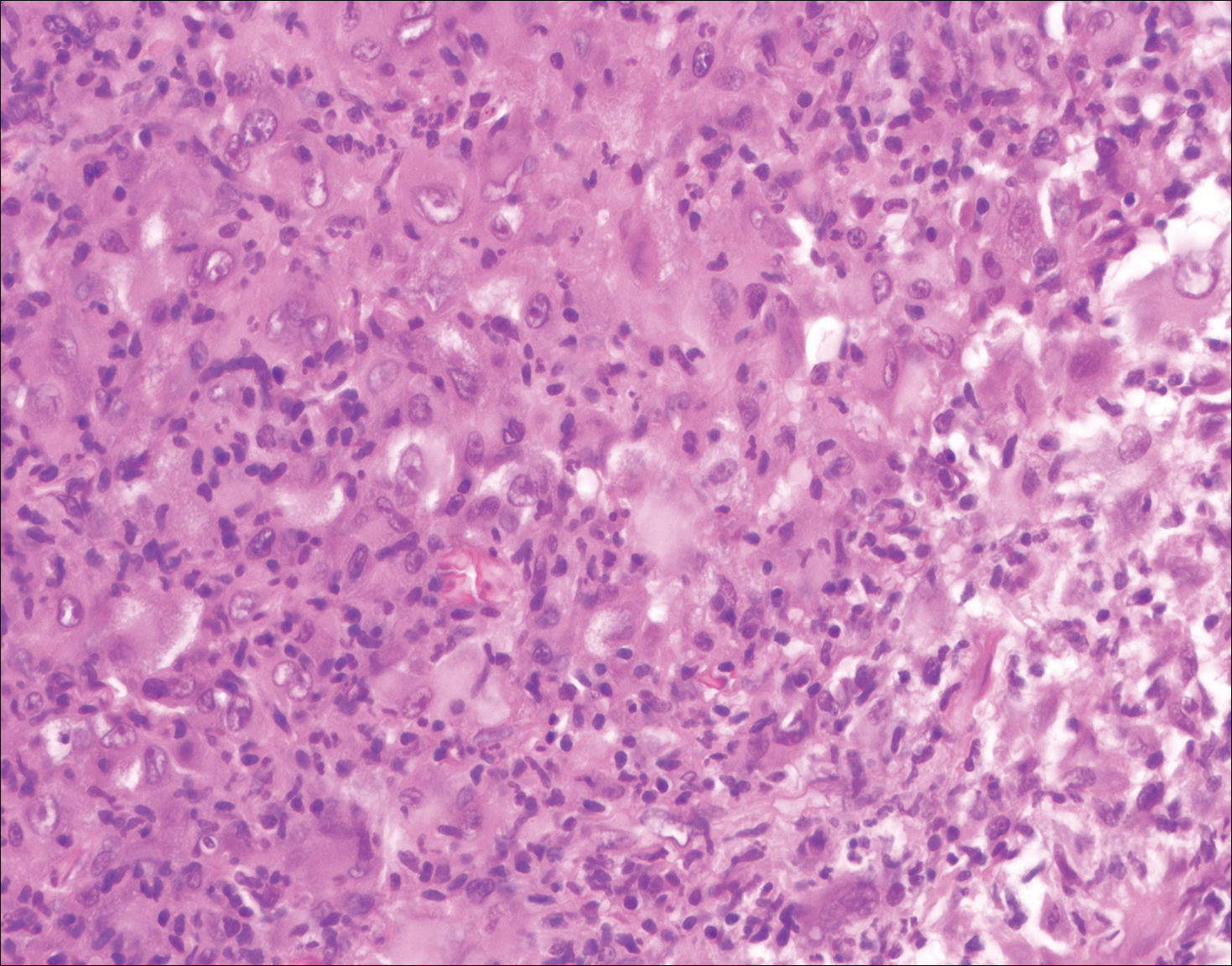
Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.
- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.

Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3

Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.

Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.

Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.

The Diagnosis: Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare histiocytic proliferative disorder of unknown etiology. It has 2 forms: limited cutaneous and systemic. The systemic form, also known as sinus histiocytosis with massive lymphadenopathy, affects the lymph nodes and other organs at times. The disease is characterized by a proliferation of histiocytes in the lymph nodes, most commonly in the cervical basin1; however, the inguinal, axillary, mediastinal, or para-aortic nodes also may be affected.1,2 The skin is the most common site of extranodal disease, seen in approximately 10% of cases.1 Cutaneous involvement often is in the facial area but also can be found on the trunk, ears, neck, arms, legs, and genitals. Clinically, skin lesions appear as papules, plaques, and/or nodules.2
Histopathologic examination of Rosai-Dorfman disease generally shows a dense sheetlike dermal infiltrate of large polygonal histiocytes (Figure 1). Histiocytes may display pale pink or clear cytoplasm. The pathognomonic finding is emperipolesis, which consists of histiocytes with engulfed lymphocytes, erythrocytes, plasma cells, and/or granulocytes surrounded by a clear halo. Immunohistochemical staining also is characteristic, with lesional histiocytes showing expression of S-100 protein (Figure 1, inset) and CD68. The associated inflammatory infiltrate is mixed, containing primarily plasma cells but also lymphocytes, neutrophils, and eosinophils.

Blastomycosis (Figure 2) is a systemic infection due to inhalation of Blastomyces dermatitidis conidia. Primary infection occurs in the lungs, and with dissemination the skin is the most common subsequently involved organ.3 Cutaneous blastomycosis shows pseudoepitheliomatous hyperplasia with neutrophilic microabscesses and a dense dermal infiltrate containing suppurative granulomatous inflammation. The nonpigmented yeast phase typically is 8 to 15 µm in length with a refractile cell wall and characteristic single, broad-based budding.3

Granuloma faciale (Figure 3) is a rare disease with unknown etiology characterized by reddish brown plaques or nodules most commonly occurring on the face.4,5 Histology shows a dense nodular dermal infiltrate with a grenz zone. The infiltrate is mixed, containing mostly neutrophils with leukocytoclasis and eosinophils. Leukocytoclastic vasculitis is present with associated extravasated erythrocytes. In chronic fibrosing granuloma faciale, lesions can demonstrate fibrosis and hemosiderin deposition, similar to erythema elevatum diutinum.

Juvenile xanthogranuloma (Figure 4) is a common histiocytic disease of early childhood, though adult cases have been reported.6 Tumors are found on the head and trunk and are typically firm, reddish yellow papules or nodules.6,7 Histologic examination shows a nodular infiltrate of foamy histiocytes in the superficial dermis. Touton-type multinucleated giant cells with a peripheral rim of xanthomatized foamy cytoplasm and a wreathlike arrangement of nuclei are characteristic. Associated eosinophils are seen. No emperipolesis is present.

Reticulohistiocytoma (Figure 5) is a benign dermal lesion that presents as solitary or less commonly multiple red-brown papules or nodules.8 Lesions consist of well-delineated nodular aggregates of histiocytes containing a finely granular eosinophilic ground glass cytoplasm. Few, if any, eosinophils are found. The lack of Touton multinucleated giant cells or emperipolesis and lack of expression of S-100 protein helps to distinguish reticulohistiocytoma from other entities in the differential diagnosis.

- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
- Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73.
- Kutlubay Z, Bairamov O, Sevim A, et al. Rosai-Dorfman disease: a case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014;36:353-357.
- James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Wolff K, Johnson R, Saavedra AP. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Marcoval J, Moreno A, Peyrí J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Tanz WS, Schwartz RA, Janniger CK. Juvenile xanthogranuloma. Cutis. 1994;54:241-245.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatology Online J. 2014;20. pii:doj_21725.
A 59-year-old man presented with itchy and mildly painful nodules on the head and neck of 7 months' duration. The patient denied fever, chills, unintentional weight loss, night sweats, and other systemic symptoms. Physical examination revealed multiple firm pink-orange nodules of varying sizes distributed on the scalp, face, and neck. Right-sided, painless, bulky cervical lymphadenopathy also was noted. An incisional biopsy was performed.
The Atopic Dermatitis Biologic Era Has Begun
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
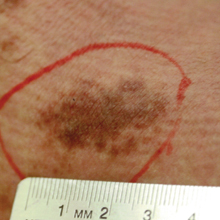
Hyperpigmented Patch on the Leg
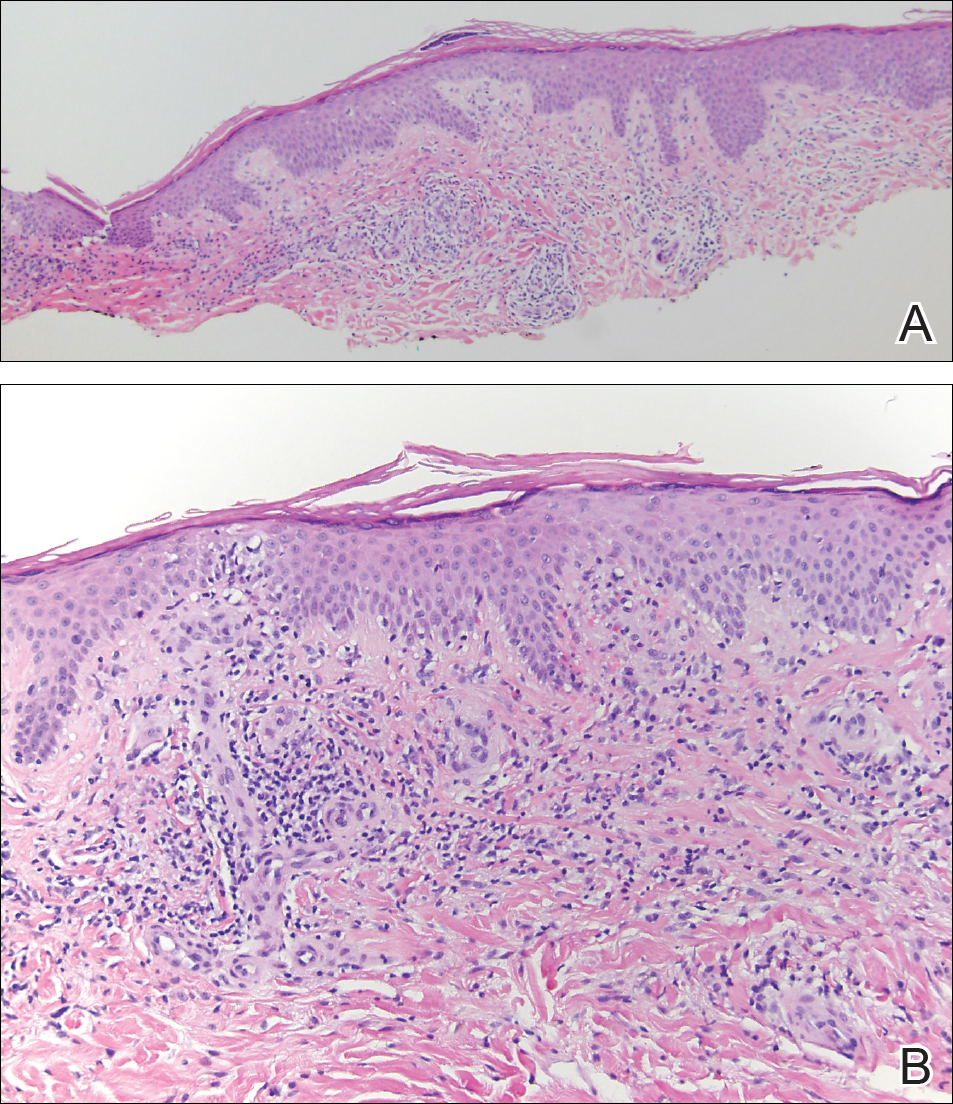
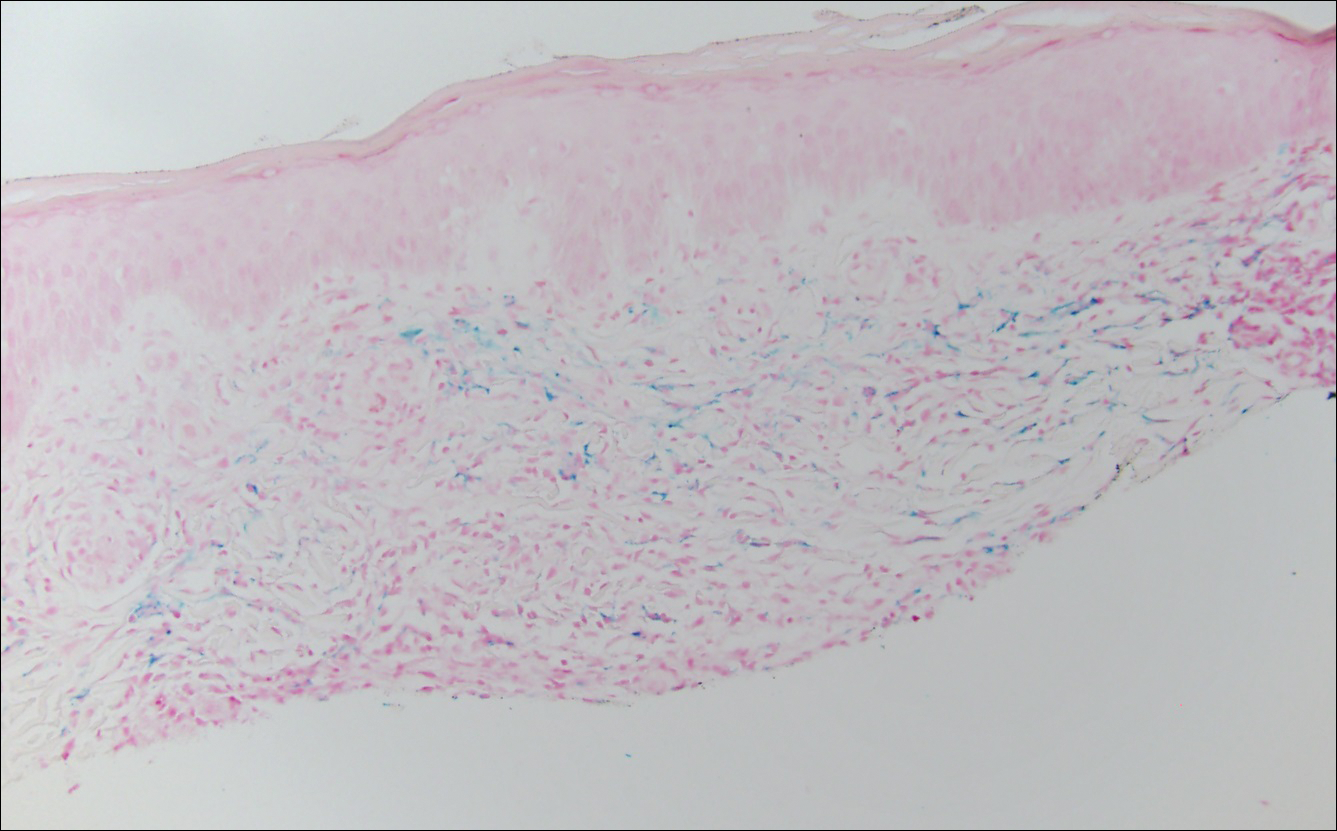
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).
Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
The Diagnosis: Lichen Aureus
The clinicopathological findings were diagnostic of lichen aureus (LA). Microscopic examination revealed a relatively sparse, superficial, perivascular and interstitial lymphohistiocytic infiltrate with scattered siderophages in the upper dermis. Extravasation of red blood cells also was noted (Figure 1). An immunohistochemical stain for Melan-A highlighted a normal number and distribution of single melanocytes at the dermoepidermal junction with no evidence of pagetoid scatter. A Perls Prussian blue stain for iron demonstrated abundant hemosiderin in the dermis (Figure 2).


Pigmented purpuric dermatosis (PPD) describes a group of cutaneous lesions that are characterized by petechiae and pigmentary changes. These lesions most commonly present on the lower limbs; however, other sites have been reported.1 This group includes several major clinical forms such as Schamberg disease, LA, purpura annularis telangiectodes of Majocchi, eczematidlike purpura of Doucas and Kapetanakis, and lichenoid PPD of Gougerot and Blum. Lesions typically demonstrate a striking golden brown color clinically and by definition occur in the absence of platelet defects or vasculitis.1
Factors implicated in the pathogenesis of pigmented purpura include gravitational dependency, venous stasis, infection, and drugs.2 It is suggested that cellular immunity may play a role in the development of the disease based on the presence of CD4+ T lymphocytes in the infiltrate and the expression of HLA-DR by these lymphocytes and the keratinocytes.3 Lichen aureus differs in that it relates to increased intravascular pressure from an incompetent valve in an underlying perforating vein.4
Lichen aureus, also referred to as lichen purpuricus, is one major variant of PPD. The name reflects both the characteristic golden brown color and the histopathologic pattern of inflammation.1 Lichen aureus usually presents as a unilateral, asymptomatic, confined single lesion located mainly on the leg,1 though it can develop at other sites or as a localized group of lesions. Extensive lesions have been reported5 and cases with a segmental distribution have been described.6 In contrast, Schamberg disease demonstrates pinhead-sized reddish lesions giving the characteristic cayenne pepper pigmentation. These lesions coalesce to form thumbprint patches that progress proximally.1 Majocchi purpura is annular and telangiectatic, while lichenoid purpura of Gougerot and Blum presents with flat-topped, polygonal, violaceous papules that turn brown over time.
Some authors have championed a role for dermoscopy in diagnosis of LA.7 By dermoscopy, LA demonstrates a diffuse copper background reflecting the lymphohistiocytic dermal infiltrate, red dots and globules representing the extravasated red blood cells and the dilated swollen vessels, and grey dots that reflect the hemosiderin present in the dermis.8
Histologically, LA demonstrates a superficial perivascular infiltrate composed mainly of CD4+ lymphocytes surrounding the superficial capillaries. Over time, red cell extravasation leads to the formation of hemosiderin-laden macrophages, which can be highlighted with Perls Prussian blue stain. A bandlike infiltrate with thin strands of collagen separating it from the epidermis also may be noted.9
An important consideration in the differential diagnosis of PPD is mycosis fungoides (MF). Mycosis fungoides is a cutaneous T-cell lymphoma that clinically presents as a single or multiple hypopigmented or hyperpigmented patches or as erythematous scaly lesions in the patch or plaque stage. These lesions eventually may evolve into tumor stage.10 Mycosis fungoides may mimic PPD clinically and/or histopathologically, and rarely PPD also may precede MF.11 Involvement of the trunk, especially the lower abdomen and buttock region, favors a diagnosis of MF. Typically, histopathologic examination of MF demonstrates an epidermotropic lymphocytic infiltrate composed of atypical cerebriform lymphocytes overlying papillary dermal fibrosis. Although classic MF would be difficult to confuse with PPD, the atrophic lichenoid pattern of MF may show remarkable overlap with PPD.12 Such cases require clinicopathologic correlation, immunophenotyping of the epidermotropic lymphocytes, and occasionally T-cell clonality studies.
Lichen aureus is a chronic persistent disease unless the underlying incompetent perforator vessel is ligated. Various treatments have been used for other forms of pigmented purpura including topical corticosteroids, topical tacrolimus, systemic vasodilators such as prostacyclin and pentoxifylline, and phototherapy.1 Clinical follow-up is recommended for lesions that show some clinical or histopathological overlap with MF. Additional biopsies also may prove useful in establishing a definitive diagnosis in ambiguous cases.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Newton RC, Raimer SS. Pigmented purpuric eruptions. Dermatol Clin. 1985;3:165-169.
- Aiba S, Tagami H. Immunohistologic studies in Schamberg's disease. evidence for cellular immune reaction in lesional skin. Arch Dermatol. 1988;124:1058-1062.
- English J. Lichen aureus. J Am Acad Dermatol. 1985;12(2, pt 1):377-379.
- Duhra P, Tan CY. Lichen aureus. Br J Dermatol. 1986;114:395.
- Moche J, Glassman S, Modi D, et al. Segmental lichen aureus: a report of two cases treated with methylprednisolone aceponate. Australas J Dermatol. 2011;52:E15-E18.
- Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
- Portela PS, Melo DF, Ormiga P, et al. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253-255.
- Smoller BR, Kamel OW. Pigmented purpuric eruptions: immunopathologic studies supportive of a common immunophenotype. J Cutan Pathol. 1991;18:423-427.
- Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. a progress report. Am J Clin Pathol. 1999;111(1 suppl 1):S8-S12.
- Hanna S, Walsh N, D'Intino Y, et al. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354.
- Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
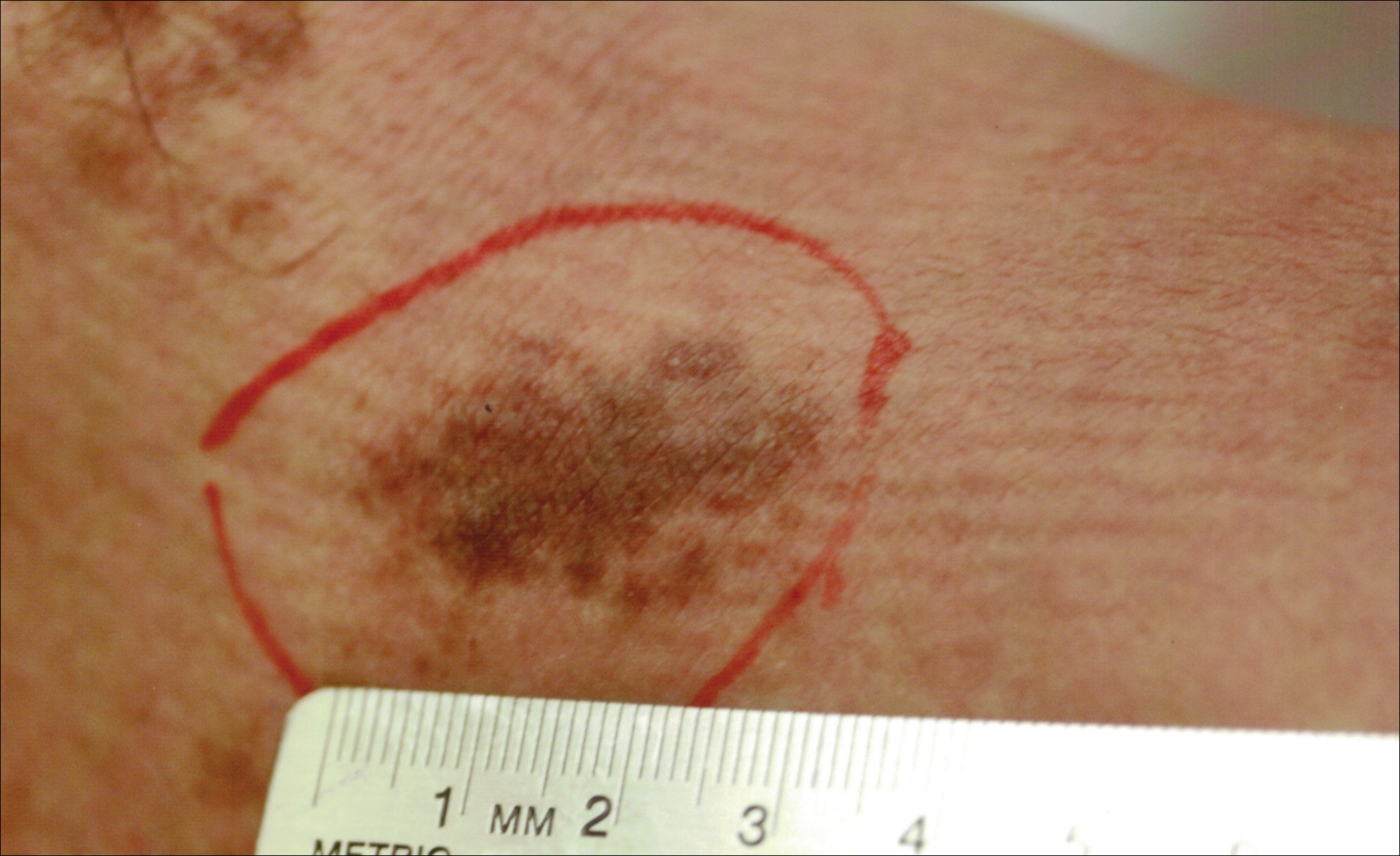
A 32-year-old man presented with an asymptomatic pigmented lesion on the left foot that developed over the course of 4 months. Physical examination revealed a 4-cm asymmetrical, deeply pigmented macule on the left foot. A shave biopsy of the lesion was performed.
Optical Coherence Tomography in Dermatology
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
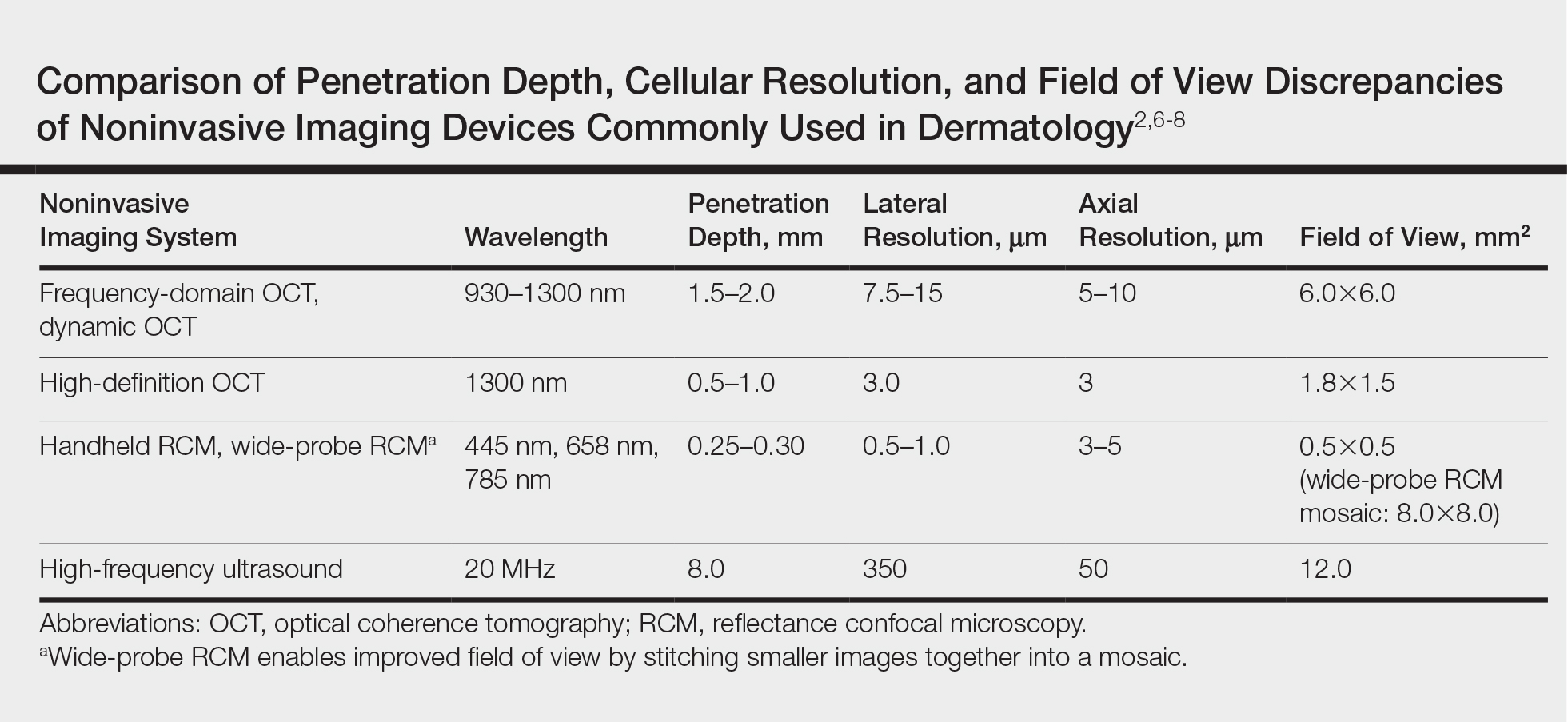
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12

Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12

Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Practice Points
- Optical coherence tomography (OCT) technology has considerable utility in research and clinical settings given its high resolution, wide field of view, moderate penetration depth, straightforward image acquisition, and accessibility to anatomically challenging areas.
- Potential benefits of OCT include its ability to noninvasively diagnose and monitor nonmelanoma skin cancers as well as to delineate presurgical margins and elucidate the course and mechanism of action of skin conditions at the bedside.
- Limitations of OCT include device cost, lack of reimbursement, and training, as well as restricted ability to image advanced deep tumors and differentiate melanocytic lesions.
- Optical coherence tomography recently received 2 category III Current Procedural Terminology (CPT) codes to track its utilization in clinical practice and will hopefully receive category I CPT codes within the next 5 years.
Incorporating New Atopic Dermatitis Medications in Your Practice
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.