User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Pushing the Limits: Developing a New Standard of Care for Psoriasis
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
We are now in the midst of a second revolution in the care of patients with psoriasis. Since biologic therapies for psoriasis were first introduced in 2003 with the approval of alefacept, the psoriasis treatment paradigm has shifted and continues to evolve. Interestingly, the first 2 biologic agents approved for psoriasis, alefacept and efalizumab, are no longer on the market in the United States.
We certainly have made progress since the early days of psoriasis treatment. Over the years, we have come to understand the nature of psoriasis as a systemic inflammatory condition rather than as simply a skin disease. With this knowledge, we have continued to identify systemic comorbidities associated with psoriasis, including cardiovascular risk, diabetes, and metabolic syndrome. It is therefore the role of the dermatologist to serve as the gatekeeper for these individuals and help to screen for comorbidities of psoriasis, as well as provide appropriate counseling and referral.
Additionally, psoriasis therapies have been approved for new segments of the population. In 2016, the US Food and Drug Administration approved a supplemental biologics license application for use of etanercept in children aged 4 years and older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Last year, the US Food and Drug Administration also approved an expanded indication for ustekinumab for the treatment of adolescents (aged 12 years and older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Another treatment development included the approval of apremilast as a new oral therapeutic option for psoriasis patients. This agent, which is approved for both psoriasis and psoriatic arthritis, has become an attractive therapy for many patients who are new to systemic treatment. Many patients prefer an oral medication and like the fact that no routine laboratory monitoring is required. Often patients leave their dermatologist’s office with 2- to 4-weeks’ worth of samples and can begin their course immediately.
A treat-to-target approach also has been established for psoriasis. In 2016, the Medical Board of the National Psoriasis Foundation1 created specific treatment goals in order to make achieving clear or almost clear skin the new standard of care. A consensus-building study conducted among 25 psoriasis experts revealed that the most preferred instrument for evaluating disease severity was body surface area (BSA). The time at which most participants preferred to evaluate patient response after starting a new psoriasis therapy was 3 months, and an acceptable response at this timepoint was considered to be either BSA involvement of 3% or less or improvement in BSA involvement of 75% or more compared to baseline. The target response at 3 months after starting treatment was BSA involvement of 1% or less. During the maintenance period, evaluation every 6 months was most preferred, and the target response at every 6-month follow-up evaluation was BSA involvement of 1% or less.1 These standards enable and encourage both clinicians and patients to maximize their treatment success.
Over the past several years, a variety of new biologic agents also have come to the market, including 3 IL-17 inhibitors (ixekizumab, brodalumab, and secukinumab) and one IL-23 inhibitor (guselkumab). All of these agents have added new options to the armamentarium for psoriasis treatment and are highly effective. Overall, the clinical improvement and safety profiles for these agents are promising, and these new drugs may be equal to or more efficacious than the currently available therapeutic options for psoriasis treatment; however, long-term studies are still needed to further establish the safety and efficacy profiles for these biologic agents. Even more novel therapies are in development, as will be discussed by Lee et al2 in this issue.
It is the purpose of this special issue to review new standards of care for psoriasis in 2018. We hope that you find this issue enjoyable and informative.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis [published online November 28, 2016]. J Am Acad Dermatol. 2017;76:290-298.
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(suppl 3):5-9.
Asymptomatic Subcutaneous Nodule on the Cheek
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
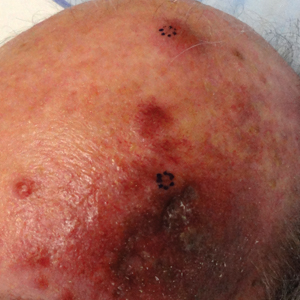
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
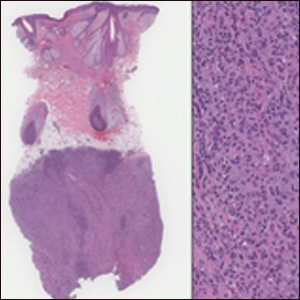
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2
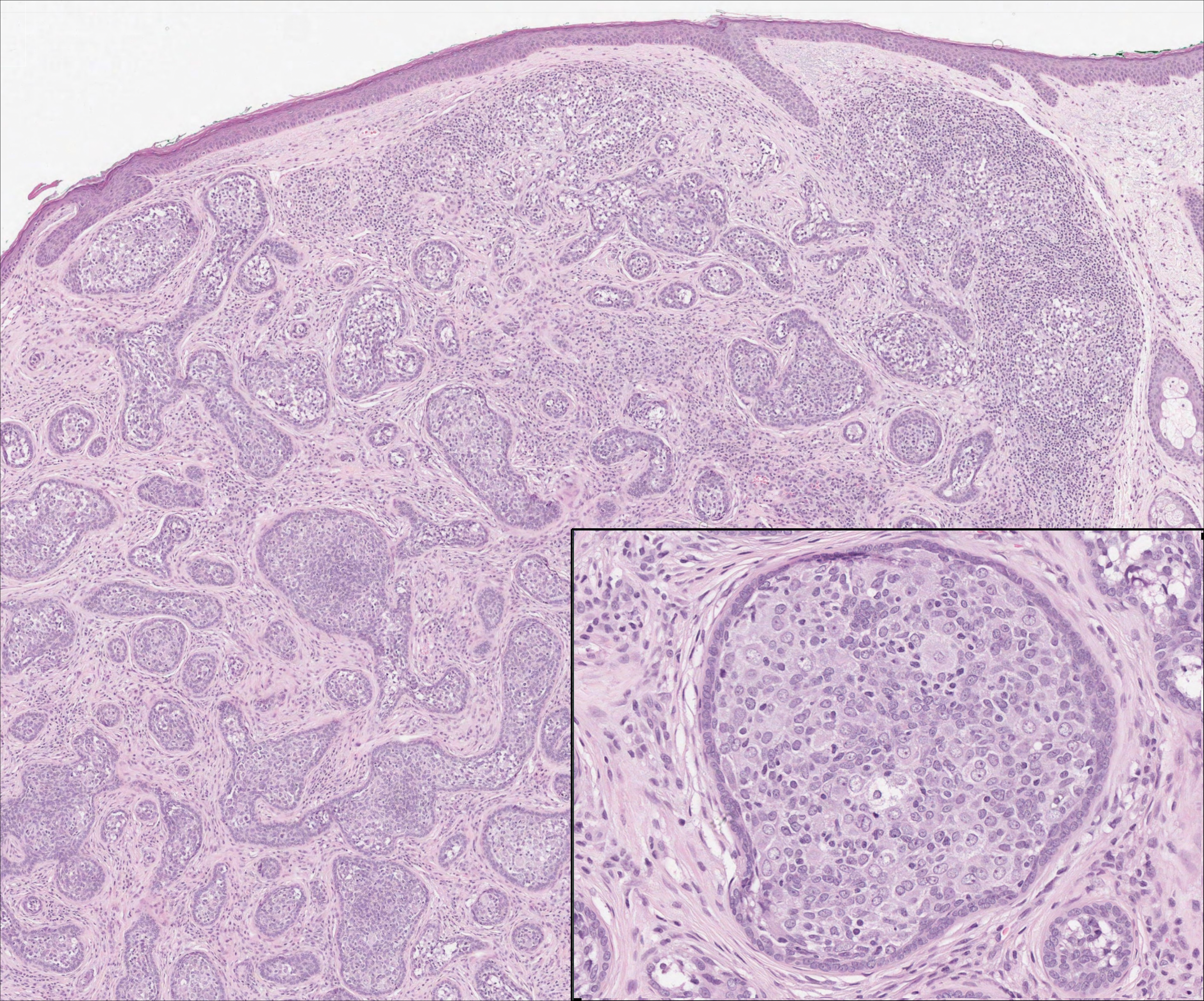
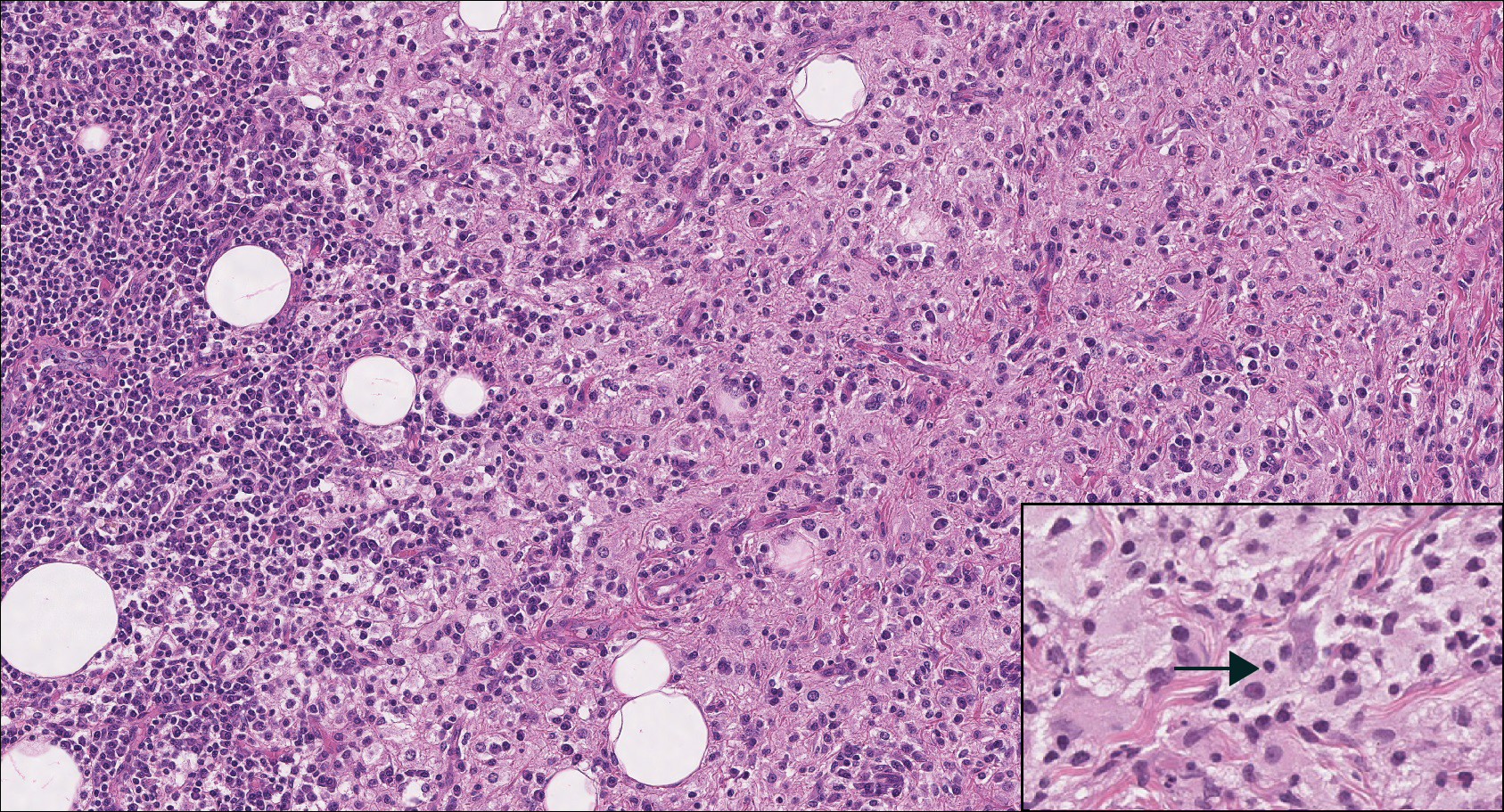
The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.
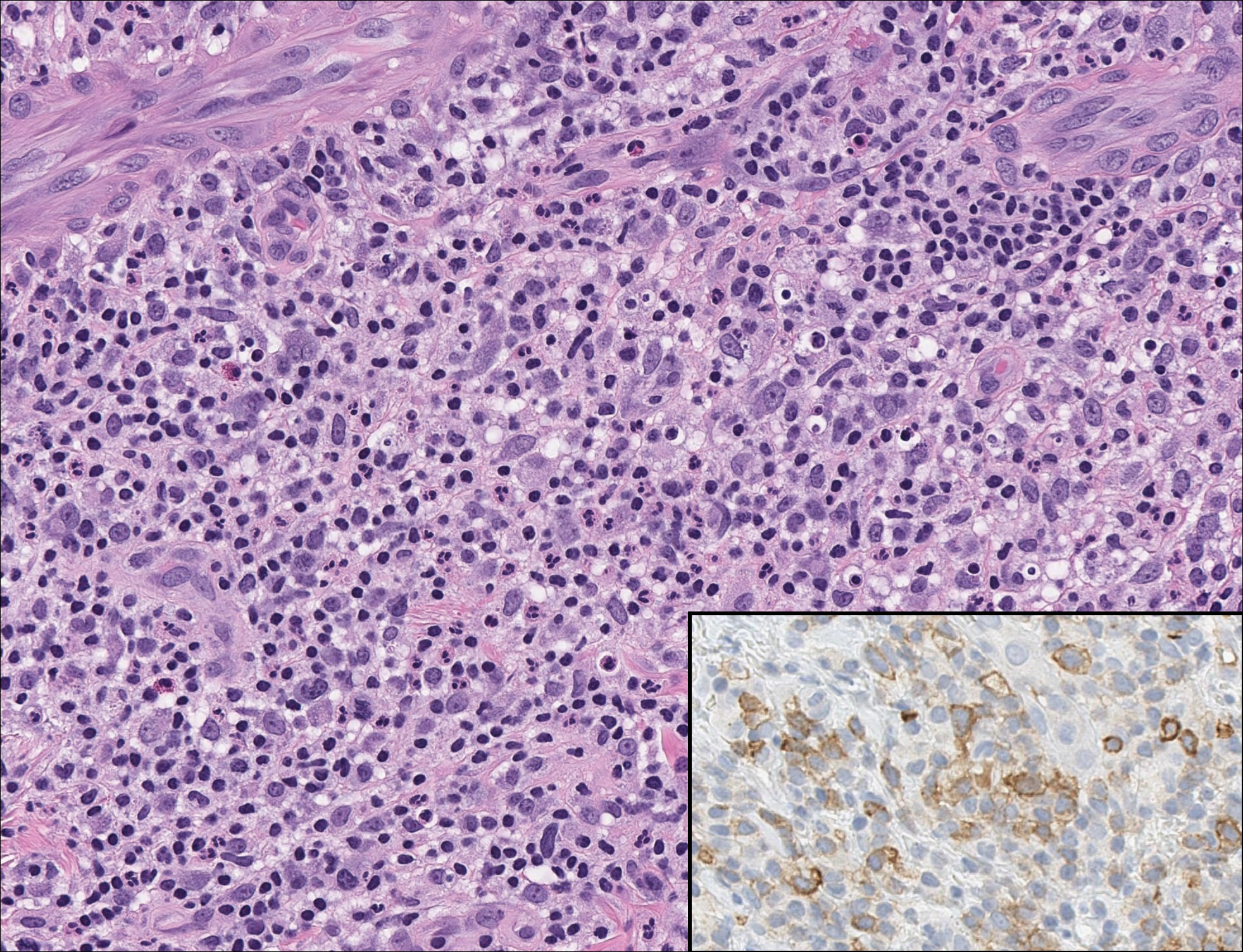
Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.
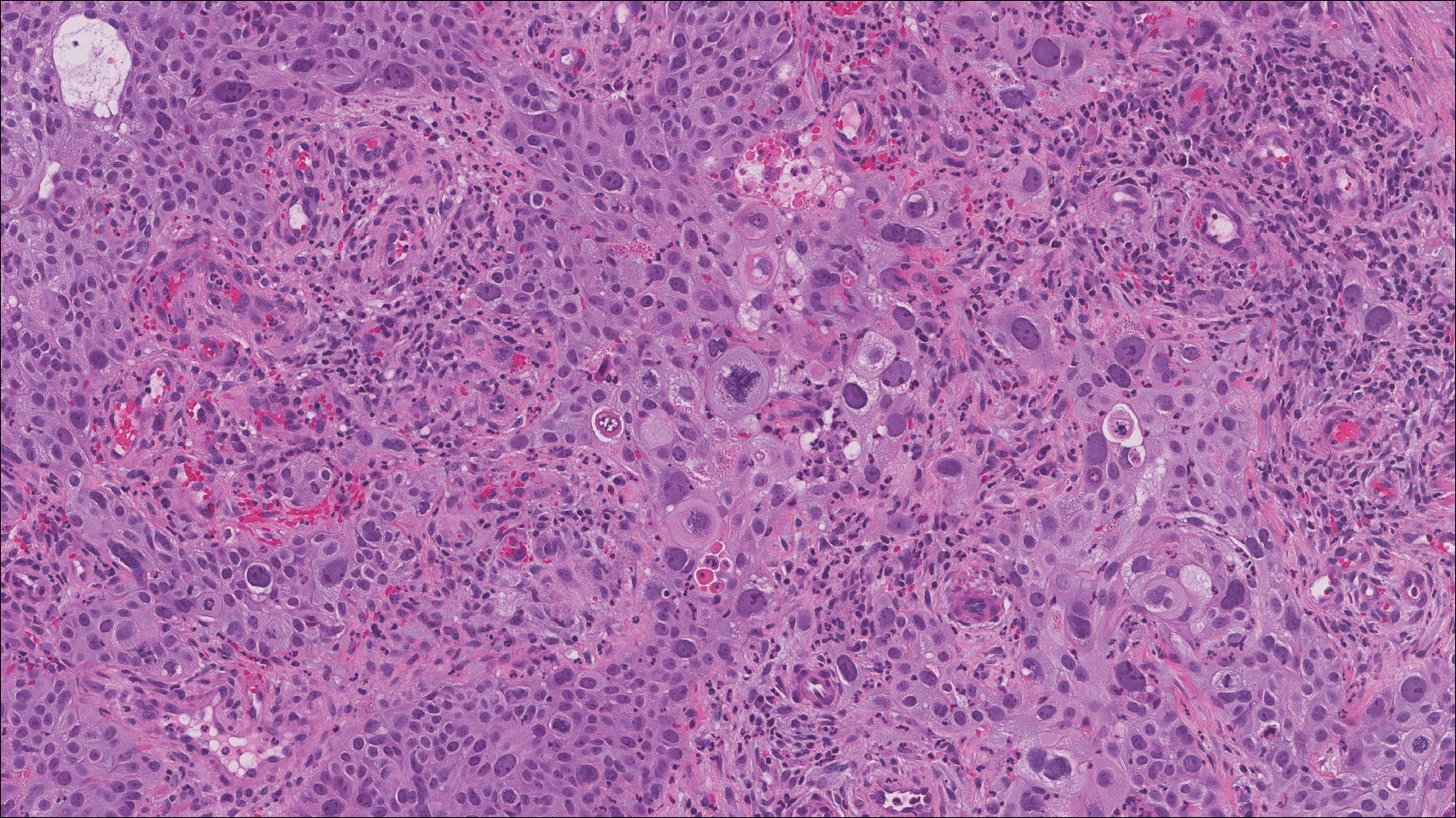
Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

The Diagnosis: Lymphoepitheliomalike Carcinoma of the Skin
The term lymphoepitheliomalike carcinoma of the skin (LELCS) initially was proposed by Swanson et al1 in 1988 when they described 5 patients with cutaneous neoplasms histologically resembling nasopharyngeal carcinoma, also known as lymphoepithelioma. A PubMed search of articles indexed for MEDLINE using the term lymphoepitheliomalike carcinoma of the skin revealed over 60 cases of LELCS since 1988. However, unlike nasopharyngeal carcinoma, LELCS has not been associated with Epstein-Barr virus, with the exception of 1 known reported case.2 The clinical appearance of LELCS is nonspecific but usually presents as a flesh-colored to erythematous nodule, as was seen in the current case. Lesions commonly are found on the head and neck in middle-aged to elderly patients with a slight male predominance.2
On histology, LELCS is characterized by aggregations of large, atypical epithelioid cells surrounded by a dense lymphoplasmocytic infiltrate (right quiz image). The neoplasm tends to reside within the deep dermis and/or subcutis1 without appreciable epidermal involvement (left quiz image). The atypical epithelioid cells demonstrate positive immunoreactivity for cytokeratins (right quiz image inset), p40/p63, and epithelial membrane antigen,3 and the surrounding lymphocytic infiltrate stains positively for leukocyte common antigen. The tumor histogenesis still is unknown, although an epidermal origin has been suggested given its staining pattern.2 Other investigators have postulated on an adnexal origin, citing the tumor's dermal location along with case reports describing possible glandular, sebaceous, or follicular differentiation.2,4
Treatment for LELCS can include either standard surgical excision or Mohs micrographic surgery, with radiation reserved for lymph node involvement, tumor recurrence, or poor surgical candidates.2,3,5 With appropriate therapy, prognosis may be considered favorable. Data from 49 LELCS patients presenting from 1988 and 2008 showed that 36 (73.5%) had no evidence of recurrence after treatment with standard surgical excision, 4 (8.2%) had local recurrence, and 6 (12.2%) developed lymph node metastasis, which led to death in 1 (2.0%) patient.2
Given the histologic similarity of LELCS to nasopharyngeal carcinoma, it is important to rule out the possibility of cutaneous metastasis, which can be done by testing for Epstein-Barr virus and performing either computed tomography imaging or comprehensive laryngoscopic examination of the head and neck region. In the current case, the patient was referred for laryngoscopy, at which time no suspicious lesions were identified. He subsequently underwent treatment with Mohs micrographic surgery, and the tumor was cleared after 2 surgical stages. At 5-month follow-up, the patient continued to do well with no signs of clinical recurrence.
Cutaneous lymphadenoma may be included in the differential diagnosis for LELCS on histopathology. This neoplasm is characterized by a well-circumscribed dermal proliferation of basaloid tumor islands within a fibrotic stroma (Figure 1). The basaloid cells may display peripheral palisading, and lymphocytes often are seen infiltrating the tumor lobules and the surrounding stroma (Figure 1 inset). Clinically, cutaneous lymphadenomas are slowly growing nodules that typically occur in young to middle-aged patients,4,6 unlike LELCS, which is more commonly observed in middle-aged to elderly patients.2

The dense lymphocytic infiltrate seen in LELCS may obscure the neoplastic epithelioid cells and in doing so may mimic a lymphoproliferative disorder, such as lymphomatoid papulosis (LyP). Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder consisting of recurrent crops of self-resolving papulonodules occurring on the trunk, arms, and legs. The average age of onset is in the third to fourth decades of life. Histology is dependent on the subtype; type A, the most common subtype, displays a wedge-shaped dermal infiltrate consisting of small lymphocytes (Figure 2) admixed with larger CD30+ atypical lymphocytes with prominent nucleoli (Figure 2 inset).7 Bizarre, binucleated forms resembling Reed-Sternberg cells also may be observed along with hallmark cells, which contain a horseshoe-shaped nucleus. The presence of admixed neutrophils and eosinophils also are common in type A LyP, a feature that is not characteristic of LELCS. Moreover, the atypical cells in LyP would not stain positively for epithelial markers as they would in LELCS.

Rosai-Dorfman disease is a rare condition that usually presents with painless cervical lymphadenopathy, typically in the first and second decades of life. Skin involvement can be seen in a small subset of extranodal cases, but cutaneous involvement alone is uncommon. On histopathology, cutaneous lesions are characterized by a dense dermal infiltrate of atypical histiocytes with vesicular nuclei and pale cytoplasm admixed with inflammatory cells, including lymphocytes, neutrophils, and plasma cells (Figure 3). Intracytoplasmic inflammatory cells or emperipolesis often is appreciated (Figure 3 inset).8,9 The atypical histiocytes stain positively for S100 and negatively for CD1a.

Lymphoepitheliomalike carcinoma of the skin sometimes is considered to be a poorly differentiated, inflamed variant of squamous cell carcinoma (SCC).10 A number of features may allow distinction of a primary cutaneous SCC from LELCS; for instance, SCC is more likely to have an epidermal connection and at least focal signs of squamous differentiation,11 which can include the presence of poorly differentiated epithelial cells with mitoses (Figure 4), keratin pearls, dyskeratotic cells, or intercellular bridges.12 Moreover, SCCs have a more variable surrounding inflammatory infiltrate compared to LELCS.

- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
- Swanson SA, Cooper PH, Mills SE, et al. Lymphoepithelioma-like carcinoma of the skin. Mod Pathol. 1988;1:359-365.
- Aoki R, Mitsui H, Harada K, et al. A case of lymphoepithelioma-like carcinoma of the skin associated with Epstein-Barr virus infection. J Am Acad Dermatol. 2010;62:681-684.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Requena L, Sánchez Yus E, Jiménez E, et al. Lymphoepithelioma-like carcinoma of the skin: a light-microscopic and immunohistochemical study. J Cutan Pathol. 1994;21:541-548.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Santa Cruz DJ, Barr RJ, Headington JT. Cutaneous lymphadenoma. Am J Surg Pathol. 1991;15:101-110.
- Patterson JW. Cutaneous infiltrates--lymphomatous and leukemic. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1186-1189.
- Patterson JW. Cutaneous infiltrates--nonlymphoid. In: Patterson JW, Hosler GA, eds. Weedon's Skin Pathology. 4th ed. London, United Kingdom: Churchill Livingstone; 2016:1158.
- Skiljo M, Garcia-Lora E, Tercedor J, et al. Purely cutaneous Rosai-Dorfman disease. Dermatology. 1995;191:49-51.
- Wang G, Bordeaux JS, Rowe DJ, et al. Lymphoepithelioma-like carcinoma vs inflamed squamous cell carcinoma of the skin. JAMA Dermatol. 2014;150:1367-1368.
- Hall G, Duncan A, Azurdia R, et al. Lymphoepithelioma-like carcinoma of the skin: a case with lymph node metastases at presentation. Am J Dermatopathol. 2006;28:211-215.
- Lind AC, Breer WA, Wick MR. Lymphoepithelioma-like carcinoma of the skin with apparent origin in the epidermis--a pattern or an entity? a case report. Cancer. 1999;85:884-890.
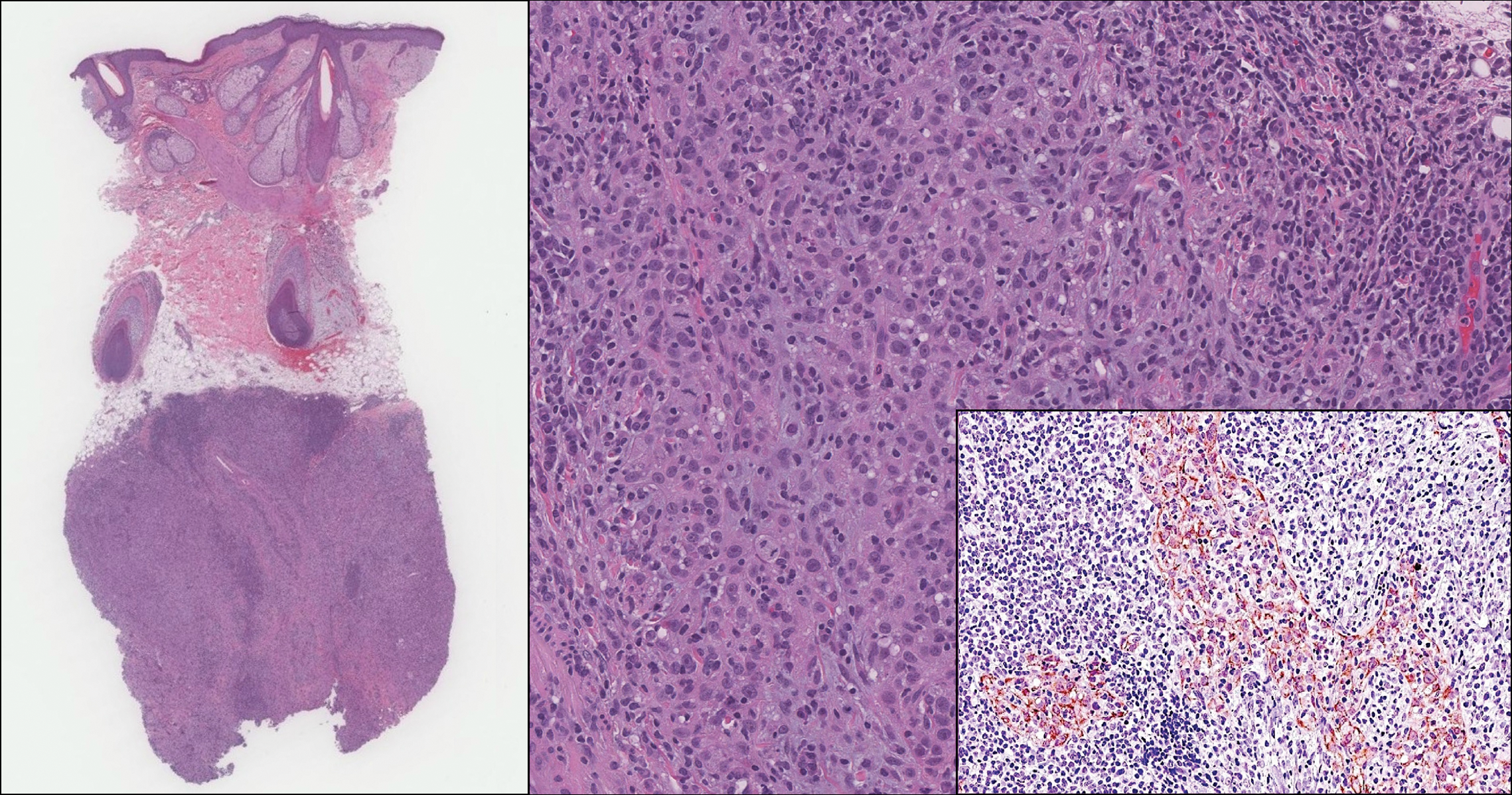
An 81-year-old man with history of melanoma and nonmelanoma skin cancer presented with a subcutaneous nodule on the left cheek of 3 months' duration. The lesion was reportedly asymptomatic and measured 2.6×2.9 cm. A punch biopsy of the lesion was obtained for histopathologic evaluation.
Bullous Eruption in 2 Brothers
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
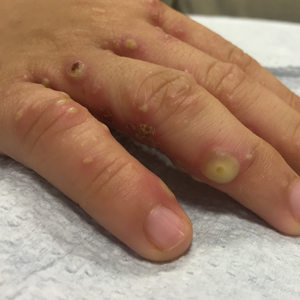
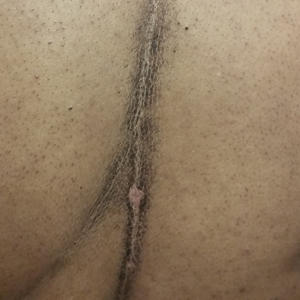
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
The Diagnosis: Bullous Scabies
Scabies infection is caused by the mite Sarcoptes scabiei var hominis. It is commonly transmitted via direct skin-to-skin contact.1 Classic manifestations include pruritus that worsens at night. It commonly presents with burrows and papules in the interdigital web spaces, as well as flexor surfaces of the wrists, elbows, axillae, buttocks, and genitalia. Pruritus occurs from infestation and delayed hypersensitivity reaction to mites. The recommended treatment of classic scabies is permethrin cream 5% for all occupants of the household and a repeat application for just the patients in 1 week. Posttreatment pruritus can last up to 3 weeks.2 At-risk populations include school-aged children and patients in long-term care facilities.
In our case, bullous lesions in a classic distribution with potassium hydroxide preparation of a scabietic mite (Figure) confirmed the diagnosis of bullous scabies. Treatment of bullous scabies is the same as classic scabies. Both patients were treated with 1 application of permethrin cream 5% before we evaluated them. We instructed to repeat application in 7 days for both boys and all family members.

Bullae may be secondary to hypersensitivity response3 or superinfection with Staphylococcus aureus causing bullous impetigo.4 Bullous scabies may present a diagnostic challenge and requires a high index of suspicion. Although childhood bullous pemphigoid can involve the palms and soles, patients usually present in infancy. Diagnoses such as dyshidrotic eczema and bullous tinea can present with pustules on the hands and feet; however, involvement of the genitalia would be uncommon.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
- Chosidow O. Clinical practices. scabies. N Engl J Med. 2006;354:1718-1727.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- Ansarin H, Jalali MH, Mazloomi S, et al. Scabies presenting with bullous pemphigoid-like lesions. Dermatol Online J. 2006;12:19.
- Herman PS. Letter: scabies and bullae. JAMA. 1975;231:1134.
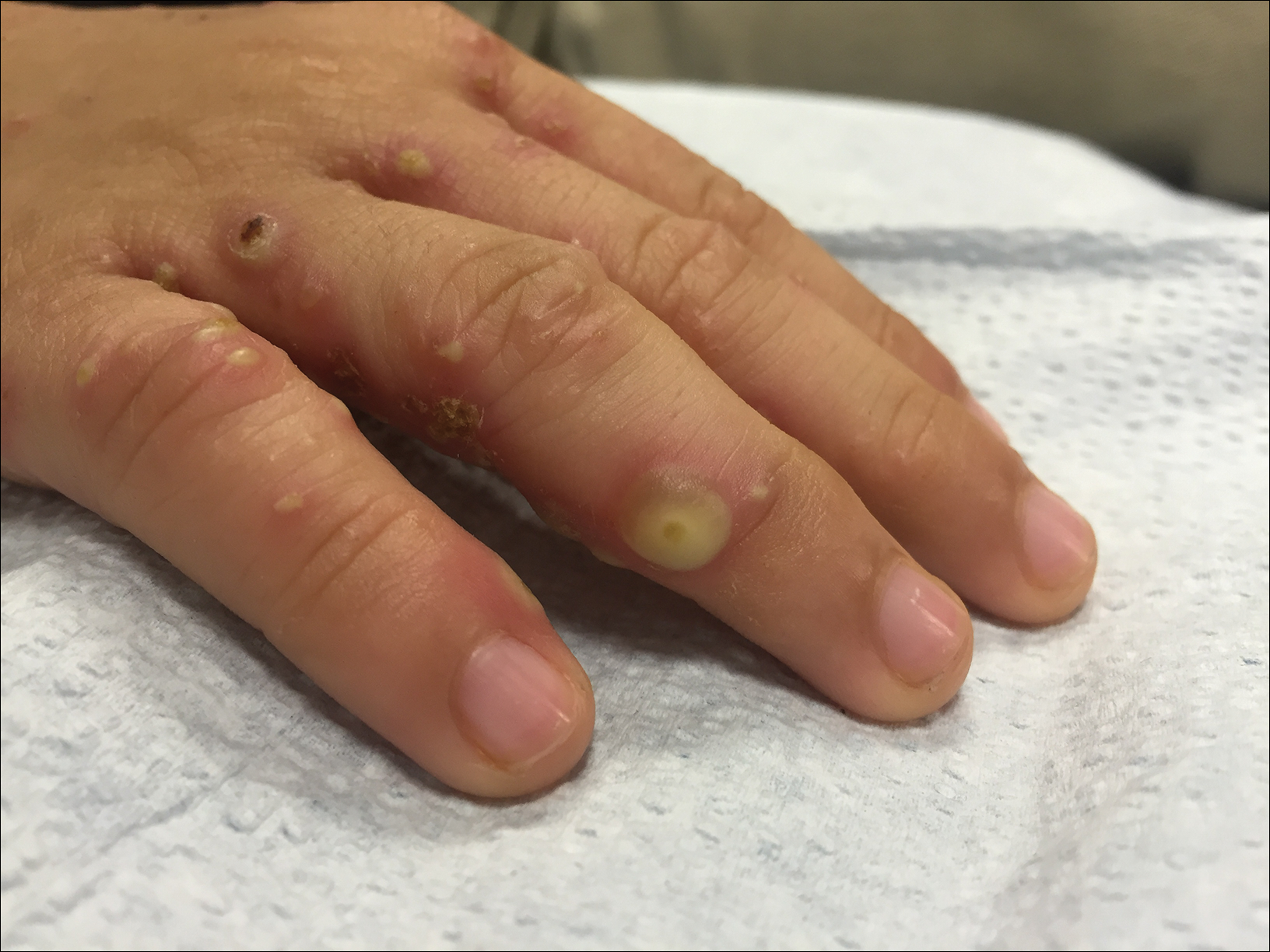
Brothers aged 7 and 8 years with a history of atopic dermatitis presented to the emergency department with similar diffuse pruritic eruptions of 1 week's duration. They previously were treated with permethrin cream 5% without improvement. Two days prior to presentation they developed painful pustules on the hands and feet. No other family members were affected. Physical examination revealed numerous yellow pustules and vesicles in the interdigital web spaces, elbows, and knees. Notably, the penis and scrotum also were involved in both brothers. A potassium hydroxide preparation of small pustules was obtained.
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
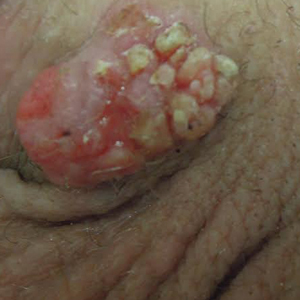
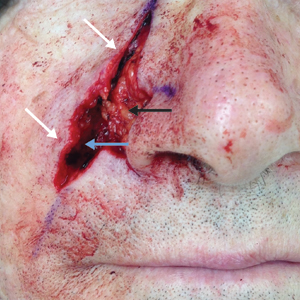
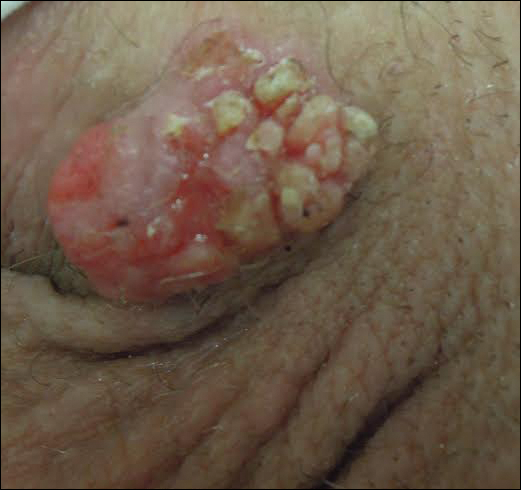
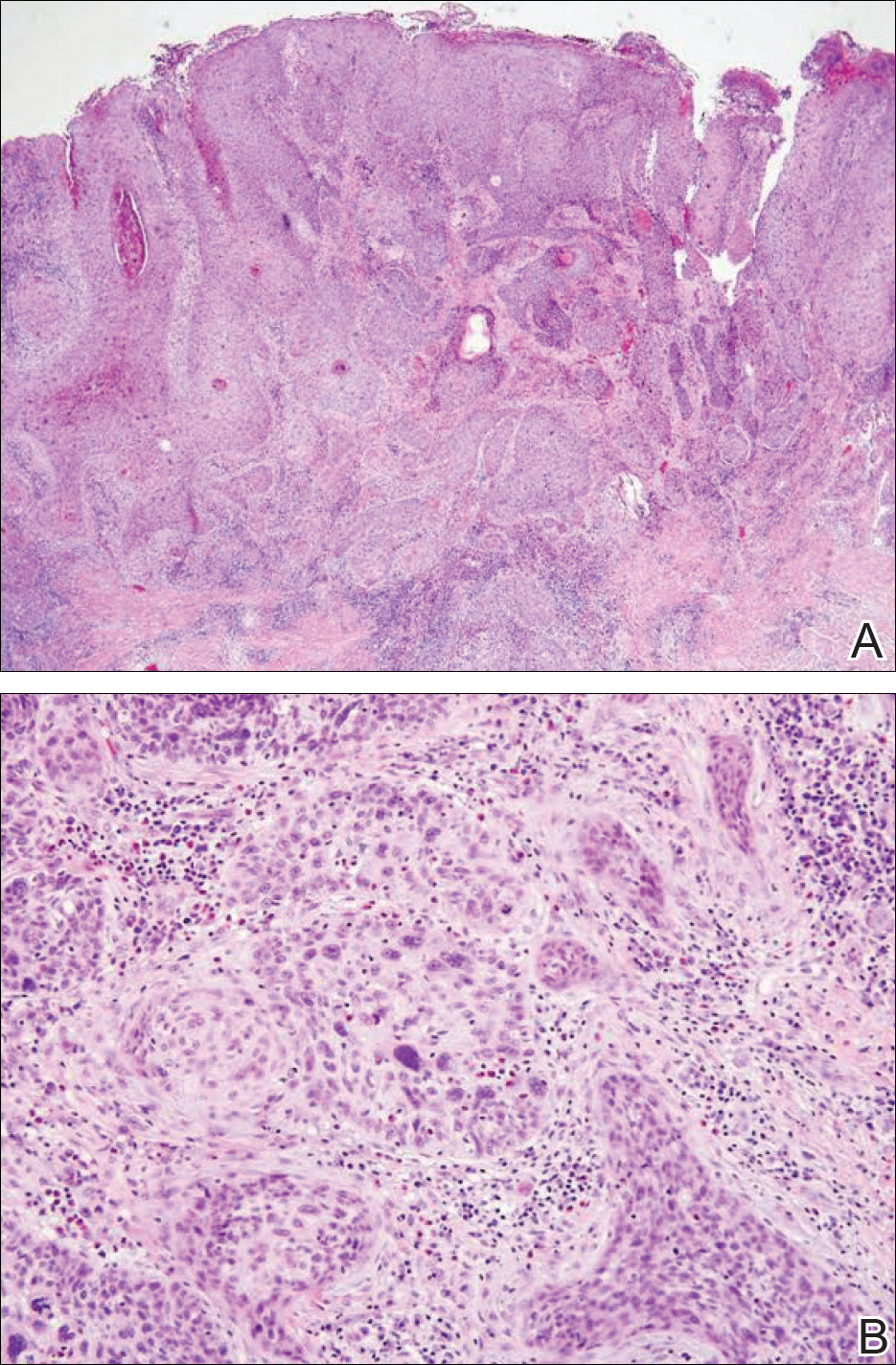
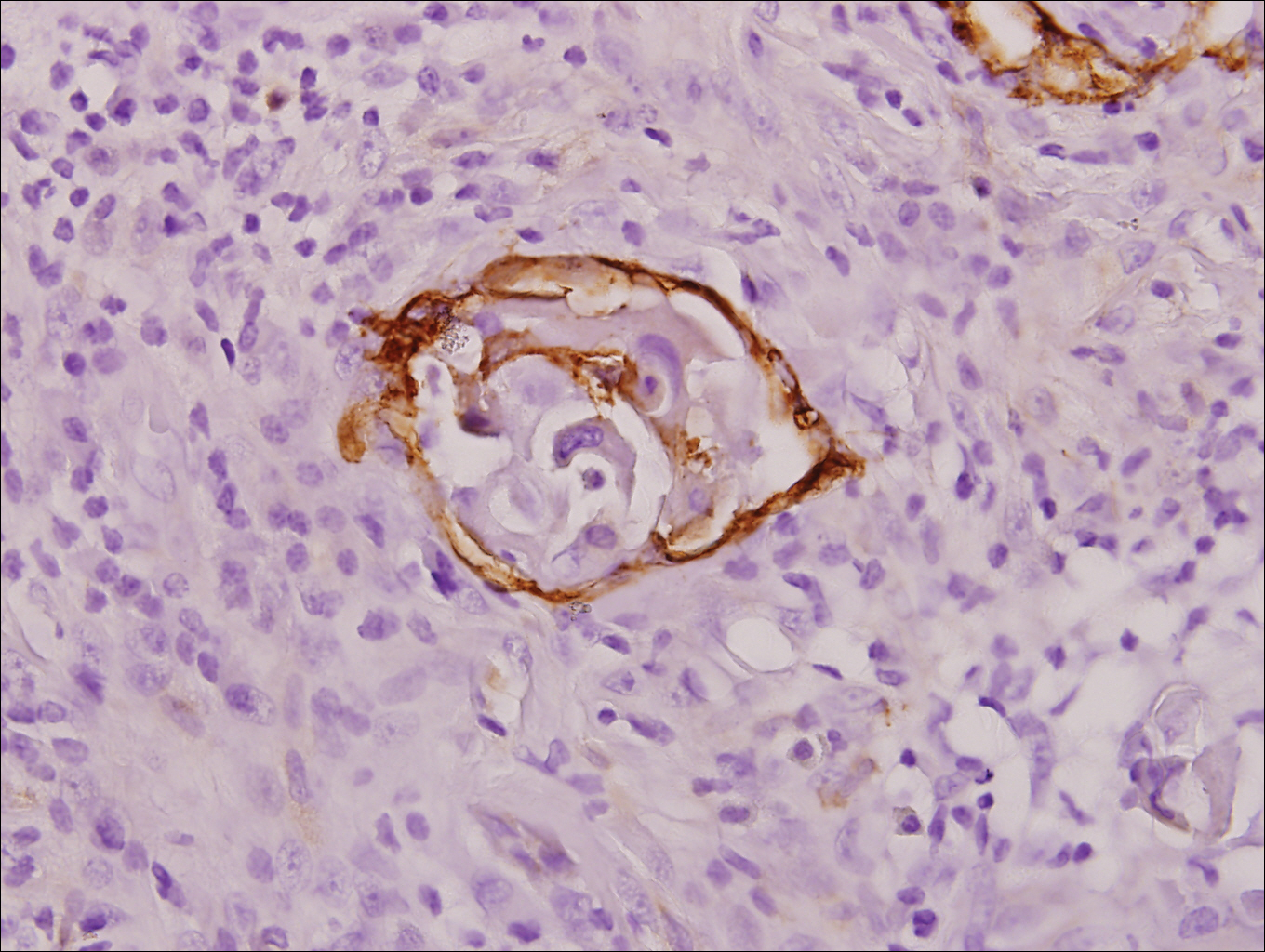
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.
Diffuse Cutaneous Breast Cancer Metastases Resembling Subcutaneous Nodules With No Surface Changes
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.
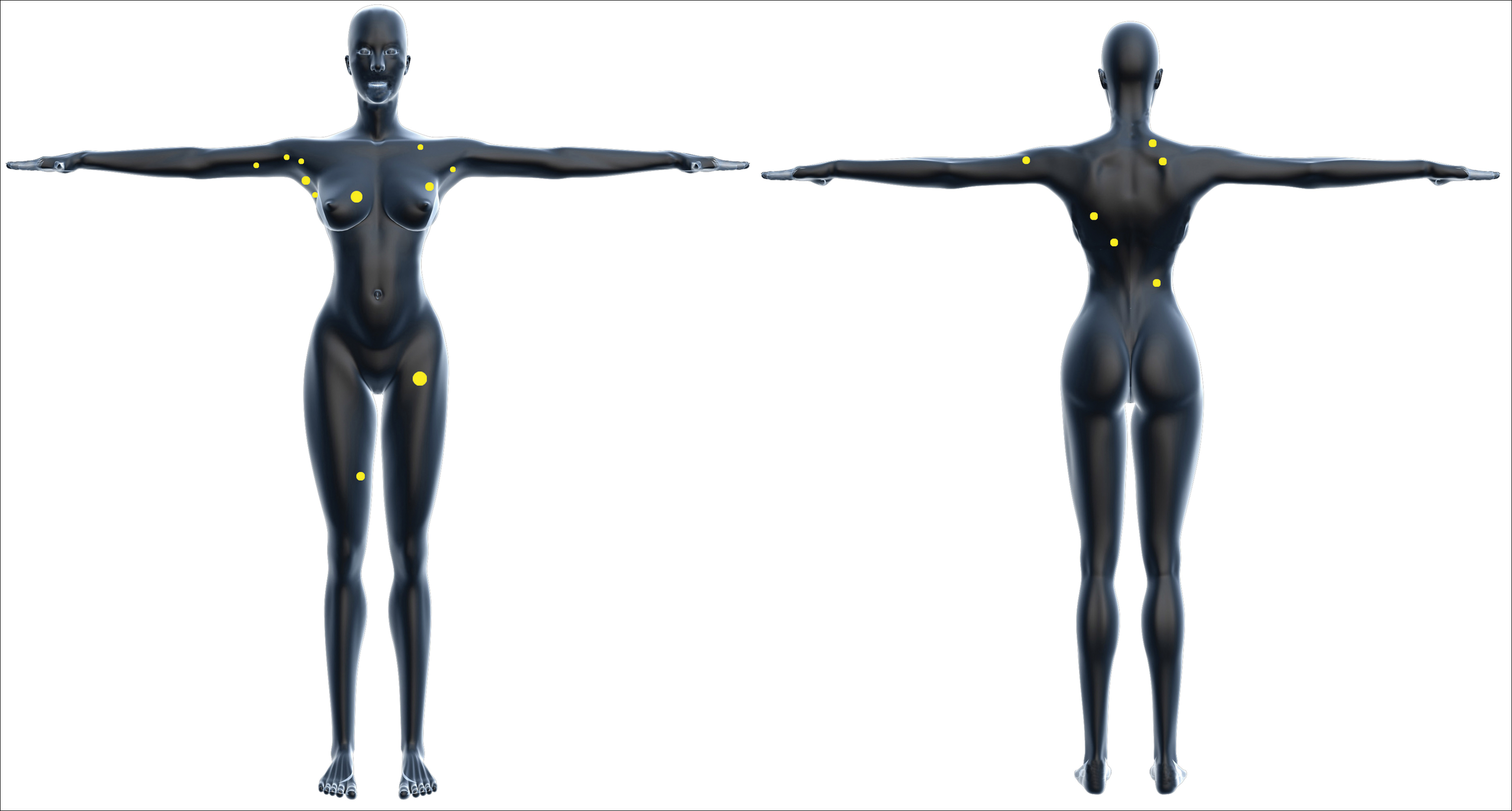
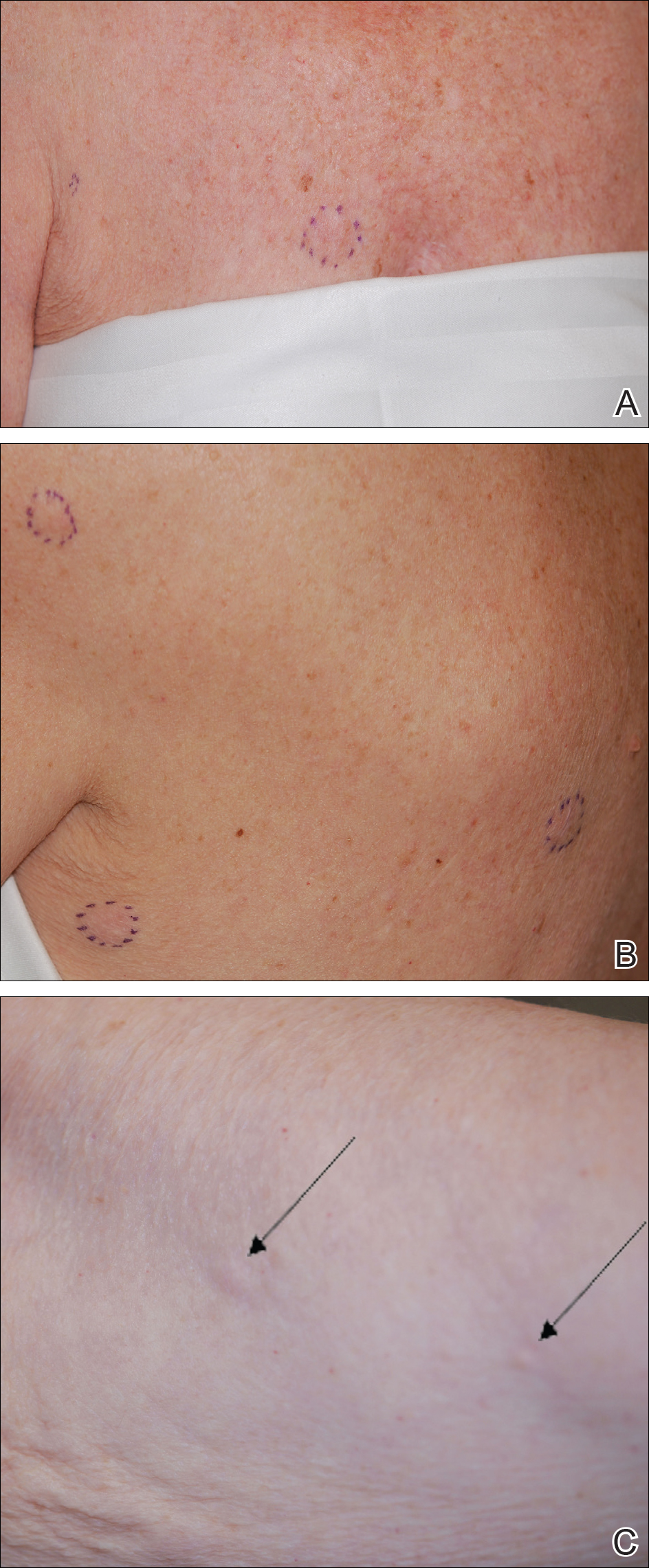
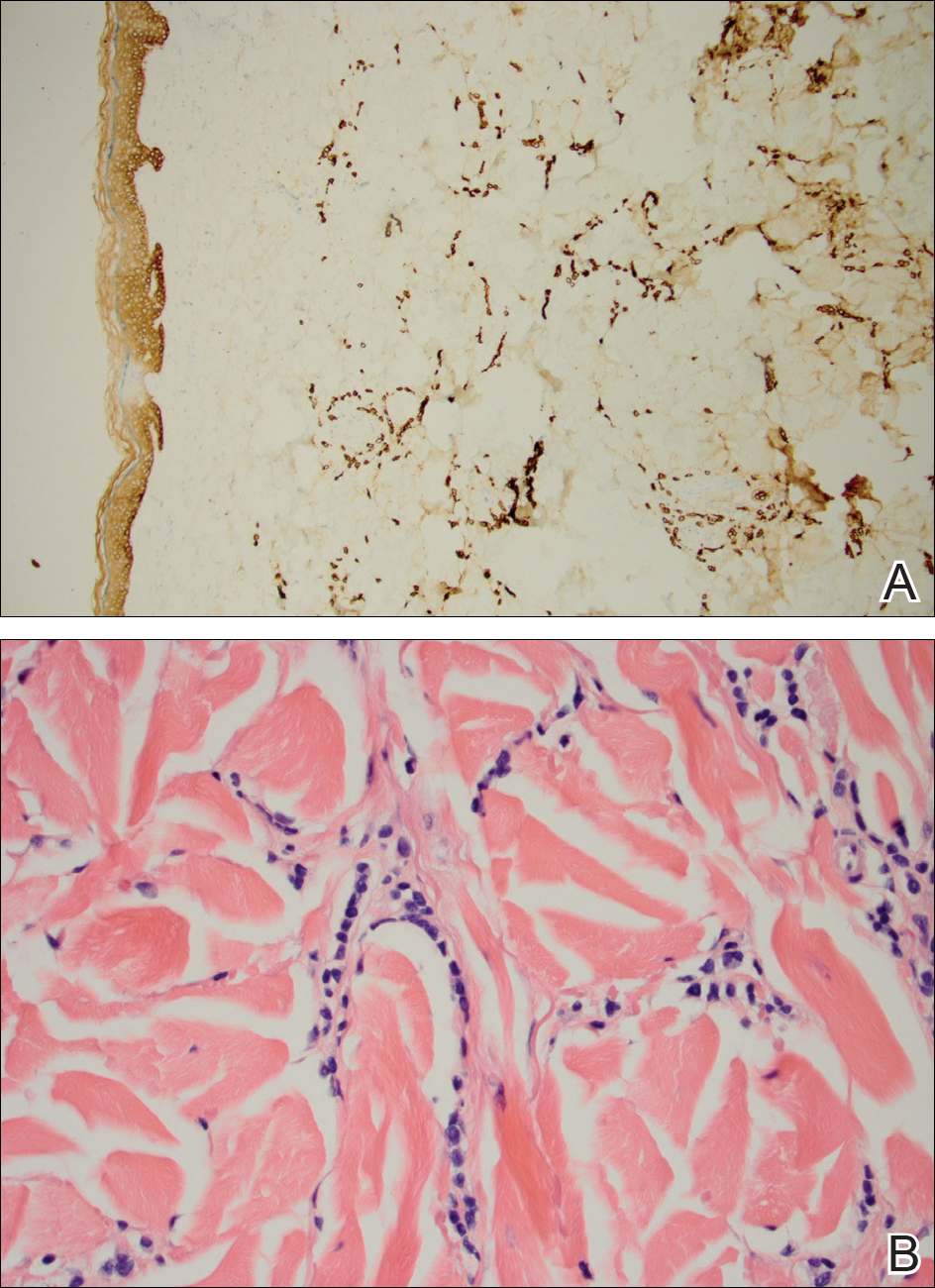
After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.
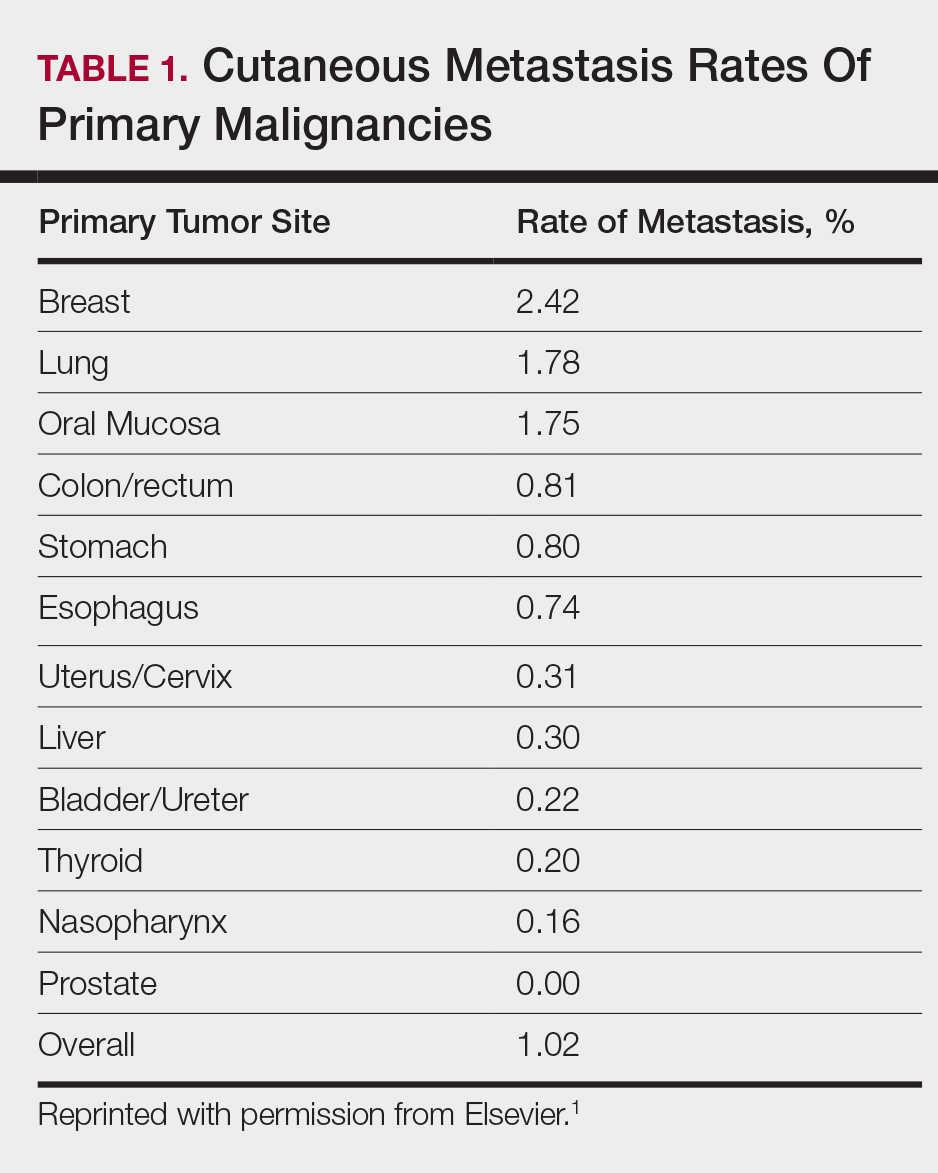
The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.
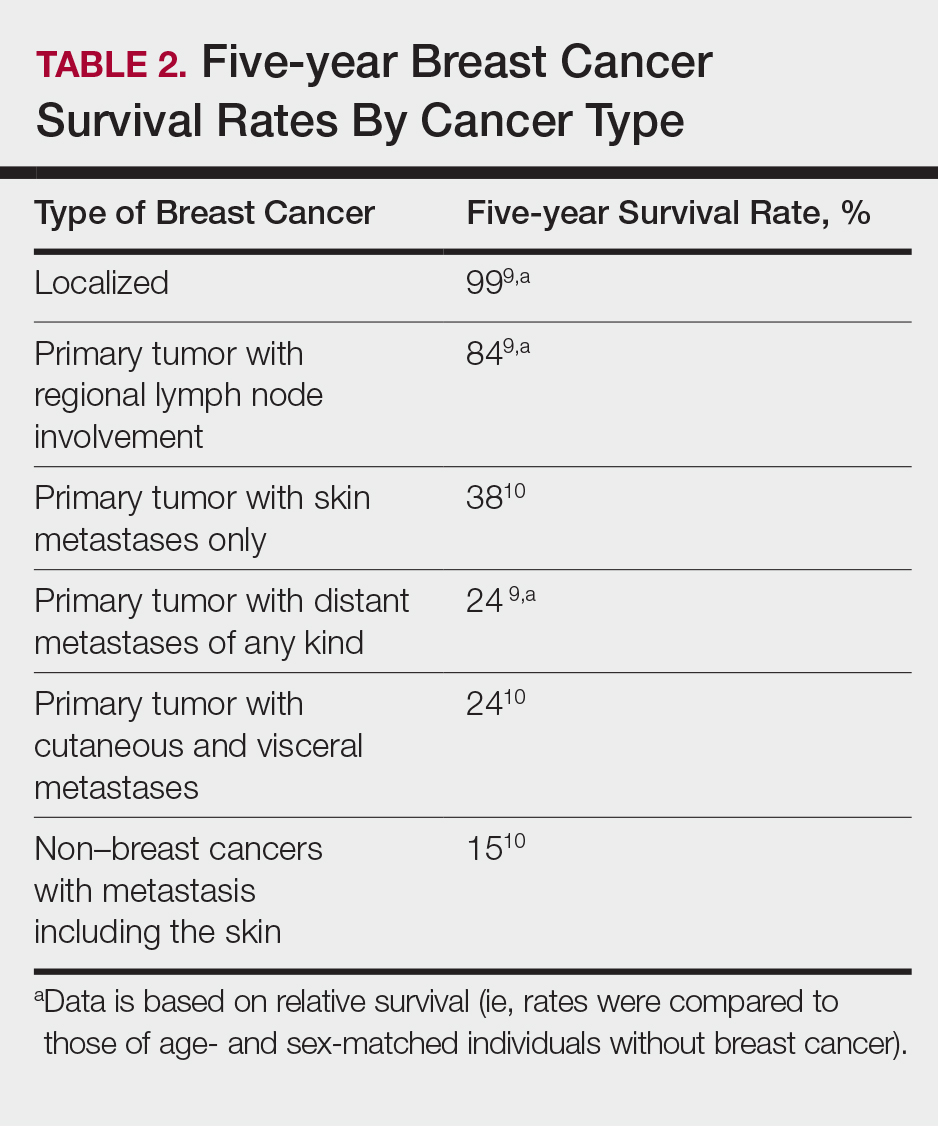
Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Practice Points
- Although breast cancer has the highest rate of cutaneous metastasis among internal malignancies, cutaneous metastases occur in only a small minority of breast cancer patients.
- Cutaneous metastases from breast cancer typically do not carry as poor a prognosis as those in other internal malignancies.
- The clinical presentation of cutaneous metastases from breast cancer can be varied. In our patient, the metastases were subtle and resembled subcutaneous nodules lacking surface changes, thus making them best detectable by palpation.
- While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response, the cutaneous response may be poorer due to the skin’s relatively weaker blood supply.
Deepithelialized Flaps and Grafts: Applications in Dermatologic Surgery
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15
Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).
Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Practice Points
- Deepithelialized flaps should be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.
- Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and reconstruction in areas of high mechanical tension.
Digital Strategies For Dermatology Patient Education
Technology offers new opportunities that can both enhance and challenge the physician-patient relationship, including the ways in which patients are educated. Ensuring dermatology patients are appropriately educated about their conditions can improve clinical care and treatment adherence, increase patient satisfaction, and potentially decrease medical costs. There are various digital methods by which physicians can deliver information to their patients, and while there are benefits and drawbacks to each, many Americans turn to the Internet for health information—a practice that is only predicted to become more prevalent.1
Dermatologists should strive to keep up with this trend by staying informed about the digital patient education options that are available and which tools they can use to more effectively share their knowledge with patients. Electronic health education has a powerful potential, but it is up to physicians to direct patients to the appropriate resources and education tools that will support their clear understanding of all elements of care.
Effective patient education can transform the role of the patient from passive recipient to active participant in his/her care and subsequently supports the physician-patient relationship. The benefits of patient education are timely and valuable with the new pay-for-performance model instated by the Medicare Access and CHIP Reauthorization Act and the Merit-based Incentive Payment System.2 In dermatology, patient education alone can essentially be a management strategy for numerous conditions (eg, identifying which triggers patients with contact dermatitis should avoid). On the other hand, a lack of patient knowledge can result in perceived noncompliance or treatment failure, when in reality there has simply been a communication gap between the physician and the patient. For example, if a patient notices little to no improvement of a fungal infection after applying ketoconazole shampoo 2% to affected areas and immediately rinsing, this does not necessarily constitute a treatment failure, as the patient should have been educated on the importance of leaving the shampoo on for 5 minutes before rinsing. One study alluded to this communication gap, revealing physicians’ tendency to overestimate how effectively they are communicating with their patients.3
Successful patient education ultimately is dependent on both the content provided and the method of delivery. In one survey of 2636 Internet users, 72% of respondents admitted to searching online for health information within the previous year; however, the same survey showed that physicians remain patients’ most trusted source of information.4 Physicians can use digital education methods to fulfill patient needs by providing them with and directing them to credible up-to-date sources.
Physicians can use electronic medical record (EMR) systems to electronically deliver health information to patients by directly communicating via an online patient portal. Allowing patients to engage with their health care providers electronically has been shown to increase patient satisfaction, promote adherence to preventative and treatment recommendations, improve clinical outcomes, and lower medical costs.5 The online portal can provide direct links for patients to digital resources; for example, MedlinePlus Connect (https://medlineplus.gov/connect/overview.html) is a free service that connects patients to MedlinePlus, an authoritative, up-to-date health information resource for consumer health information; however, many EMR systems lack quality dermatology content, as there is a greater emphasis on primary care, and patient usage of these online portals also is notoriously low.6 Dermatologists can work with EMR vendors to enhance the dermatology content for patient portals, and in some cases, specialty-specific content may be available for an additional fee. Clinicians can make their patients aware of the online portal and incentivize its use by sending an informational email, including a link on their practice’s website, promoting the portal during check-in and check-out at office visits, making tablets or kiosks available in the waiting room for sign-up, hanging posters in the examination rooms, and explaining the portal’s useful features during consultations with patients.
Mobile apps have revolutionized the potential for dermatologists to streamline patient education to a large population. In a 2014 review of 365 dermatology mobile apps, 13% were categorized as educational aids, adding to the realm of possibilities for digital patient education. For example, these apps may provide information on specific dermatologic conditions and medications, help users perform skin cancer checks, and provide reminders for when to administer injections for those on biologics. However, a drawback of medical mobile apps is that, to date, the US Food and Drug Administration has not released formal guidelines for their development.
It would be impractical for busy dermatologists to keep up with the credibility of every mobile app available in a growing market, but one solution could be for physicians to stay informed on only the most popular and most reviewed apps to keep in their digital toolbox. In 2014, the most reviewed dermatology app was the Dermatology app, which provided a guide to common dermatologic conditions and included images and a list of symptoms.7 To help keep physicians up to date on the most reliable dermatology apps, specialty societies, journal task forces, or interested dermatologists, residents, or medical students could publish updated literature on the most popular and most reviewed dermatology apps for patient education annually or biannually.
A practice’s website is a prime place for physicians to direct patients to educational content. Although many dermatology practice websites offer clinical information, the content often is focused on cosmetic procedures or is designed for search engine optimization to support online marketing and therefore may not be helpful to patients trying to understand a specific condition or treatment. Links to trusted resources, such as dermatology journals or medical societies, may be added but also would direct patients away from the practice’s website and would not allow physicians to customize the information he or she would like to share with their patients. Dermatologists should consider investing time and money into customizing educational material for their websites so patients can access health information from the source they trust most: their own physician.
Many of these digital options are useful for patients who want to access education material outside of the physician’s office, but digital opportunities to enhance point-of-care education also are available. In 2016, the American Academy of Dermatology partnered with ContextMedia:Health with the goal of delivering important decision enhancement technologies, educational content, and intelligence to patients and dermatologists for use before and during the consultation.8 ContextMedia:Health’s digital wallboard tablets are an engaging way to visually explain conditions and treatments to patients during the consultation, thus empowering physicians and patients to make decisions together and helping patients to be better advocates of their own health care. The downside is that health care workers must devote time and resources to be trained in using these devices.
The increasing availability of technology for electronic health information can be both beneficial and challenging for dermatologists. Physicians should explore and familiarize themselves with the tools that are available and assess their effectiveness by communicating with patients about their perception and understanding of their conditions. Digital delivery of health information is not meant to replace other methods of patient education but to supplement and reinforce them that which is verbally discussed during the office visit. Electronic health education demonstrates powerful potential, but it is up to the physician to direct patients to the appropriate resources and educational tools that will support a clear understanding of all elements of care.
Acknowledgment
The authors would like to thank Dr. Mark Becker (Berkeley, California) for helpful discussion and reviewing this manuscript.
- Explosive growth in healthcare apps raises oversight questions. Association of American Medical Colleges website. https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed January 16, 2017.
- What’s MACRA? Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Updated November 16, 2017. Accessed February 26, 2018.
- Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med. 2004;79:495-507.
- Dutta-Bergman M. Trusted online sources of health information: differences in demographics, health beliefs, and health-information orientation [published online September 25, 2003]. J Med Internet Res. 2003;5:E21.
- Griffin A, Skinner A, Thornhill J, et al. Patient portals: who uses them? what features do they use? and do they reduce hospital readmissions? Appl Clin Inform. 2016;7:489-501.
- Lin CT, Wittevrongel L, Moore L, et al. An internet-based patient-provider communication system: randomized controlled trial. J Med Internet Res. 2005;7:E47.
- Patel S, Eluri M, Boyers LN, et al. Update on mobile applications in dermatology [published online November 9, 2014]. Dermatol Online J. 2014;21. pii:13030/qt1zc343js.
- American Academy of Dermatology selects ContextMedia:Health as patient education affinity partner. American Academy of Dermatology website. https://www.aad.org/media/news-releases/aad-selects-patient-education-affinity-partner. Published November 14, 2016. Accessed February 25, 2018.
Technology offers new opportunities that can both enhance and challenge the physician-patient relationship, including the ways in which patients are educated. Ensuring dermatology patients are appropriately educated about their conditions can improve clinical care and treatment adherence, increase patient satisfaction, and potentially decrease medical costs. There are various digital methods by which physicians can deliver information to their patients, and while there are benefits and drawbacks to each, many Americans turn to the Internet for health information—a practice that is only predicted to become more prevalent.1
Dermatologists should strive to keep up with this trend by staying informed about the digital patient education options that are available and which tools they can use to more effectively share their knowledge with patients. Electronic health education has a powerful potential, but it is up to physicians to direct patients to the appropriate resources and education tools that will support their clear understanding of all elements of care.
Effective patient education can transform the role of the patient from passive recipient to active participant in his/her care and subsequently supports the physician-patient relationship. The benefits of patient education are timely and valuable with the new pay-for-performance model instated by the Medicare Access and CHIP Reauthorization Act and the Merit-based Incentive Payment System.2 In dermatology, patient education alone can essentially be a management strategy for numerous conditions (eg, identifying which triggers patients with contact dermatitis should avoid). On the other hand, a lack of patient knowledge can result in perceived noncompliance or treatment failure, when in reality there has simply been a communication gap between the physician and the patient. For example, if a patient notices little to no improvement of a fungal infection after applying ketoconazole shampoo 2% to affected areas and immediately rinsing, this does not necessarily constitute a treatment failure, as the patient should have been educated on the importance of leaving the shampoo on for 5 minutes before rinsing. One study alluded to this communication gap, revealing physicians’ tendency to overestimate how effectively they are communicating with their patients.3
Successful patient education ultimately is dependent on both the content provided and the method of delivery. In one survey of 2636 Internet users, 72% of respondents admitted to searching online for health information within the previous year; however, the same survey showed that physicians remain patients’ most trusted source of information.4 Physicians can use digital education methods to fulfill patient needs by providing them with and directing them to credible up-to-date sources.
Physicians can use electronic medical record (EMR) systems to electronically deliver health information to patients by directly communicating via an online patient portal. Allowing patients to engage with their health care providers electronically has been shown to increase patient satisfaction, promote adherence to preventative and treatment recommendations, improve clinical outcomes, and lower medical costs.5 The online portal can provide direct links for patients to digital resources; for example, MedlinePlus Connect (https://medlineplus.gov/connect/overview.html) is a free service that connects patients to MedlinePlus, an authoritative, up-to-date health information resource for consumer health information; however, many EMR systems lack quality dermatology content, as there is a greater emphasis on primary care, and patient usage of these online portals also is notoriously low.6 Dermatologists can work with EMR vendors to enhance the dermatology content for patient portals, and in some cases, specialty-specific content may be available for an additional fee. Clinicians can make their patients aware of the online portal and incentivize its use by sending an informational email, including a link on their practice’s website, promoting the portal during check-in and check-out at office visits, making tablets or kiosks available in the waiting room for sign-up, hanging posters in the examination rooms, and explaining the portal’s useful features during consultations with patients.
Mobile apps have revolutionized the potential for dermatologists to streamline patient education to a large population. In a 2014 review of 365 dermatology mobile apps, 13% were categorized as educational aids, adding to the realm of possibilities for digital patient education. For example, these apps may provide information on specific dermatologic conditions and medications, help users perform skin cancer checks, and provide reminders for when to administer injections for those on biologics. However, a drawback of medical mobile apps is that, to date, the US Food and Drug Administration has not released formal guidelines for their development.
It would be impractical for busy dermatologists to keep up with the credibility of every mobile app available in a growing market, but one solution could be for physicians to stay informed on only the most popular and most reviewed apps to keep in their digital toolbox. In 2014, the most reviewed dermatology app was the Dermatology app, which provided a guide to common dermatologic conditions and included images and a list of symptoms.7 To help keep physicians up to date on the most reliable dermatology apps, specialty societies, journal task forces, or interested dermatologists, residents, or medical students could publish updated literature on the most popular and most reviewed dermatology apps for patient education annually or biannually.
A practice’s website is a prime place for physicians to direct patients to educational content. Although many dermatology practice websites offer clinical information, the content often is focused on cosmetic procedures or is designed for search engine optimization to support online marketing and therefore may not be helpful to patients trying to understand a specific condition or treatment. Links to trusted resources, such as dermatology journals or medical societies, may be added but also would direct patients away from the practice’s website and would not allow physicians to customize the information he or she would like to share with their patients. Dermatologists should consider investing time and money into customizing educational material for their websites so patients can access health information from the source they trust most: their own physician.
Many of these digital options are useful for patients who want to access education material outside of the physician’s office, but digital opportunities to enhance point-of-care education also are available. In 2016, the American Academy of Dermatology partnered with ContextMedia:Health with the goal of delivering important decision enhancement technologies, educational content, and intelligence to patients and dermatologists for use before and during the consultation.8 ContextMedia:Health’s digital wallboard tablets are an engaging way to visually explain conditions and treatments to patients during the consultation, thus empowering physicians and patients to make decisions together and helping patients to be better advocates of their own health care. The downside is that health care workers must devote time and resources to be trained in using these devices.
The increasing availability of technology for electronic health information can be both beneficial and challenging for dermatologists. Physicians should explore and familiarize themselves with the tools that are available and assess their effectiveness by communicating with patients about their perception and understanding of their conditions. Digital delivery of health information is not meant to replace other methods of patient education but to supplement and reinforce them that which is verbally discussed during the office visit. Electronic health education demonstrates powerful potential, but it is up to the physician to direct patients to the appropriate resources and educational tools that will support a clear understanding of all elements of care.
Acknowledgment
The authors would like to thank Dr. Mark Becker (Berkeley, California) for helpful discussion and reviewing this manuscript.
Technology offers new opportunities that can both enhance and challenge the physician-patient relationship, including the ways in which patients are educated. Ensuring dermatology patients are appropriately educated about their conditions can improve clinical care and treatment adherence, increase patient satisfaction, and potentially decrease medical costs. There are various digital methods by which physicians can deliver information to their patients, and while there are benefits and drawbacks to each, many Americans turn to the Internet for health information—a practice that is only predicted to become more prevalent.1
Dermatologists should strive to keep up with this trend by staying informed about the digital patient education options that are available and which tools they can use to more effectively share their knowledge with patients. Electronic health education has a powerful potential, but it is up to physicians to direct patients to the appropriate resources and education tools that will support their clear understanding of all elements of care.
Effective patient education can transform the role of the patient from passive recipient to active participant in his/her care and subsequently supports the physician-patient relationship. The benefits of patient education are timely and valuable with the new pay-for-performance model instated by the Medicare Access and CHIP Reauthorization Act and the Merit-based Incentive Payment System.2 In dermatology, patient education alone can essentially be a management strategy for numerous conditions (eg, identifying which triggers patients with contact dermatitis should avoid). On the other hand, a lack of patient knowledge can result in perceived noncompliance or treatment failure, when in reality there has simply been a communication gap between the physician and the patient. For example, if a patient notices little to no improvement of a fungal infection after applying ketoconazole shampoo 2% to affected areas and immediately rinsing, this does not necessarily constitute a treatment failure, as the patient should have been educated on the importance of leaving the shampoo on for 5 minutes before rinsing. One study alluded to this communication gap, revealing physicians’ tendency to overestimate how effectively they are communicating with their patients.3
Successful patient education ultimately is dependent on both the content provided and the method of delivery. In one survey of 2636 Internet users, 72% of respondents admitted to searching online for health information within the previous year; however, the same survey showed that physicians remain patients’ most trusted source of information.4 Physicians can use digital education methods to fulfill patient needs by providing them with and directing them to credible up-to-date sources.
Physicians can use electronic medical record (EMR) systems to electronically deliver health information to patients by directly communicating via an online patient portal. Allowing patients to engage with their health care providers electronically has been shown to increase patient satisfaction, promote adherence to preventative and treatment recommendations, improve clinical outcomes, and lower medical costs.5 The online portal can provide direct links for patients to digital resources; for example, MedlinePlus Connect (https://medlineplus.gov/connect/overview.html) is a free service that connects patients to MedlinePlus, an authoritative, up-to-date health information resource for consumer health information; however, many EMR systems lack quality dermatology content, as there is a greater emphasis on primary care, and patient usage of these online portals also is notoriously low.6 Dermatologists can work with EMR vendors to enhance the dermatology content for patient portals, and in some cases, specialty-specific content may be available for an additional fee. Clinicians can make their patients aware of the online portal and incentivize its use by sending an informational email, including a link on their practice’s website, promoting the portal during check-in and check-out at office visits, making tablets or kiosks available in the waiting room for sign-up, hanging posters in the examination rooms, and explaining the portal’s useful features during consultations with patients.
Mobile apps have revolutionized the potential for dermatologists to streamline patient education to a large population. In a 2014 review of 365 dermatology mobile apps, 13% were categorized as educational aids, adding to the realm of possibilities for digital patient education. For example, these apps may provide information on specific dermatologic conditions and medications, help users perform skin cancer checks, and provide reminders for when to administer injections for those on biologics. However, a drawback of medical mobile apps is that, to date, the US Food and Drug Administration has not released formal guidelines for their development.
It would be impractical for busy dermatologists to keep up with the credibility of every mobile app available in a growing market, but one solution could be for physicians to stay informed on only the most popular and most reviewed apps to keep in their digital toolbox. In 2014, the most reviewed dermatology app was the Dermatology app, which provided a guide to common dermatologic conditions and included images and a list of symptoms.7 To help keep physicians up to date on the most reliable dermatology apps, specialty societies, journal task forces, or interested dermatologists, residents, or medical students could publish updated literature on the most popular and most reviewed dermatology apps for patient education annually or biannually.
A practice’s website is a prime place for physicians to direct patients to educational content. Although many dermatology practice websites offer clinical information, the content often is focused on cosmetic procedures or is designed for search engine optimization to support online marketing and therefore may not be helpful to patients trying to understand a specific condition or treatment. Links to trusted resources, such as dermatology journals or medical societies, may be added but also would direct patients away from the practice’s website and would not allow physicians to customize the information he or she would like to share with their patients. Dermatologists should consider investing time and money into customizing educational material for their websites so patients can access health information from the source they trust most: their own physician.
Many of these digital options are useful for patients who want to access education material outside of the physician’s office, but digital opportunities to enhance point-of-care education also are available. In 2016, the American Academy of Dermatology partnered with ContextMedia:Health with the goal of delivering important decision enhancement technologies, educational content, and intelligence to patients and dermatologists for use before and during the consultation.8 ContextMedia:Health’s digital wallboard tablets are an engaging way to visually explain conditions and treatments to patients during the consultation, thus empowering physicians and patients to make decisions together and helping patients to be better advocates of their own health care. The downside is that health care workers must devote time and resources to be trained in using these devices.
The increasing availability of technology for electronic health information can be both beneficial and challenging for dermatologists. Physicians should explore and familiarize themselves with the tools that are available and assess their effectiveness by communicating with patients about their perception and understanding of their conditions. Digital delivery of health information is not meant to replace other methods of patient education but to supplement and reinforce them that which is verbally discussed during the office visit. Electronic health education demonstrates powerful potential, but it is up to the physician to direct patients to the appropriate resources and educational tools that will support a clear understanding of all elements of care.
Acknowledgment
The authors would like to thank Dr. Mark Becker (Berkeley, California) for helpful discussion and reviewing this manuscript.
- Explosive growth in healthcare apps raises oversight questions. Association of American Medical Colleges website. https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed January 16, 2017.
- What’s MACRA? Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Updated November 16, 2017. Accessed February 26, 2018.
- Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med. 2004;79:495-507.
- Dutta-Bergman M. Trusted online sources of health information: differences in demographics, health beliefs, and health-information orientation [published online September 25, 2003]. J Med Internet Res. 2003;5:E21.
- Griffin A, Skinner A, Thornhill J, et al. Patient portals: who uses them? what features do they use? and do they reduce hospital readmissions? Appl Clin Inform. 2016;7:489-501.
- Lin CT, Wittevrongel L, Moore L, et al. An internet-based patient-provider communication system: randomized controlled trial. J Med Internet Res. 2005;7:E47.
- Patel S, Eluri M, Boyers LN, et al. Update on mobile applications in dermatology [published online November 9, 2014]. Dermatol Online J. 2014;21. pii:13030/qt1zc343js.
- American Academy of Dermatology selects ContextMedia:Health as patient education affinity partner. American Academy of Dermatology website. https://www.aad.org/media/news-releases/aad-selects-patient-education-affinity-partner. Published November 14, 2016. Accessed February 25, 2018.
- Explosive growth in healthcare apps raises oversight questions. Association of American Medical Colleges website. https://www.aamc.org/newsroom/reporter/october2012/308516/health-care-apps.html. Accessed January 16, 2017.
- What’s MACRA? Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Updated November 16, 2017. Accessed February 26, 2018.
- Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med. 2004;79:495-507.
- Dutta-Bergman M. Trusted online sources of health information: differences in demographics, health beliefs, and health-information orientation [published online September 25, 2003]. J Med Internet Res. 2003;5:E21.
- Griffin A, Skinner A, Thornhill J, et al. Patient portals: who uses them? what features do they use? and do they reduce hospital readmissions? Appl Clin Inform. 2016;7:489-501.
- Lin CT, Wittevrongel L, Moore L, et al. An internet-based patient-provider communication system: randomized controlled trial. J Med Internet Res. 2005;7:E47.
- Patel S, Eluri M, Boyers LN, et al. Update on mobile applications in dermatology [published online November 9, 2014]. Dermatol Online J. 2014;21. pii:13030/qt1zc343js.
- American Academy of Dermatology selects ContextMedia:Health as patient education affinity partner. American Academy of Dermatology website. https://www.aad.org/media/news-releases/aad-selects-patient-education-affinity-partner. Published November 14, 2016. Accessed February 25, 2018.
Linear Terra Firma–Forme Dermatosis of the Midline Back
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.
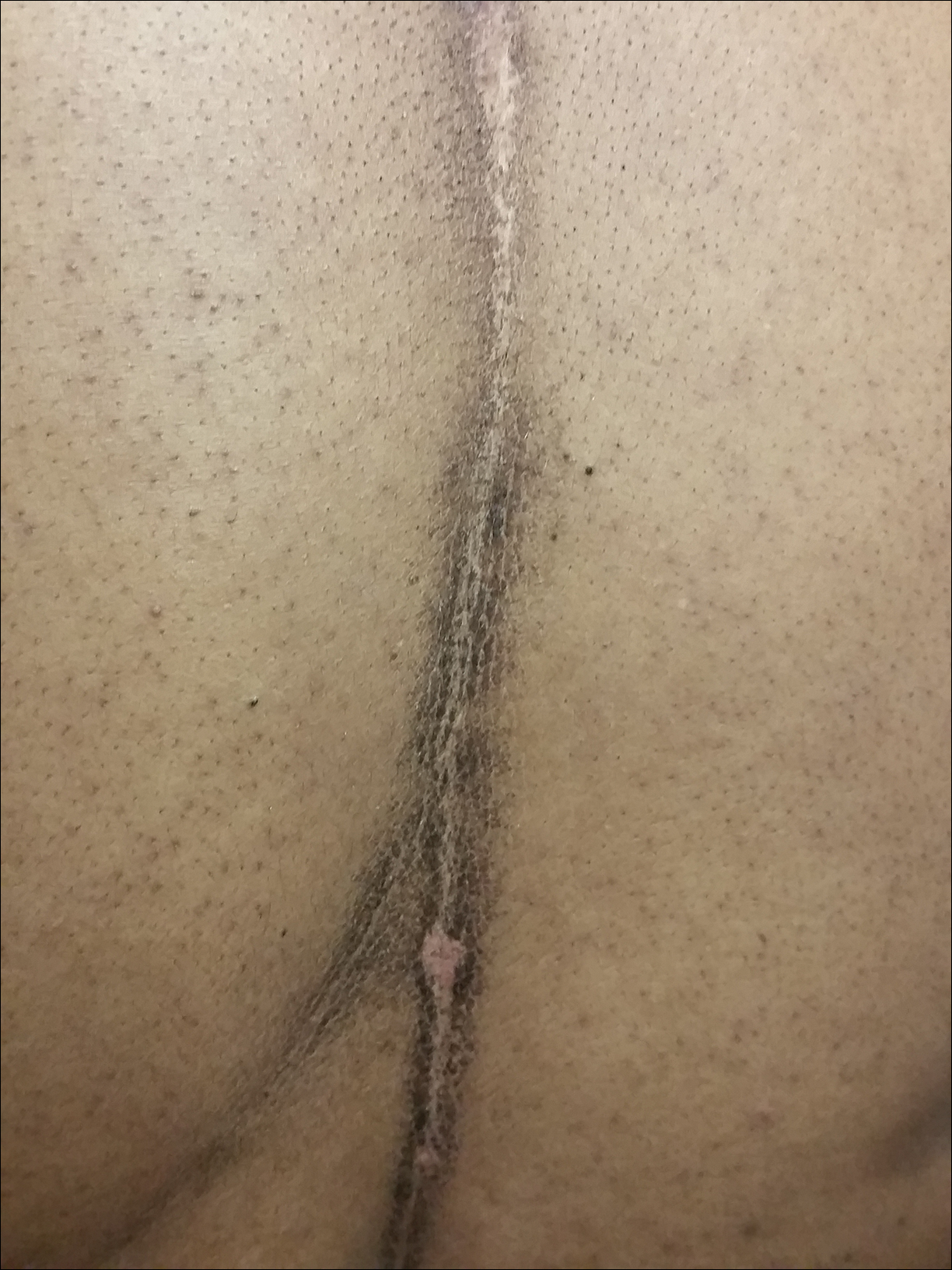
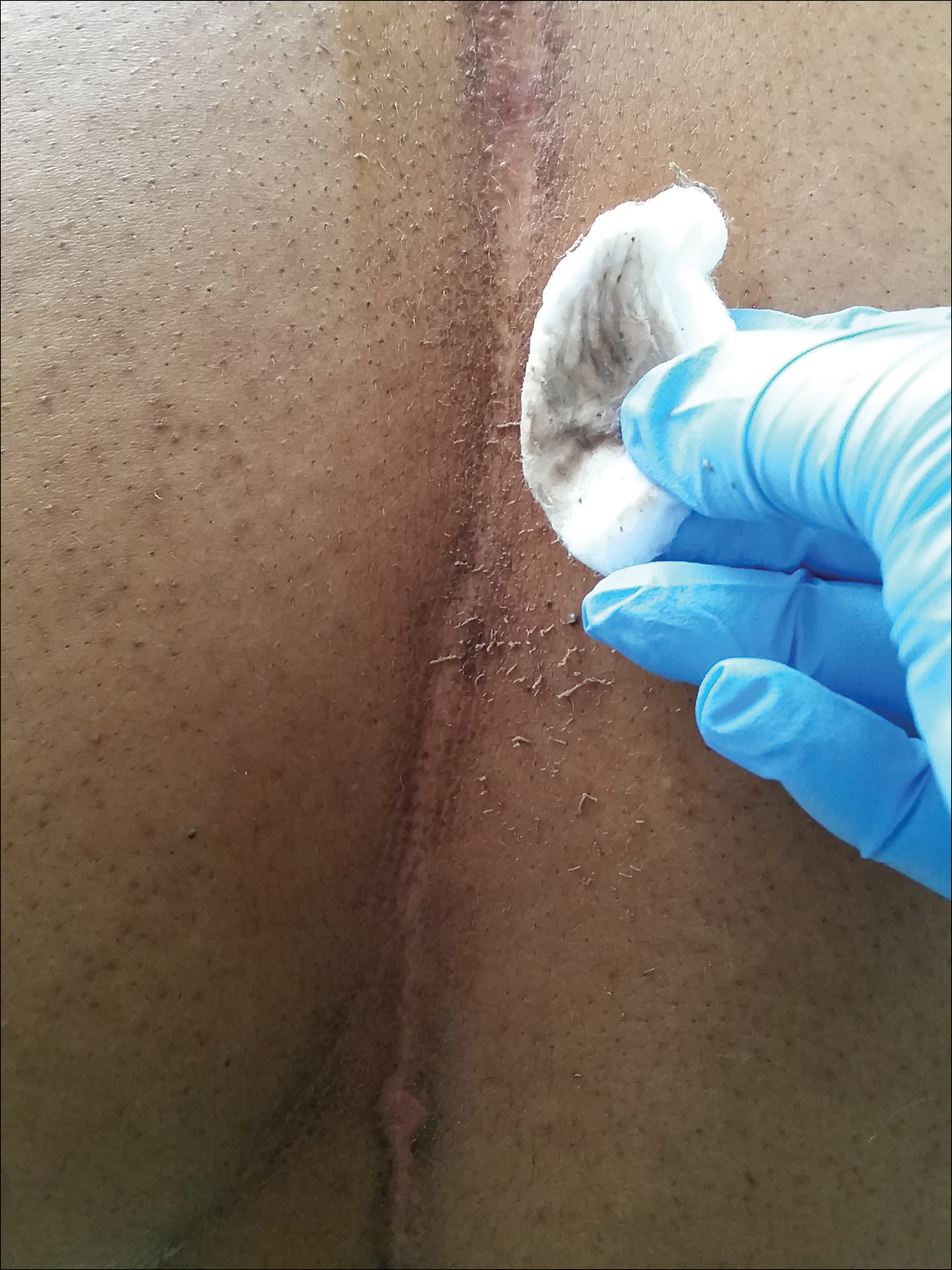
Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Practice Points
- Terra firma-forme dermatosis (TFFD) is an idiopathic condition characterized by asymptomatic hyperpigmented and hyperkeratotic plaques that are resistant to removal with soap and water.
- Diagnosis and cure of TFFD can be achieved through removal by rubbing with isopropyl alcohol.
- Increased awareness of the clinical presentation and treatment of TFFD may help patients avoid unnecessary treatment and workup and leads to immediate resolution of the condition.
Postherpetic Isotopic Responses With 3 Simultaneously Occurring Reactions Following Herpes Zoster
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.
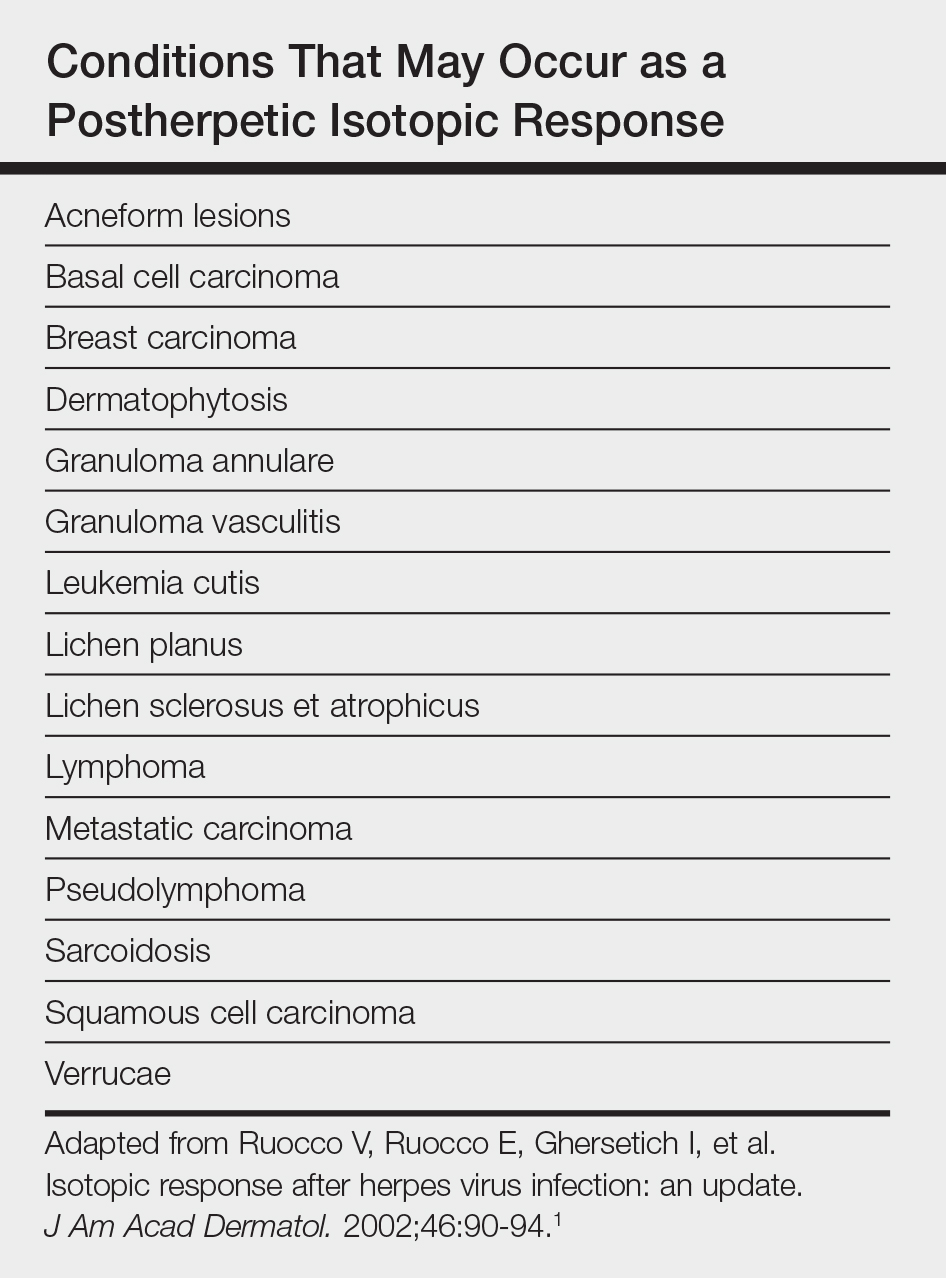
Case Report
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.
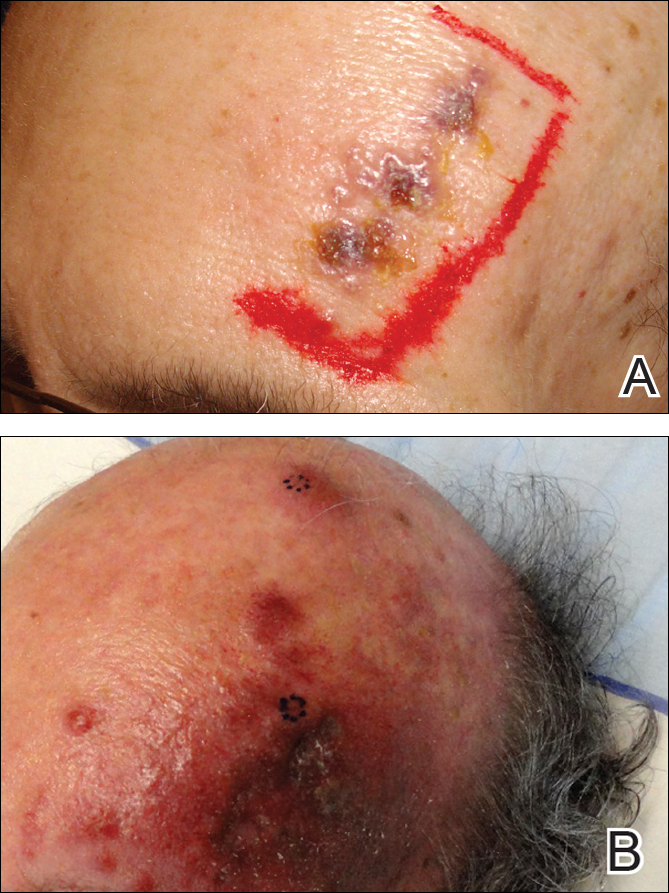
A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.
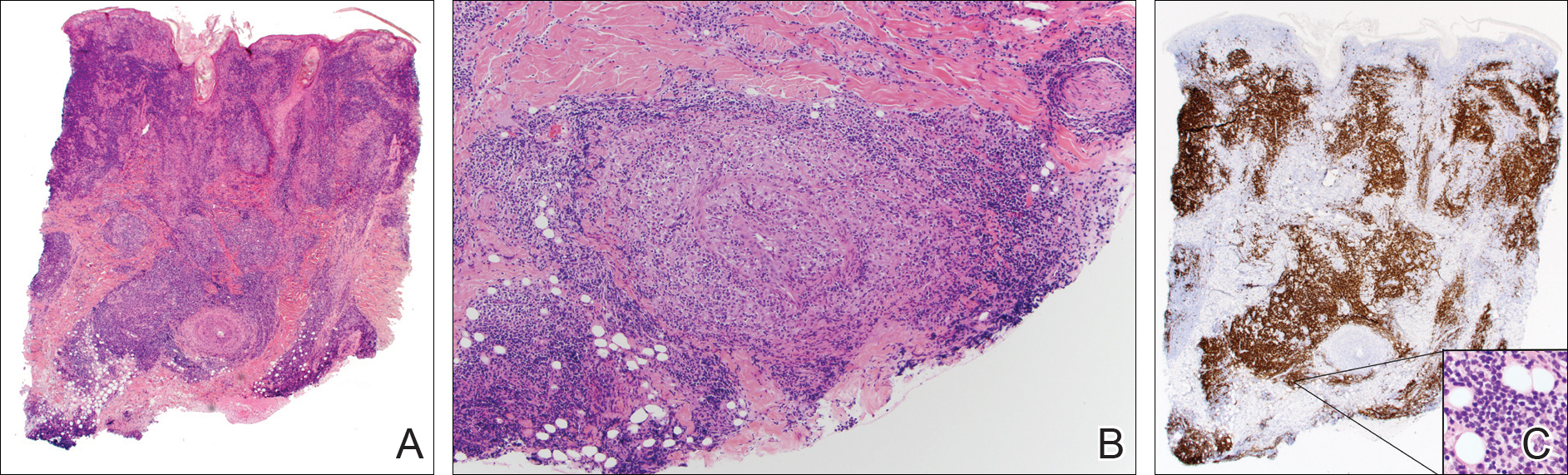
Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.

Case Report
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.

A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.

Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.

Case Report
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.

A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.

Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
Practice Points
- Multiple diseases may present in prior sites of herpes infection (postherpetic isotopic response).
- Granulomatous dermatitis is the most common postherpetic isotopic response, but other inflammatory, neoplastic, or infectious conditions also occur.
- Multiple conditions may present simultaneously at sites of herpes infection.
- Cutaneous involvement by chronic lymphocytic leukemia (CLL) can be easily overlooked in this setting.
What’s Eating You? Ixodes Tick and Related Diseases, Part 1: Life Cycle, Local Reactions, and Lyme Disease
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4
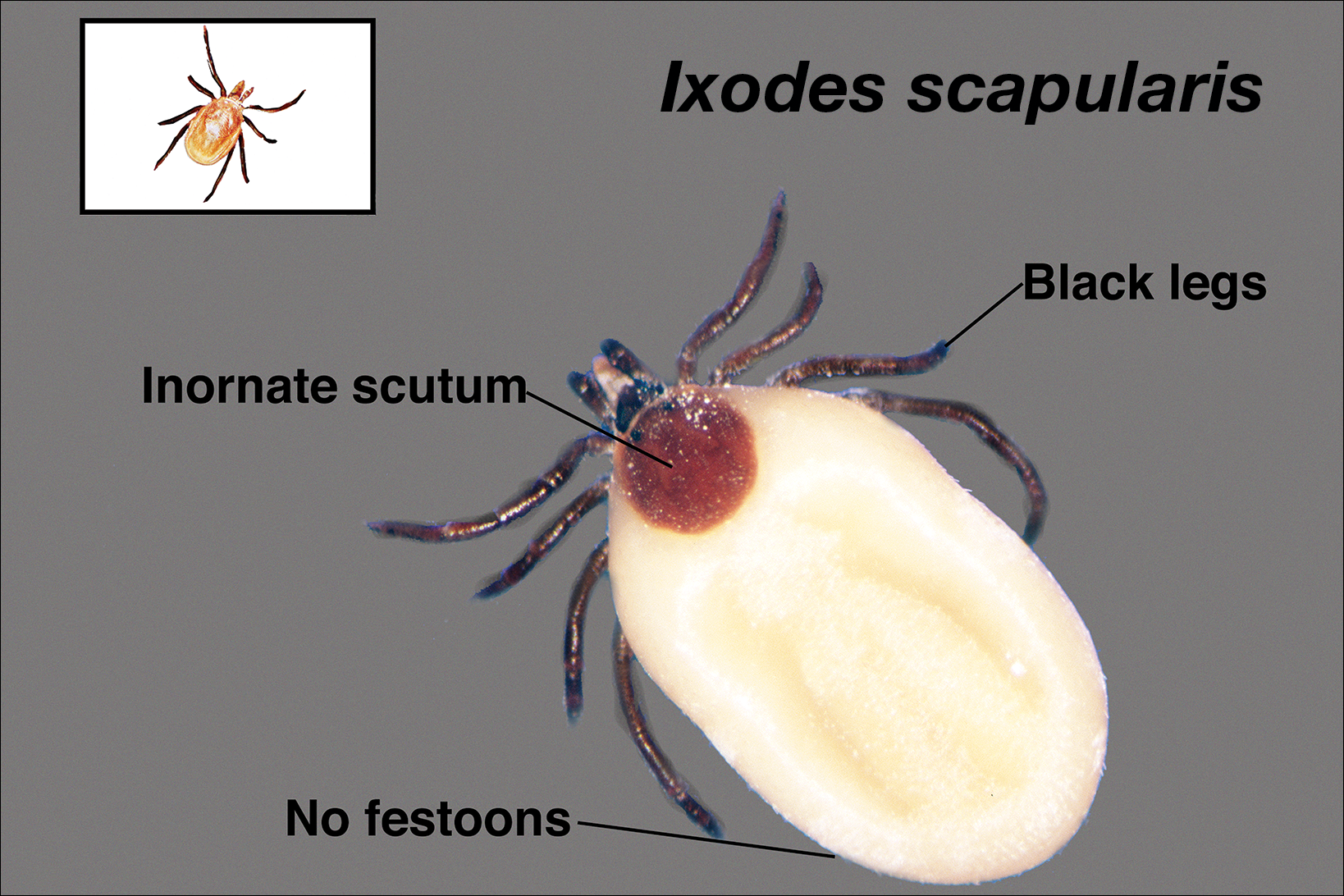
Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27
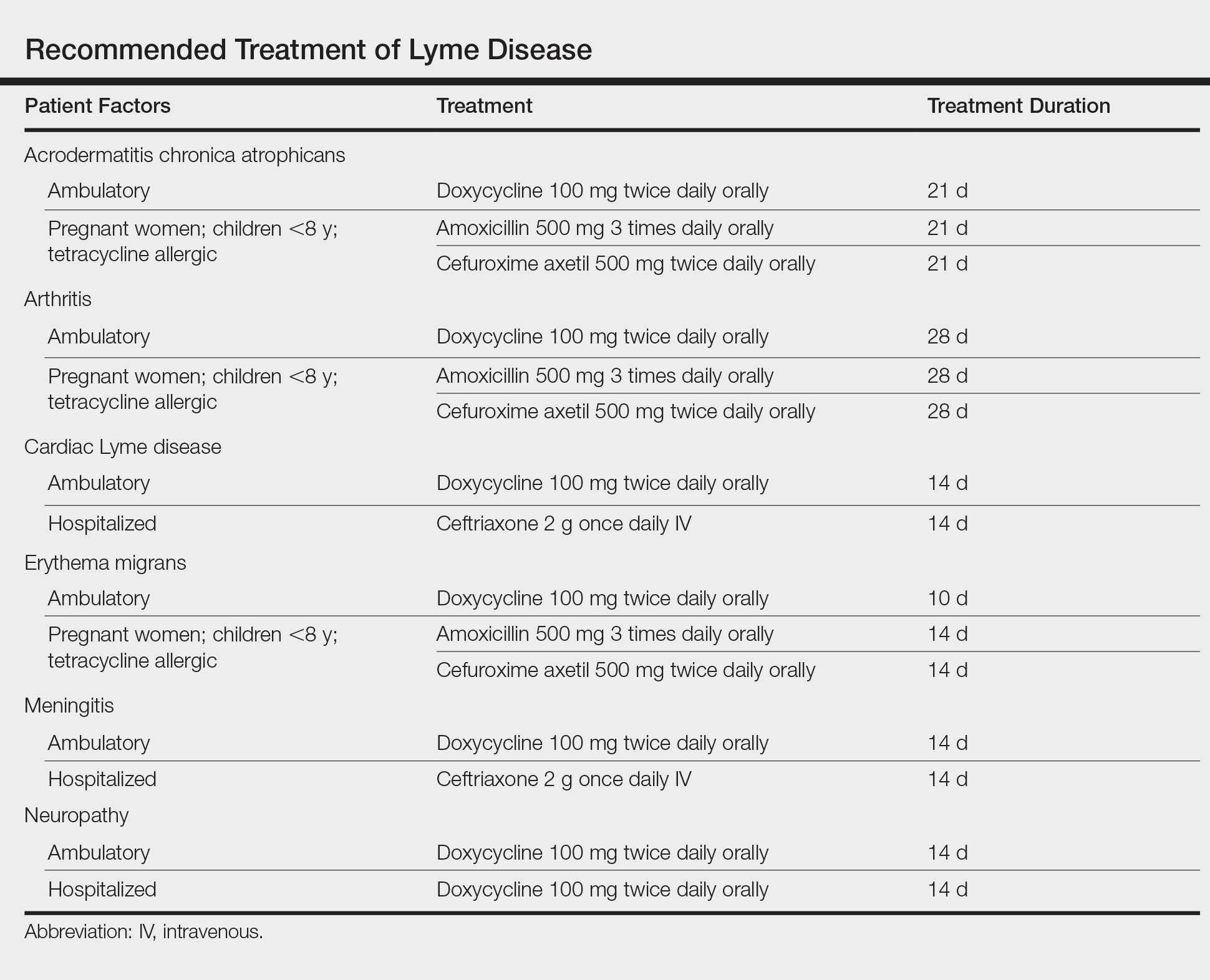
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Practice Points
- Lyme disease is transmitted by Ixodes ticks in the northeastern, midwestern, and far western United States.
- Most tick-borne illnesses, including Lyme disease, respond to treatment with doxycycline.
- Babesiosis, a malarialike illness, can be transmitted concurrently with Lyme disease.