User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Maria A. Oquendo, MD, PhD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
Early interventions for psychosis
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
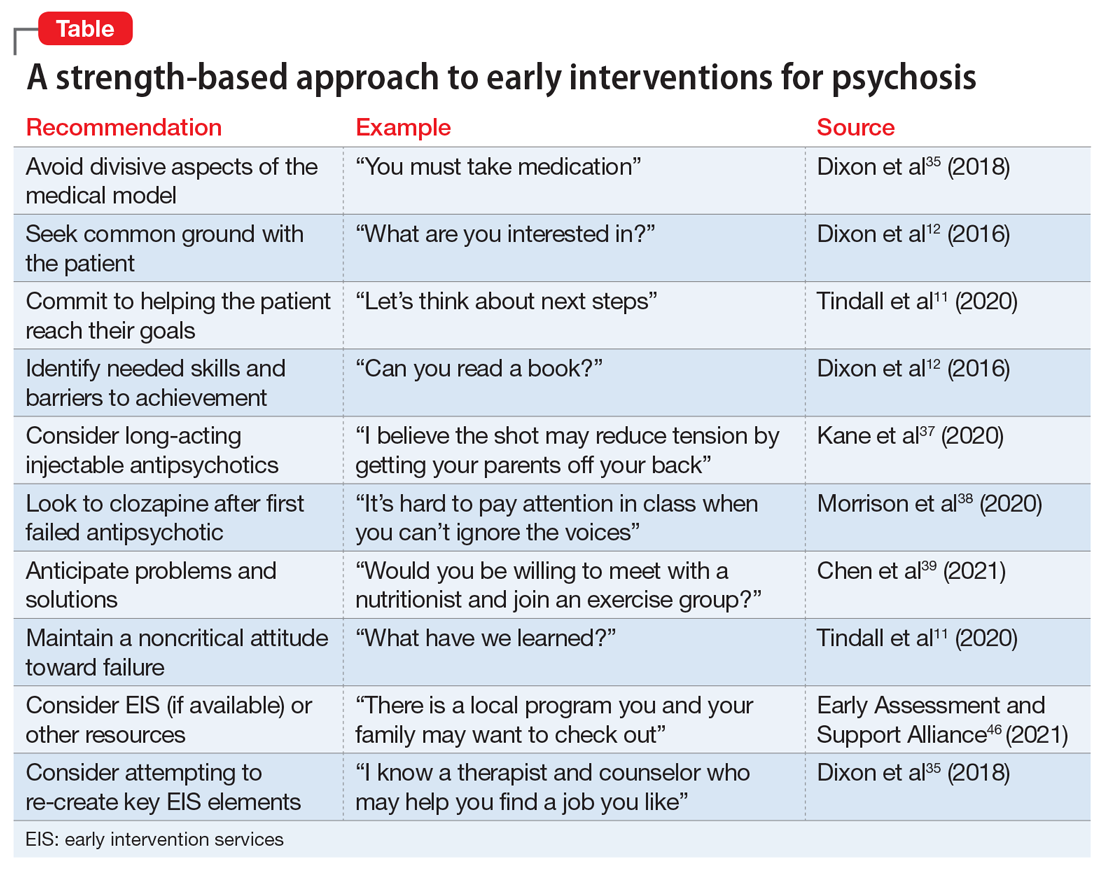
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).

Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).

Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).

Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Nontraditional therapies for treatment-resistant depression: Part 2
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (
Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (

Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (

Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
From famous to infamous: Psychiatric aspects of the fall from grace
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
Persistent altered mental status
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.
[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.
Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
CASE Sluggish, weak, and incoherent
Mr. O, age 24, who has a history of schizophrenia and obesity, presents to the emergency department (ED) for altered mental status (AMS). His mother reports that he has been sluggish, weak, incoherent, had no appetite, and that on the day before admission, he was drinking excessive amounts of water and urinating every 10 minutes.
HISTORY Multiple ineffective antipsychotics
Mr. O was diagnosed with schizophrenia at age 21 and struggled with medication adherence, which resulted in multiple hospitalizations for stabilization. Trials of haloperidol, risperidone, paliperidone palmitate, and valproic acid had been ineffective. At the time of admission, his psychotropic medication regimen is fluphenazine decanoate, 25 mg injection every 2 weeks; clozapine, 50 mg/d; lithium carbonate, 300 mg twice a day; benztropine, 2 mg every night; and trazodone, 50 mg every night.
EVALUATION Fever, tachycardia, and diabetic ketoacidosis
Upon arrival to the ED, Mr. O is obtunded, unable to follow commands, and does not respond to painful stimuli. On physical exam, he has a fever of 38.4°C (reference range 35.1°C to 37.9°C); tachycardia with a heart rate of 142 beats per minute (bpm) (reference range 60 to 100); tachypnea with a respiratory rate of 35 breaths per minute (reference range 12 to 20); a blood pressure of 116/76 mmHg (reference range 90/60 to 130/80); and hypoxemia with an oxygen saturation of 90% on room air (reference range 94% to 100%).
Mr. O is admitted to the hospital and his laboratory workup indicates diabetic ketoacidosis (DKA), with a glucose of 1,700 mg/dL; anion gap of 30 (reference range 4 to 12 mmol/L); pH 7.04 (reference range 7.32 to 7.42); serum bicarbonate 6 (reference range 20 to 24 mEq/L); beta-hydroxybutyrate 11.04 (reference range 0 to 0.27 mmol/L); urine ketones, serum osmolality 407 (reference range 280 to 300 mOsm/kg); and an elevated white blood cell count of 18.4 (reference range 4.5 to 11.0 × 109/L). A CT scan of the head is negative for acute pathology.
Initially, all psychotropic medications are held. On Day 3 of hospitalization, psychiatry is consulted and clozapine, 50 mg/d; lithium, 300 mg/d; and benztropine, 1 mg at night, are restarted; however, fluphenazine decanoate and trazodone are held. The team recommends IV haloperidol, 2 mg as needed for agitation; however, it is never administered.
Imaging rules out deep vein thrombosis, cardiac dysfunction, and stroke, but a CT chest scan is notable for bilateral lung infiltrates, which suggests aspiration pneumonia.
Mr. O is diagnosed with diabetes, complicated by DKA, and is treated in the intensive care unit (ICU). Despite resolution of the DKA, he remains altered with fever and tachycardia.
Continue to: On Day 6 of hospitalization...
On Day 6 of hospitalization, Mr. O continues to be tachycardic and obtunded with nuchal rigidity. The team decides to transfer Mr. O to another hospital for a higher level of care and continued workup of his persistent AMS.
Immediately upon arrival at the second hospital, infectious disease and neurology teams are consulted for further evaluation. Mr. O’s AMS continues despite no clear signs of infection or other neurologic insults.
[polldaddy:10930631]
The authors’ observations
Based on Mr. O’s psychiatric history and laboratory results, the first medical team concluded his initial AMS was likely secondary to DKA; however, the AMS continued after the DKA resolved. At the second hospital, Mr. O’s treatment team continued to dig for answers.
EVALUATION Exploring the differential diagnosis
At the second hospital, Mr. O is admitted to the ICU with fever (37.8°C), tachycardia (120 bpm), tachypnea, withdrawal from painful stimuli, decreased reflexes, and muscle rigidity, including clenched jaw. The differential diagnoses include meningitis, sepsis from aspiration pneumonia, severe metabolic encephalopathy with prolonged recovery, central pontine myelinolysis, anoxic brain injury, and subclinical seizures.
Empiric vancomycin, 1.75 g every 12 hours; ceftriaxone, 2 g/d; and acyclovir, 900 mg every 8 hours are started for meningoencephalitis, and all psychotropic medications are discontinued. Case reports have documented a relationship between hyperglycemic hyperosmolar syndrome (HHS) and malignant hyperthermia in rare cases1; however, HHS is ruled out based on Mr. O’s laboratory results.A lumbar puncture and imaging rules out CNS infection. Antibiotic treatment is narrowed to ampicillin-sulbactam due to Mr. O’s prior CT chest showing concern for aspiration pneumonia. An MRI of the brain rules out central pontine myelinolysis, acute stroke, and anoxic brain injury, and an EEG shows nonspecific encephalopathy. On Day 10 of hospitalization, a neurologic exam shows flaccid paralysis and bilateral clonus, and Mr. O is mute. On Day 14 of hospitalization, his fever resolves, and his blood cultures are negative. On Day 15 of hospitalization, Mr. O’s creatine kinase (CK) level is elevated at 1,308 U/L (reference range 26 to 192 U/L), suggesting rhabdomyolysis.
Continue to: Given the neurologic exam findings...
Given the neurologic exam findings, and the limited evidence of infection, the differential diagnosis for Mr. O’s AMS is broadened to include catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, and autoimmune encephalitis. The psychiatry team evaluates Mr. O for catatonia. He scores 14 on the Bush-Francis Catatonia Rating Scale, with findings of immobility/stupor, mutism, staring, autonomic instability, and withdrawal indicating the presence of catatonia.2
The authors’ observations
When Mr. O was transferred to the second hospital, the primary concern was to rule out meningitis due to his unstable vitals, obtunded mental state, and nuchal rigidity. A comprehensive infectious workup, including lumbar puncture, was imperative because infection can not only lead to AMS, but also precipitate episodes of DKA. Mr. O’s persistently abnormal vital signs indicated an underlying process may have been missed by focusing on treating DKA.
TREATMENT Finally, the diagnosis is established
A lorazepam challenge is performed, and Mr. O receives 4 mg of lorazepam over 24 hours with little change in his catatonia symptoms. Given his persistent fever, tachycardia, and an elevated CK levels in the context of recent exposure to antipsychotic medications, Mr. O is diagnosed with NMS (Table 13,4 ) and is started on bromocriptine, 5 mg 3 times daily.

[polldaddy:10930632]
The authors’ observations
Mr. O’s complicated medical state—starting with DKA, halting the use of antipsychotic medications, and the suspicion of catatonia due to his history of schizophrenia—all distracted from the ultimate diagnosis of NMS as the cause of his enduring AMS and autonomic instability. Catatonia and NMS have overlapping symptomatology, including rigidity, autonomic instability, and stupor, which make the diagnosis of either condition complicated. A positive lorazepam test to diagnose catatonia is defined as a marked reduction in catatonia symptoms (typically a 50% reduction) as measured on a standardized rating scale.5 However, a negative lorazepam challenge does not definitely rule out catatonia because some cases are resistant to benzodiazepines.6
NMS risk factors relevant in this case include male sex, young age, acute medical illness, dehydration, and exposure to multiple psychotropic medications, including 2 antipsychotics, clozapine and fluphenazine.7 DKA is especially pertinent due to its acute onset and cause of significant dehydration. NMS can occur at any point of antipsychotic exposure, although the risk is highest during the initial weeks of treatment and during dosage changes. Unfortunately, Mr. O’s treatment team was unable to determine whether his medication had been recently changed, so it is not known what role this may have played in the development of NMS. Although first-generation antipsychotics are considered more likely to cause NMS, second-generation antipsychotics (SGAs) dominate the treatment of schizophrenia and bipolar disorder, and these medications also can cause NMS.8 As occurred in this case, long-acting injectable antipsychotics can be easily forgotten when not administered in the hospital, and their presence in the body persists for weeks. For example, the half-life of fluphenazine decanoate is approximately 10 days, and the half-life of haloperidol decanoate is 21 days.9
Continue to: OUTCOME Improvement with bromocriptine
OUTCOME Improvement with bromocriptine
After 4 days of bromocriptine, 5 mg 3 times daily, Mr. O is more alert, able to say “hello,” and can follow 1-step commands. By Day 26 of hospitalization, his CK levels decrease to 296 U/L, his CSF autoimmune panel is negative, and he is able to participate in physical therapy. After failing multiple swallow tests, Mr. O requires a percutaneous endoscopic gastrostomy (PEG) tube. He is discharged from the hospital to a long-term acute care facility with the plan to taper bromocriptine and restart a psychotropic regimen with his outpatient psychiatrist. At the time of discharge, he is able to sit at the edge of the bed independently, state his name, and respond to questions with multiple-word answers.
[polldaddy:10930633]
The authors’ observations
The most common pharmacologic treatments for NMS are dantrolene, bromocriptine, benzodiazepines (lorazepam or diazepam), and amantadine.3 Mild cases of NMS should be treated with discontinuation of all antipsychotics, supportive care, and benzodiazepines.3 Bromocriptine or amantadine are more appropriate for moderate cases and dantrolene for severe cases of NMS.3 All antipsychotics should be discontinued while a patient is experiencing an episode of NMS; however, once the NMS has resolved, clinicians must thoroughly evaluate the risks and benefits of restarting antipsychotic medication. After a patient has experienced an episode of NMS, clinicians generally should avoid prescribing the agent(s) that caused NMS and long-acting injections, and slowly titrate a low-potency SGA such as quetiapine.10Table 23,11,12 outlines the pharmacologic treatment of NMS.

Bottom Line
Neuroleptic malignant syndrome (NMS) should always be part of the differential diagnosis in patients with mental illness and altered mental status. The risk of NMS is especially high in patients with acute medical illness and exposure to antipsychotic medications.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Acyclovir • Zovirax
Amantadine • Gocovri
Ampicillin-sulbactam • Unasyn
Aripiprazole • Abilify Maintena
Benztropine • Cogentin
Bromocriptine • Cycloset, Parlodel
Ceftriaxone • Rocephin
Clozapine • Clozaril
Dantrolene • Dantrium
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate sodium • Depakote
Trazodone • Oleptro
Vancomycin • Vancocin
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
1. Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9-14.e1-2. doi: 10.1016/j.jpeds.2010.09.048
2. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185. doi: 10.1007/s11920-010-0113-y
3. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
4. Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
5. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. doi:10.3389/fpsyt.2014.00181
6. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380. doi:10.1176/jnp.2009.21.4.371
7. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004;22(2):389-411. doi:10.1016/j.ncl.2003.12.006
8. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. 2015;13(3):395-406. doi:10.2174/1570159x13999150424113345
9. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59. doi:10.1007/s40263-020-00779-5
10. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
11. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
12. Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
Intimate partner violence: Assessment in the era of telehealth
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf
Treating major depressive disorder after limited response to an initial agent
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Toy soldier syndrome: A consequence of parental cognitive dissonance
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Childhood and adolescence are periods with marked psychobehavioral development of the brain. The sense of self, identity, and role are established. This process is not without risk because brain regions governing reward, impulsivity, and sensation-seeking are relatively more developed and influential than higher-order cognitive regions regulating behavioral inhibition, decision-making, and planning, which continue to mature into one’s early to mid-20s. Consequently, while the developing brain is “under construction” by forging new pathways and taking advantage of its immense neuroplasticity, it is also prone to psychological insults under exposure to stressful events, attitudes, and behaviors, including those that can arise in the family.1
Most people would agree that there is no stronger emotion than parental love. The origins of this powerful biobehavioral bonding with a child have been attributed to maternal release of oxytocin, known colloquially as the “love hormone,” during the birthing process, and to both biological parents experiencing psychosocial attachment with their infant. Therefore, common sense dictates that parents would do anything to protect their offspring, and that no parent would deliberately behave in a manner that harms their child.
Common sense notwithstanding, reports of both child neglect and abuse are common. States have established agencies to protect children from their own parents. The answers to the question “Whose kids are they?” and under what circumstances the state has the authority to warn or reprimand parents, or even temporarily or permanently separate minors from their parents, are complex and vary by state.
In this commentary, we describe harmful actions committed by parents with the intention of protecting the impressionable minds of their children from malevolent forces or intrusive and unhealthy ideas. Second, we examine how to protect a minor from parental actions that are well-meaning but potentially harmful.
Parent-child communication
Delusional family interactions. Originally described in 1877 as “folie à deux,”2 shared madness is an extreme and uncommon parental psychiatric condition harmful to a child’s mental health. It is primarily characterized by parental-initiated delusions shared with the child that are typically persecutory and attributed to danger from vengeful folks or grandiose in nature. The question of whether the “folie” or “madness” is contagious arises due to the propensity of the child to adopt these delusions under an imposed insular or restrictive environment. Separating the child from the environment dominated by the delusional adult usually is sufficient to reverse the symptoms due to reality testing.
Normative familial communication. In contrary to a delusional familial interaction, normative family traditions and values are a unifying psychosocial force and a source of bonding and loyalty from an early age. A ubiquitous example is the support of a local sports team, and the emotional turmoil associated with the team’s wins and losses, accompanied by “hating” a rival. These family rituals are commonly devoid of emotional negative consequences for an impressionable young mind unless the child is exposed to unsportsmanlike emotional, verbal, or aggressive behavior by an adult at home in front of the television or in the stands at a game.
Continue to: Unfortunately...
Unfortunately, the “love-hate” dichotomy rooted in family-generated traditions of loyalty is becoming more evident in today’s turbulent sociopolitical environment. Children and young adolescents are not prepared to cope with the stressful effects of repeated exposure to intense conflictual events at home when parents adopt opposing sociopolitical ideologies. Furthermore, a parent might intentionally expose their child to emotionally conflictual circumstances in the name of a perceived value that might create and exacerbate stress, fear, and self-loathing. Ironically, by doing what a parent believes is right for their child, they might be transforming the child without their consent into a variant of a “toy soldier by proxy.” Such a child is a tool expected to follow the parental pathway and belief system without questioning, or even having the cognitive ability to do so, given their ongoing bio-behavioral and moral developmental phase.3
This new normative exposure to conflictual situations at the will of the parent is not only limited to watching them remotely but also may include participating in what is meant to be a peaceful protest or march. As we all witnessed in 2020, such events can easily deteriorate into unsafe environments rife with lawlessness and uncontrolled violence. This has included clashes between opposing groups who are matched in zeal and conviction, as well as opposition to or endangerment by law enforcement personnel trying to restore order by force. This is not where a responsible parent should take their child. Furthermore, there is the danger of loss of privacy of children exposed by media following their participation in public activity. This may lead to hate mail as that would further confuse and jeopardize a peaceful lifestyle, which is highly desirable for a developing child.
Cognitive dissonance. Have these parents temporarily allowed the limbic system to trump the restraints of the prefrontal cortex, as exhibited by an impulsive and risky behavior driven by poor insight? Have these parents thoughtfully weighed the balance between the merit of a child’s exposure to such conflictual circumstances and the peril of negative emotional consequences? This is illustrated by a mother who has been taking her preadolescent son to demonstrations regularly because “I want him to see how democracy works.”
Might this be a case of cognitive dissonance (CD) that amounts to unwitting mental child abuse if it happens repeatedly? According to the CD theory, there is a tendency to seek consistency between cognitions (eg, beliefs, opinions) and attitudes or behaviors. Inconsistency between these variables is termed “dissonance.”4,5 The importance attached to the dissonant belief affects the severity of the dissonance. The dissonance occurs when a parent must choose between 2 incompatible beliefs or actions. A classic demonstration of CD is when an adult requests that an adolescent follows his instructions (eg, “do not smoke or drink alcohol”), yet the adult does not act accordingly (eg, they smoke or drink). Role modeling demonstrated by such a discrepancy is a cause of confusion in the child. In terms of this article, the CD is between what the parent believes is an important learning experience by exercising the perceived right to pass to the child the parental value system vs compromising the protection of the child by exposing them to the potential negative consequences of a risky situation.
What can parents and therapists do?
Usually, parents mean well. It is important to communicate to parents the importance of refraining from forcing their children to join their battles. Calculating risks based on an intuitive approach is flawed because doing so is based on beliefs and emotions that originated in the limbic system (“I feel that”…) and are neither precise nor accurate.6 Teaching our youth in the school system how to think (eg, the science of logic and history of science) vs what to think (ie, indoctrination) is a key to healthy cognitive development. Furthermore, children need to have the time, space, and opportunities (learning moments) to develop this capacity. It is not until approximately age 16 that abstract thinking capabilities are developed. Cognitive dissonance can be eliminated by reducing the valence of the conflicting beliefs or by removing the conflicting attitude or behavior.
As parents and as mental health professionals, we should carry the necessary burden of responsibility to prevent the risk of “lost childhood” due to parental emotional zeal and righteousness that lead to early exposure to damaging adversity. We cannot afford to turn our children into exploitable tools (ie, toy soldiers) in conflicts they do not fully grasp.
1. Bagot KS, Kaminer Y. Harm reduction for youth in treatment with substance use disorders: one size does not fit all. Curr Addict Rep. 2018;5:379-385.
2. Arnon D, Patel A, Tan GM. The nosological significance of Folie à Deux: a review of the literature. Ann Gen Psychiatry. 2006;5:11.
3. Kohlberg L. The philosophy of moral development: the nature and validity of moral stages. Harper & Row; 1984.
4. Festinger L. A theory of cognitive dissonance. Stanford University Press; 1957.
5. Festinger L. Cognitive dissonance. Sci Am. 1962;207:93-102.
6. Henderson SW, Gerson R, Phillips B. What is “high risk” and what are we actually supposed to do about it? J Am Acad Child Adolesc Psychiatry. 2019;58(6):561-564.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Childhood and adolescence are periods with marked psychobehavioral development of the brain. The sense of self, identity, and role are established. This process is not without risk because brain regions governing reward, impulsivity, and sensation-seeking are relatively more developed and influential than higher-order cognitive regions regulating behavioral inhibition, decision-making, and planning, which continue to mature into one’s early to mid-20s. Consequently, while the developing brain is “under construction” by forging new pathways and taking advantage of its immense neuroplasticity, it is also prone to psychological insults under exposure to stressful events, attitudes, and behaviors, including those that can arise in the family.1
Most people would agree that there is no stronger emotion than parental love. The origins of this powerful biobehavioral bonding with a child have been attributed to maternal release of oxytocin, known colloquially as the “love hormone,” during the birthing process, and to both biological parents experiencing psychosocial attachment with their infant. Therefore, common sense dictates that parents would do anything to protect their offspring, and that no parent would deliberately behave in a manner that harms their child.
Common sense notwithstanding, reports of both child neglect and abuse are common. States have established agencies to protect children from their own parents. The answers to the question “Whose kids are they?” and under what circumstances the state has the authority to warn or reprimand parents, or even temporarily or permanently separate minors from their parents, are complex and vary by state.
In this commentary, we describe harmful actions committed by parents with the intention of protecting the impressionable minds of their children from malevolent forces or intrusive and unhealthy ideas. Second, we examine how to protect a minor from parental actions that are well-meaning but potentially harmful.
Parent-child communication
Delusional family interactions. Originally described in 1877 as “folie à deux,”2 shared madness is an extreme and uncommon parental psychiatric condition harmful to a child’s mental health. It is primarily characterized by parental-initiated delusions shared with the child that are typically persecutory and attributed to danger from vengeful folks or grandiose in nature. The question of whether the “folie” or “madness” is contagious arises due to the propensity of the child to adopt these delusions under an imposed insular or restrictive environment. Separating the child from the environment dominated by the delusional adult usually is sufficient to reverse the symptoms due to reality testing.
Normative familial communication. In contrary to a delusional familial interaction, normative family traditions and values are a unifying psychosocial force and a source of bonding and loyalty from an early age. A ubiquitous example is the support of a local sports team, and the emotional turmoil associated with the team’s wins and losses, accompanied by “hating” a rival. These family rituals are commonly devoid of emotional negative consequences for an impressionable young mind unless the child is exposed to unsportsmanlike emotional, verbal, or aggressive behavior by an adult at home in front of the television or in the stands at a game.
Continue to: Unfortunately...
Unfortunately, the “love-hate” dichotomy rooted in family-generated traditions of loyalty is becoming more evident in today’s turbulent sociopolitical environment. Children and young adolescents are not prepared to cope with the stressful effects of repeated exposure to intense conflictual events at home when parents adopt opposing sociopolitical ideologies. Furthermore, a parent might intentionally expose their child to emotionally conflictual circumstances in the name of a perceived value that might create and exacerbate stress, fear, and self-loathing. Ironically, by doing what a parent believes is right for their child, they might be transforming the child without their consent into a variant of a “toy soldier by proxy.” Such a child is a tool expected to follow the parental pathway and belief system without questioning, or even having the cognitive ability to do so, given their ongoing bio-behavioral and moral developmental phase.3
This new normative exposure to conflictual situations at the will of the parent is not only limited to watching them remotely but also may include participating in what is meant to be a peaceful protest or march. As we all witnessed in 2020, such events can easily deteriorate into unsafe environments rife with lawlessness and uncontrolled violence. This has included clashes between opposing groups who are matched in zeal and conviction, as well as opposition to or endangerment by law enforcement personnel trying to restore order by force. This is not where a responsible parent should take their child. Furthermore, there is the danger of loss of privacy of children exposed by media following their participation in public activity. This may lead to hate mail as that would further confuse and jeopardize a peaceful lifestyle, which is highly desirable for a developing child.
Cognitive dissonance. Have these parents temporarily allowed the limbic system to trump the restraints of the prefrontal cortex, as exhibited by an impulsive and risky behavior driven by poor insight? Have these parents thoughtfully weighed the balance between the merit of a child’s exposure to such conflictual circumstances and the peril of negative emotional consequences? This is illustrated by a mother who has been taking her preadolescent son to demonstrations regularly because “I want him to see how democracy works.”
Might this be a case of cognitive dissonance (CD) that amounts to unwitting mental child abuse if it happens repeatedly? According to the CD theory, there is a tendency to seek consistency between cognitions (eg, beliefs, opinions) and attitudes or behaviors. Inconsistency between these variables is termed “dissonance.”4,5 The importance attached to the dissonant belief affects the severity of the dissonance. The dissonance occurs when a parent must choose between 2 incompatible beliefs or actions. A classic demonstration of CD is when an adult requests that an adolescent follows his instructions (eg, “do not smoke or drink alcohol”), yet the adult does not act accordingly (eg, they smoke or drink). Role modeling demonstrated by such a discrepancy is a cause of confusion in the child. In terms of this article, the CD is between what the parent believes is an important learning experience by exercising the perceived right to pass to the child the parental value system vs compromising the protection of the child by exposing them to the potential negative consequences of a risky situation.
What can parents and therapists do?
Usually, parents mean well. It is important to communicate to parents the importance of refraining from forcing their children to join their battles. Calculating risks based on an intuitive approach is flawed because doing so is based on beliefs and emotions that originated in the limbic system (“I feel that”…) and are neither precise nor accurate.6 Teaching our youth in the school system how to think (eg, the science of logic and history of science) vs what to think (ie, indoctrination) is a key to healthy cognitive development. Furthermore, children need to have the time, space, and opportunities (learning moments) to develop this capacity. It is not until approximately age 16 that abstract thinking capabilities are developed. Cognitive dissonance can be eliminated by reducing the valence of the conflicting beliefs or by removing the conflicting attitude or behavior.
As parents and as mental health professionals, we should carry the necessary burden of responsibility to prevent the risk of “lost childhood” due to parental emotional zeal and righteousness that lead to early exposure to damaging adversity. We cannot afford to turn our children into exploitable tools (ie, toy soldiers) in conflicts they do not fully grasp.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Childhood and adolescence are periods with marked psychobehavioral development of the brain. The sense of self, identity, and role are established. This process is not without risk because brain regions governing reward, impulsivity, and sensation-seeking are relatively more developed and influential than higher-order cognitive regions regulating behavioral inhibition, decision-making, and planning, which continue to mature into one’s early to mid-20s. Consequently, while the developing brain is “under construction” by forging new pathways and taking advantage of its immense neuroplasticity, it is also prone to psychological insults under exposure to stressful events, attitudes, and behaviors, including those that can arise in the family.1
Most people would agree that there is no stronger emotion than parental love. The origins of this powerful biobehavioral bonding with a child have been attributed to maternal release of oxytocin, known colloquially as the “love hormone,” during the birthing process, and to both biological parents experiencing psychosocial attachment with their infant. Therefore, common sense dictates that parents would do anything to protect their offspring, and that no parent would deliberately behave in a manner that harms their child.
Common sense notwithstanding, reports of both child neglect and abuse are common. States have established agencies to protect children from their own parents. The answers to the question “Whose kids are they?” and under what circumstances the state has the authority to warn or reprimand parents, or even temporarily or permanently separate minors from their parents, are complex and vary by state.
In this commentary, we describe harmful actions committed by parents with the intention of protecting the impressionable minds of their children from malevolent forces or intrusive and unhealthy ideas. Second, we examine how to protect a minor from parental actions that are well-meaning but potentially harmful.
Parent-child communication
Delusional family interactions. Originally described in 1877 as “folie à deux,”2 shared madness is an extreme and uncommon parental psychiatric condition harmful to a child’s mental health. It is primarily characterized by parental-initiated delusions shared with the child that are typically persecutory and attributed to danger from vengeful folks or grandiose in nature. The question of whether the “folie” or “madness” is contagious arises due to the propensity of the child to adopt these delusions under an imposed insular or restrictive environment. Separating the child from the environment dominated by the delusional adult usually is sufficient to reverse the symptoms due to reality testing.
Normative familial communication. In contrary to a delusional familial interaction, normative family traditions and values are a unifying psychosocial force and a source of bonding and loyalty from an early age. A ubiquitous example is the support of a local sports team, and the emotional turmoil associated with the team’s wins and losses, accompanied by “hating” a rival. These family rituals are commonly devoid of emotional negative consequences for an impressionable young mind unless the child is exposed to unsportsmanlike emotional, verbal, or aggressive behavior by an adult at home in front of the television or in the stands at a game.
Continue to: Unfortunately...
Unfortunately, the “love-hate” dichotomy rooted in family-generated traditions of loyalty is becoming more evident in today’s turbulent sociopolitical environment. Children and young adolescents are not prepared to cope with the stressful effects of repeated exposure to intense conflictual events at home when parents adopt opposing sociopolitical ideologies. Furthermore, a parent might intentionally expose their child to emotionally conflictual circumstances in the name of a perceived value that might create and exacerbate stress, fear, and self-loathing. Ironically, by doing what a parent believes is right for their child, they might be transforming the child without their consent into a variant of a “toy soldier by proxy.” Such a child is a tool expected to follow the parental pathway and belief system without questioning, or even having the cognitive ability to do so, given their ongoing bio-behavioral and moral developmental phase.3
This new normative exposure to conflictual situations at the will of the parent is not only limited to watching them remotely but also may include participating in what is meant to be a peaceful protest or march. As we all witnessed in 2020, such events can easily deteriorate into unsafe environments rife with lawlessness and uncontrolled violence. This has included clashes between opposing groups who are matched in zeal and conviction, as well as opposition to or endangerment by law enforcement personnel trying to restore order by force. This is not where a responsible parent should take their child. Furthermore, there is the danger of loss of privacy of children exposed by media following their participation in public activity. This may lead to hate mail as that would further confuse and jeopardize a peaceful lifestyle, which is highly desirable for a developing child.
Cognitive dissonance. Have these parents temporarily allowed the limbic system to trump the restraints of the prefrontal cortex, as exhibited by an impulsive and risky behavior driven by poor insight? Have these parents thoughtfully weighed the balance between the merit of a child’s exposure to such conflictual circumstances and the peril of negative emotional consequences? This is illustrated by a mother who has been taking her preadolescent son to demonstrations regularly because “I want him to see how democracy works.”
Might this be a case of cognitive dissonance (CD) that amounts to unwitting mental child abuse if it happens repeatedly? According to the CD theory, there is a tendency to seek consistency between cognitions (eg, beliefs, opinions) and attitudes or behaviors. Inconsistency between these variables is termed “dissonance.”4,5 The importance attached to the dissonant belief affects the severity of the dissonance. The dissonance occurs when a parent must choose between 2 incompatible beliefs or actions. A classic demonstration of CD is when an adult requests that an adolescent follows his instructions (eg, “do not smoke or drink alcohol”), yet the adult does not act accordingly (eg, they smoke or drink). Role modeling demonstrated by such a discrepancy is a cause of confusion in the child. In terms of this article, the CD is between what the parent believes is an important learning experience by exercising the perceived right to pass to the child the parental value system vs compromising the protection of the child by exposing them to the potential negative consequences of a risky situation.
What can parents and therapists do?
Usually, parents mean well. It is important to communicate to parents the importance of refraining from forcing their children to join their battles. Calculating risks based on an intuitive approach is flawed because doing so is based on beliefs and emotions that originated in the limbic system (“I feel that”…) and are neither precise nor accurate.6 Teaching our youth in the school system how to think (eg, the science of logic and history of science) vs what to think (ie, indoctrination) is a key to healthy cognitive development. Furthermore, children need to have the time, space, and opportunities (learning moments) to develop this capacity. It is not until approximately age 16 that abstract thinking capabilities are developed. Cognitive dissonance can be eliminated by reducing the valence of the conflicting beliefs or by removing the conflicting attitude or behavior.
As parents and as mental health professionals, we should carry the necessary burden of responsibility to prevent the risk of “lost childhood” due to parental emotional zeal and righteousness that lead to early exposure to damaging adversity. We cannot afford to turn our children into exploitable tools (ie, toy soldiers) in conflicts they do not fully grasp.
1. Bagot KS, Kaminer Y. Harm reduction for youth in treatment with substance use disorders: one size does not fit all. Curr Addict Rep. 2018;5:379-385.
2. Arnon D, Patel A, Tan GM. The nosological significance of Folie à Deux: a review of the literature. Ann Gen Psychiatry. 2006;5:11.
3. Kohlberg L. The philosophy of moral development: the nature and validity of moral stages. Harper & Row; 1984.
4. Festinger L. A theory of cognitive dissonance. Stanford University Press; 1957.
5. Festinger L. Cognitive dissonance. Sci Am. 1962;207:93-102.
6. Henderson SW, Gerson R, Phillips B. What is “high risk” and what are we actually supposed to do about it? J Am Acad Child Adolesc Psychiatry. 2019;58(6):561-564.
1. Bagot KS, Kaminer Y. Harm reduction for youth in treatment with substance use disorders: one size does not fit all. Curr Addict Rep. 2018;5:379-385.
2. Arnon D, Patel A, Tan GM. The nosological significance of Folie à Deux: a review of the literature. Ann Gen Psychiatry. 2006;5:11.
3. Kohlberg L. The philosophy of moral development: the nature and validity of moral stages. Harper & Row; 1984.
4. Festinger L. A theory of cognitive dissonance. Stanford University Press; 1957.
5. Festinger L. Cognitive dissonance. Sci Am. 1962;207:93-102.
6. Henderson SW, Gerson R, Phillips B. What is “high risk” and what are we actually supposed to do about it? J Am Acad Child Adolesc Psychiatry. 2019;58(6):561-564.
The world authority
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
It was the late 1970s. She arrived by limousine; an attractive young woman, “Jasmine,” daughter of one of the richest men in the country. She was wearing a low-cut silk blouse and was adorned with an abundance of jewelry: large earrings, a necklace, and rings on nearly every finger. She smiled broadly when I introduced myself, shook her hand, and ushered her into my office.
She moved quickly and spoke in a rapid and pressured manner. She complained of poor sleep, mood swings, and racing thoughts. I began to ask her questions. “Have you used cocaine recently?” “What about Ritalin or amphetamines?” “Do you take prednisone or any other steroids?” “Do you have thyroid disease?” “Do you or anyone in your family have a history of manic depressive illness? Who? Have they ever required hospitalization for its treatment?”
“Well, doctor, what do you think?” she asked.
“I think that you probably have manic depressive illness and that you are currently having a manic episode. You should go into the hospital and begin treatment with lithium.”
“Who is the authority on this illness?” she asked.
“…The US authority or the world authority?” I replied.
“The world authority” she answered.
“Probably Professor Mogens Schou in Copenhagen,” I said.
“I’ll go see what he thinks,” she responded.
“Okay, you do that,” I replied.
When she left, I thought, “What a grandiose young woman, I doubt I’ll hear from her again.”
Continue to: Three days...
Three days later I received an unusual phone call.
“Dr. Jaffe, this is the long-distance operator, will you hold for Dr. Schou?”
“Of course,” I replied.
“Dr. Jaffe, I’m here with your patient, a charming young woman. I told her that I am in complete agreement with your diagnosis and treatment plan. She will be flying home tomorrow.”
A few days after she arrived home, I had Jasmine hospitalized under my care at one of the local psychiatric units. She stabilized nicely on lithium and tolerated it well. She remained there for about 3 weeks and was then discharged. I began seeing her in my office for weekly visits. After a few months we started meeting every 2 weeks, and eventually monthly.
She was doing well. Her mood swings were now mild and infrequent. Her sleep had normalized. Most important, she felt a lot more in control of her life.
Jasmine offered me a small window into the world of the super-rich and powerful. Basically, what I learned was that they are just like the rest of us, only more so. All the money provides both the opportunity to do a lot more good as well as to get into a lot more trouble. When a middle-class person gets manic and goes on a spending spree, they may blow a few hundred dollars on lottery tickets and perhaps a thousand dollars on clothing or gifts they don’t need. When the very rich do this, they buy airplanes, Ferraris, and vacation homes.
Jasmine and her siblings were often pestered—usually by acquaintances, but sometimes friends—for favors, usually loans, jobs, or introductions to other famous or powerful people. Jasmine turned out to be a lovely young woman, kind, generous, loyal to her friends and with a fine sense of humor. Getting to know her well helped dispel some of my prejudices about the adult children of the super-rich. I had incorrectly assumed that she would be quite spoiled and entitled.
After working together for approximately 2 years, we said our goodbyes because I was moving to a different part of the country. She thanked me for helping her get well. I asked her if there was anything in particular that she found most helpful. She surprised me when she answered so quickly.
“Yes. When you come from a very wealthy family, most people want something from you. You never wanted anything from me except my honesty” she said.
I thanked Jasmine for her gift.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
It was the late 1970s. She arrived by limousine; an attractive young woman, “Jasmine,” daughter of one of the richest men in the country. She was wearing a low-cut silk blouse and was adorned with an abundance of jewelry: large earrings, a necklace, and rings on nearly every finger. She smiled broadly when I introduced myself, shook her hand, and ushered her into my office.
She moved quickly and spoke in a rapid and pressured manner. She complained of poor sleep, mood swings, and racing thoughts. I began to ask her questions. “Have you used cocaine recently?” “What about Ritalin or amphetamines?” “Do you take prednisone or any other steroids?” “Do you have thyroid disease?” “Do you or anyone in your family have a history of manic depressive illness? Who? Have they ever required hospitalization for its treatment?”
“Well, doctor, what do you think?” she asked.
“I think that you probably have manic depressive illness and that you are currently having a manic episode. You should go into the hospital and begin treatment with lithium.”
“Who is the authority on this illness?” she asked.
“…The US authority or the world authority?” I replied.
“The world authority” she answered.
“Probably Professor Mogens Schou in Copenhagen,” I said.
“I’ll go see what he thinks,” she responded.
“Okay, you do that,” I replied.
When she left, I thought, “What a grandiose young woman, I doubt I’ll hear from her again.”
Continue to: Three days...
Three days later I received an unusual phone call.
“Dr. Jaffe, this is the long-distance operator, will you hold for Dr. Schou?”
“Of course,” I replied.
“Dr. Jaffe, I’m here with your patient, a charming young woman. I told her that I am in complete agreement with your diagnosis and treatment plan. She will be flying home tomorrow.”
A few days after she arrived home, I had Jasmine hospitalized under my care at one of the local psychiatric units. She stabilized nicely on lithium and tolerated it well. She remained there for about 3 weeks and was then discharged. I began seeing her in my office for weekly visits. After a few months we started meeting every 2 weeks, and eventually monthly.
She was doing well. Her mood swings were now mild and infrequent. Her sleep had normalized. Most important, she felt a lot more in control of her life.
Jasmine offered me a small window into the world of the super-rich and powerful. Basically, what I learned was that they are just like the rest of us, only more so. All the money provides both the opportunity to do a lot more good as well as to get into a lot more trouble. When a middle-class person gets manic and goes on a spending spree, they may blow a few hundred dollars on lottery tickets and perhaps a thousand dollars on clothing or gifts they don’t need. When the very rich do this, they buy airplanes, Ferraris, and vacation homes.
Jasmine and her siblings were often pestered—usually by acquaintances, but sometimes friends—for favors, usually loans, jobs, or introductions to other famous or powerful people. Jasmine turned out to be a lovely young woman, kind, generous, loyal to her friends and with a fine sense of humor. Getting to know her well helped dispel some of my prejudices about the adult children of the super-rich. I had incorrectly assumed that she would be quite spoiled and entitled.
After working together for approximately 2 years, we said our goodbyes because I was moving to a different part of the country. She thanked me for helping her get well. I asked her if there was anything in particular that she found most helpful. She surprised me when she answered so quickly.
“Yes. When you come from a very wealthy family, most people want something from you. You never wanted anything from me except my honesty” she said.
I thanked Jasmine for her gift.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
It was the late 1970s. She arrived by limousine; an attractive young woman, “Jasmine,” daughter of one of the richest men in the country. She was wearing a low-cut silk blouse and was adorned with an abundance of jewelry: large earrings, a necklace, and rings on nearly every finger. She smiled broadly when I introduced myself, shook her hand, and ushered her into my office.
She moved quickly and spoke in a rapid and pressured manner. She complained of poor sleep, mood swings, and racing thoughts. I began to ask her questions. “Have you used cocaine recently?” “What about Ritalin or amphetamines?” “Do you take prednisone or any other steroids?” “Do you have thyroid disease?” “Do you or anyone in your family have a history of manic depressive illness? Who? Have they ever required hospitalization for its treatment?”
“Well, doctor, what do you think?” she asked.
“I think that you probably have manic depressive illness and that you are currently having a manic episode. You should go into the hospital and begin treatment with lithium.”
“Who is the authority on this illness?” she asked.
“…The US authority or the world authority?” I replied.
“The world authority” she answered.
“Probably Professor Mogens Schou in Copenhagen,” I said.
“I’ll go see what he thinks,” she responded.
“Okay, you do that,” I replied.
When she left, I thought, “What a grandiose young woman, I doubt I’ll hear from her again.”
Continue to: Three days...
Three days later I received an unusual phone call.
“Dr. Jaffe, this is the long-distance operator, will you hold for Dr. Schou?”
“Of course,” I replied.
“Dr. Jaffe, I’m here with your patient, a charming young woman. I told her that I am in complete agreement with your diagnosis and treatment plan. She will be flying home tomorrow.”
A few days after she arrived home, I had Jasmine hospitalized under my care at one of the local psychiatric units. She stabilized nicely on lithium and tolerated it well. She remained there for about 3 weeks and was then discharged. I began seeing her in my office for weekly visits. After a few months we started meeting every 2 weeks, and eventually monthly.
She was doing well. Her mood swings were now mild and infrequent. Her sleep had normalized. Most important, she felt a lot more in control of her life.
Jasmine offered me a small window into the world of the super-rich and powerful. Basically, what I learned was that they are just like the rest of us, only more so. All the money provides both the opportunity to do a lot more good as well as to get into a lot more trouble. When a middle-class person gets manic and goes on a spending spree, they may blow a few hundred dollars on lottery tickets and perhaps a thousand dollars on clothing or gifts they don’t need. When the very rich do this, they buy airplanes, Ferraris, and vacation homes.
Jasmine and her siblings were often pestered—usually by acquaintances, but sometimes friends—for favors, usually loans, jobs, or introductions to other famous or powerful people. Jasmine turned out to be a lovely young woman, kind, generous, loyal to her friends and with a fine sense of humor. Getting to know her well helped dispel some of my prejudices about the adult children of the super-rich. I had incorrectly assumed that she would be quite spoiled and entitled.
After working together for approximately 2 years, we said our goodbyes because I was moving to a different part of the country. She thanked me for helping her get well. I asked her if there was anything in particular that she found most helpful. She surprised me when she answered so quickly.
“Yes. When you come from a very wealthy family, most people want something from you. You never wanted anything from me except my honesty” she said.
I thanked Jasmine for her gift.