User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
The third generation of therapeutic innovation and the future of psychopharmacology
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
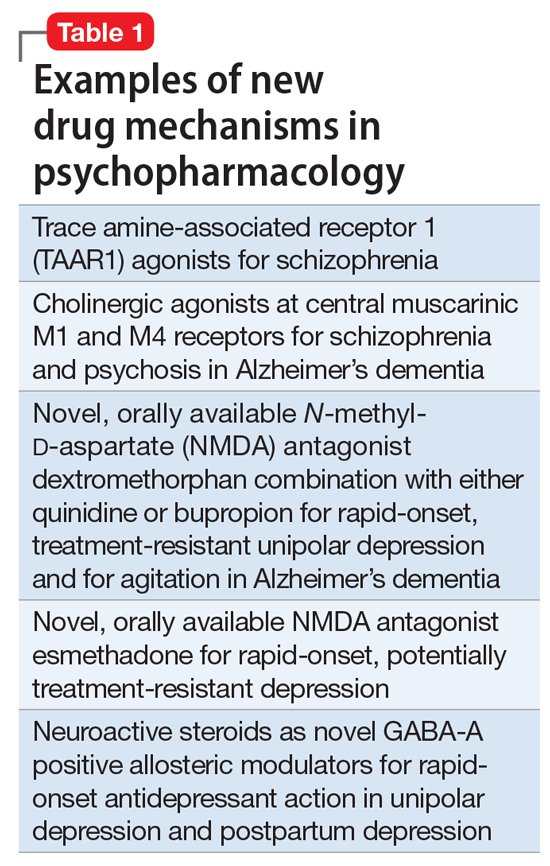
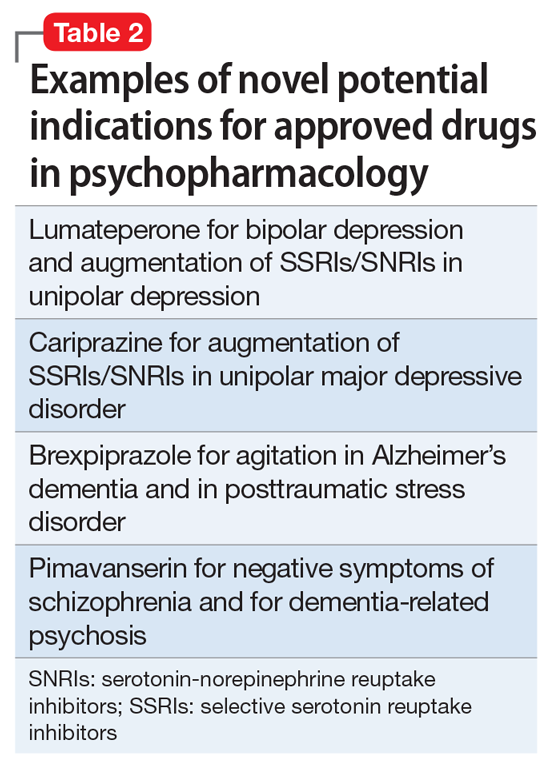
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1

Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
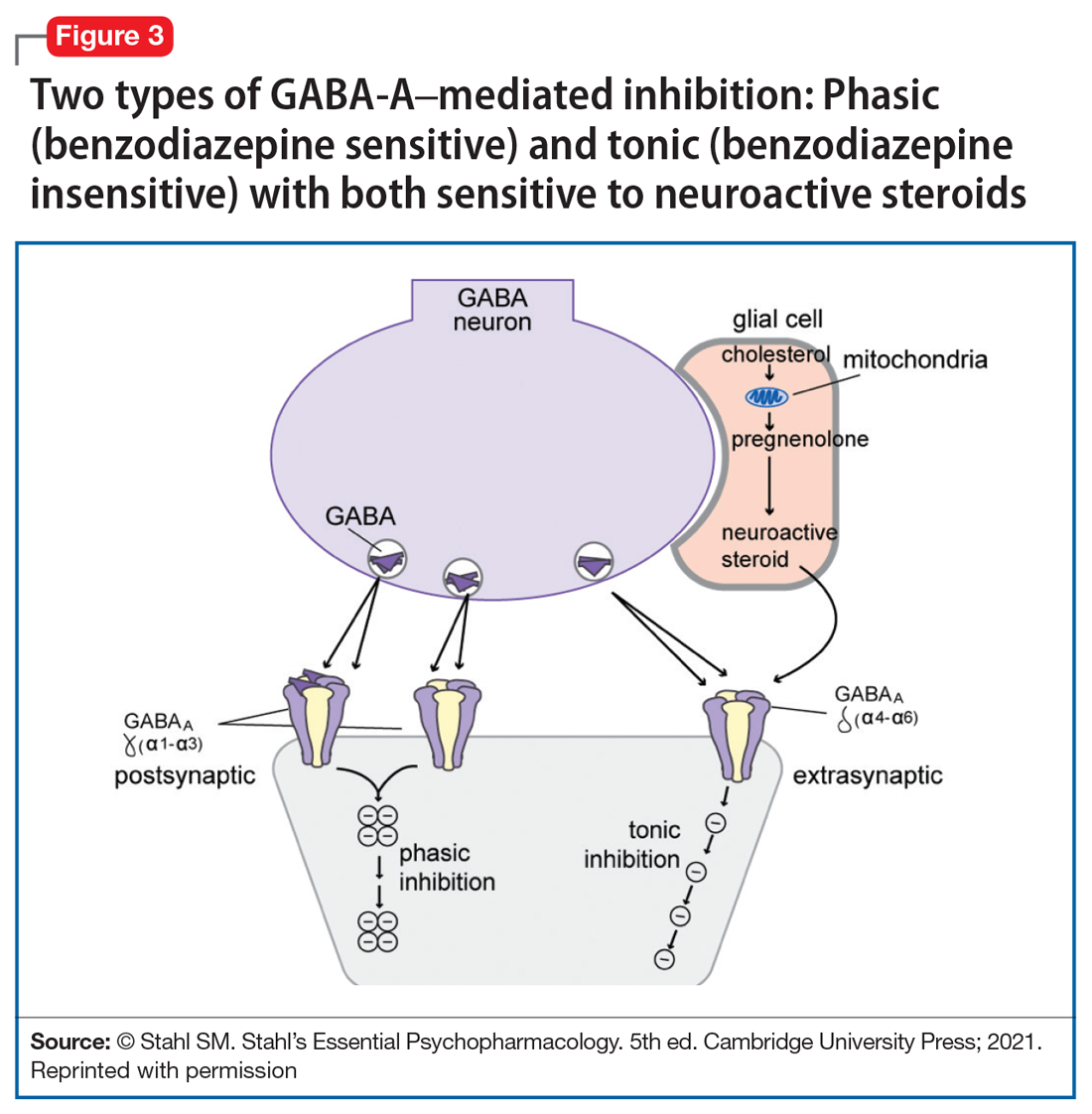
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.
The future of psychopharmacology is clearly going to be amazing.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1



Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.


The future of psychopharmacology is clearly going to be amazing.
The field of psychiatric therapeutics is now experiencing its third generation of progress. No sooner had the pace of innovation in psychiatry and psychopharmacology hit the doldrums a few years ago, following the dwindling of the second generation of progress, than the current third generation of new drug development in psychopharmacology was born.
That is, the first generation of discovery of psychiatric medications in the 1960s and 1970s ushered in the first known psychotropic drugs, such as the tricyclic antidepressants, as well as major and minor tranquilizers, such as chlorpromazine and benzodiazepines, only to fizzle out in the 1980s. By the 1990s, the second generation of innovation in psychopharmacology was in full swing, with the “new” serotonin selective reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors for depression, and the “atypical” antipsychotics for schizophrenia. However, soon after the turn of the century, pessimism for psychiatric therapeutics crept in again, and “big Pharma” abandoned their psychopharmacology programs in favor of other therapeutic areas. Surprisingly, the current “green shoots” of new ideas sprouting in our field today have not come from traditional big Pharma returning to psychiatry, but largely from small, innovative companies. These new entrepreneurial small pharmas and biotechs have found several new therapeutic targets. Furthermore, current innovation in psychopharmacology is increasingly following a paradigm shift away from DSM-5 disorders and instead to domains or symptoms of psychopathology that cut across numerous psychiatric conditions (transdiagnostic model).
So, what are the new therapeutic mechanisms of this current third generation of innovation in psychopharmacology? Not all of these can be discussed here, but 2 examples of new approaches to psychosis deserve special mention because, for the first time in 70 years, they turn away from blocking postsynaptic dopamine D2 receptors to treat psychosis and instead stimulate receptors in other neurotransmitter systems that are linked to dopamine neurons in a network “upstream.” That is, trace amine-associated receptor 1 (TAAR1) agonists target the pre-synaptic dopamine neuron, where dopamine synthesis and release are too high in psychosis, and cause dopamine synthesis to be reduced so that blockade of postsynaptic dopamine receptors is no longer necessary (Table 1 and Figure 1).1 Similarly, muscarinic cholinergic 1 and 4 receptor agonists target excitatory cholinergic neurons upstream, and turn down their stimulation of dopamine neurons, thereby reducing dopamine release so that postsynaptic blockade of dopamine receptors is also not necessary to treat psychosis with this mechanism (Table 1 and Figure 2).1 A similar mechanism of reducing upstream stimulation of dopamine release by serotonin has led to demonstration of antipsychotic actions of blocking this stimulation at serotonin 2A receptors (Table 2), and multiple approaches to enhancing deficient glutamate actions upstream are also under investigation for the treatment of psychosis. 1



Another major area of innovation in psychopharmacology worthy of emphasis is the rapid induction of neurogenesis that is associated with rapid reduction in the symptoms of depression, even when many conventional treatments have failed. Blockade of N-methyl-
that may hypothetically drive rapid recovery from depression.1 Proof of this concept was first shown with intravenous ketamine, and then intranasal esketamine, and now the oral NMDA antagonists dextromethorphan (combined with either bupropion or quinidine) and esmethadone (Table 1).1 Interestingly, this same mechanism may lead to a novel treatment of agitation in Alzheimer’s dementia as well.1
Continue to: Yet another mechanism...
Yet another mechanism of potentially rapid onset antidepressant action is that of the novel agents known as neuroactive steroids that have a novel action at gamma aminobutyric acid A (GABA-A) receptors that are not sensitive to benzodiazepines (as well as those that are) (Table 1 and Figure 3).1 Finally, psychedelic drugs that target serotonin receptors such as psilocybin and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) seem to also have rapid onset of both neurogenesis and antidepressant action.


The future of psychopharmacology is clearly going to be amazing.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
1. Stahl SM. Stahl’s Essential Psychopharmacology. 5th ed. Cambridge University Press; 2021.
Adolescents, THC, and the risk of psychosis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact [email protected].
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
Vaping: Understand the risks
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
From 2017 to 2018, the 30-day prevalence of “vaping” nicotine rose dramatically among 8th graders, 10th graders, 12th graders, college students, and young adults; the increase was the greatest among college students.1 As vaping has become a common phenomenon in our society, it is prudent to have a basic understanding of what vaping is, and its potential health risks.
How it works
Vaping is the inhaling and exhaling of aerosol that is produced by a device.2 Users can vape nicotine, tetrahydrocannabinol (THC), or synthetic drugs. The aerosol, often mistaken for water vapor, consists of fine particles that contain varying amounts of toxic chemicals and heavy metals that enter the lungs and bloodstream when vaping.2 In general, vaping devices consist of a mouthpiece, a battery, a cartridge for containing the e-juice/e-liquid, and a heating component that turns the e-juice/e-liquid into vapor.2 The e-juice/e-liquid usually contains a propylene glycol or vegetable glycerin-based liquid with nicotine, THC, or synthetic drugs.2 The e-juice/e-liquid also contains flavorings, additives, and other chemicals and metals (but not tobacco).2
There are 4 types of vaping devices3:
E-cigarettes. This first generation of vaping devices was introduced to US markets in 2007. E-cigarettes look similar to cigarettes and come in disposable or rechargeable forms.3 They may emit a light when the user puffs. E-cigarettes have shorter battery lives and are less expensive than other vaping devices.
Vape pens. These second-generation vaping devices resemble fountain pens. Vape pens also come in disposable and rechargeable forms.3 They can be refilled with e-juice/e-liquid.3
Vaping mods. These third-generation vaping devices were created when users modified items such as flashlights to create a more powerful vaping experience; however, these self-modifications often are unsafe. Vaping mods are larger than vape pens and e-cigarettes and include modification options. They also have large-capacity batteries that are replaceable. Vaping mods are typically rechargeable and deliver more nicotine than earlier-generation vaping devices.
Pod systems. Pod systems, such as Juul, are the latest generation of vaping devices. These small, sleek devices resemble a USB drive.3 They can be recharged on a laptop or any USB charger.3 Pods combine the portability of e-cigarettes or vape pens with the power of a mod system. There are 2 types of pod systems: open and closed. Open pod systems consist of removable pods that are filled with the user’s choice of e-juice/e-liquid and then replaced after being refilled several times. Closed pod systems are purchased pre-filled with e-juice/e-liquid and are disposable, similar to single-use coffee pods. Juul is the most popular vape brand in the United States.4 For a visual guide of the different vaping devices, see https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf
What are the risks?
Vaping is relatively new, so the long-term health effects are not well studied. Although less harmful than smoking cigarettes, vaping is still not safe because users are exposed to chemicals in the aerosol, such as nicotine, heavy metals such as lead, volatile organic compounds, and cancer-causing agents.3 Vaping nicotine can result in the same cardiac and pulmonary complications as smoking cigarettes. Vaping nicotine can also be more addictive than smoking cigarettes because users can buy cartridges with higher concentrations of nicotine or increase the vaping device’s voltage to get a greater “hit” of nicotine (or whatever substance the user is vaping.) Vaping devices can also cause unintentional injuries due to fires and explosions from defective batteries.
Vaping—particularly vaping THC—has been linked to a condition called e-cigarette, or vaping, product use-associated lung injury (EVALI).5 As of February 18, 2020, the CDC had received reports of approximately 2,800 patients with EVALI who were hospitalized or had died.5 Most EVALI cases have been linked to e-cigarette or vaping products that contained THC, particularly products obtained from informal sources such as friends, family, or in-person or online dealers.5 Vitamin E acetate, an additive in some THC-containing vaping products, has been strongly linked to EVALI.5 When ingested as a vitamin supplement or applied to the skin, vitamin E usually is harmless, but when inhaled, it may interfere with normal lung functioning.5 The CDC recommends that individuals who vape do not use products that contain THC; avoid getting vaping products from informal sources, such as friends, family, or online dealers; and not modify or add any substances to a vaping device other than as intended by the manufacturer.5
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
- Schulenberg JE, Johnston LD, O’Malley PM, et al; the University of Michigan Institute for Social Research. Monitoring the Future national survey results on drug use, 1975-2018. Volume 2. College students and adults ages 19-60. Published July 2019. Accessed November 12, 2021. http://www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf
- Partnership to End Addiction. Vaping & e-cigarettes. Last updated May 2021. Accessed November 12, 2021. https://drugfree.org/drugs/e-cigarettes-vaping/
- Centers for Disease Control and Prevention. About electronic cigarettes (e-cigarettes). Last reviewed February 24, 2020. Accessed June 20, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- Partnership to End Addiction. What parents need to know about vaping. Published May 2020. Accessed October 27, 2021. https://drugfree.org/article/what-parents-need-to-know-about-vaping/
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Last reviewed August 3, 2021. Accessed November 19, 2021. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#overview
Serotonin-mediated anxiety: How to recognize and treat it
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Dealing with a difficult boss: A ‘bossectomy’ is rarely the cure
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”
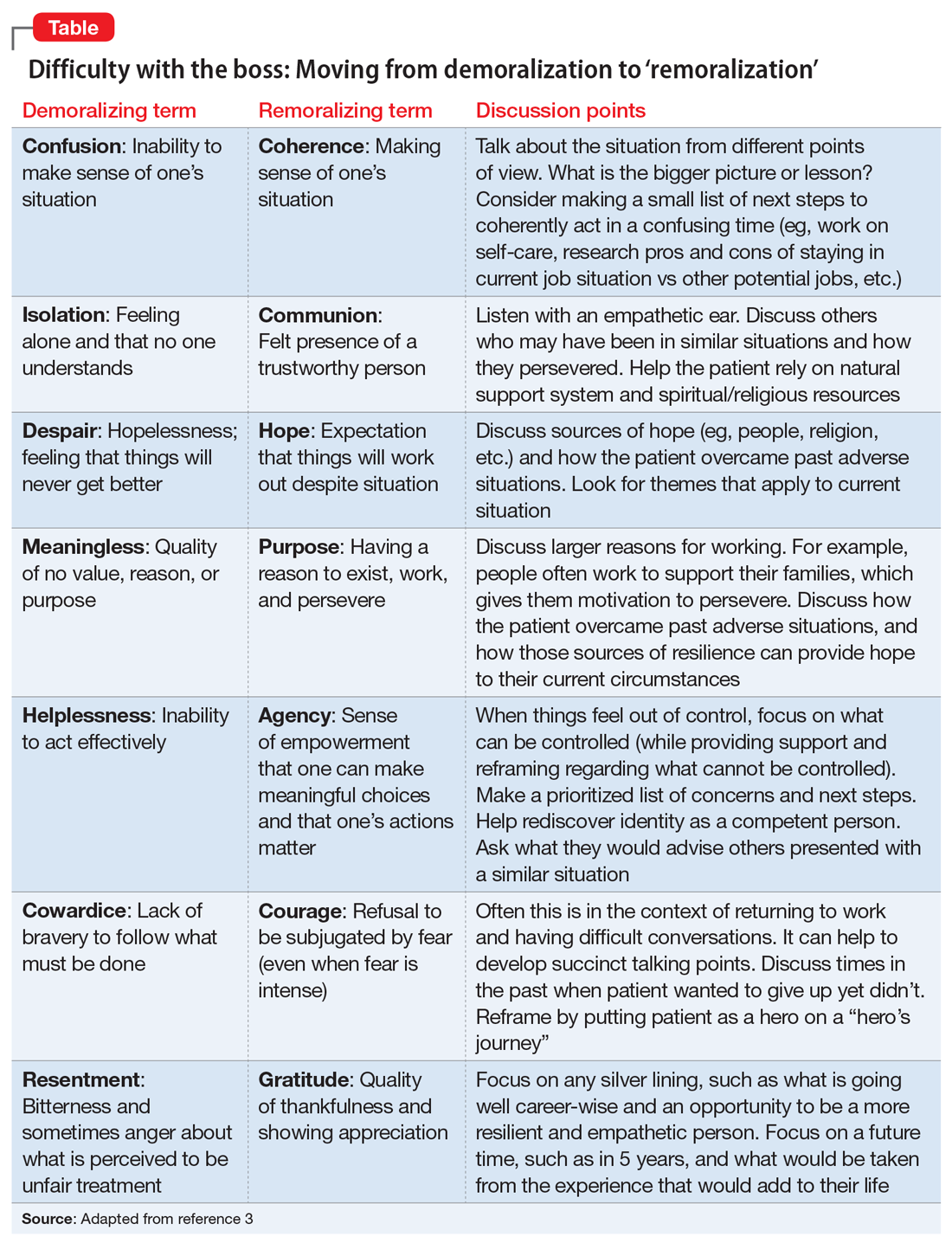
Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”

Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
Ms. D is a 48-year-old administrative assistant and married mother of 2 teenagers with a history of adjustment disorder with mixed anxiety and depressed mood. She presents with increasing anxiety, poor sleep, irritability, and occasional feelings of hopelessness in the context of feeling stuck in a “dead-end job.” She describes her main issue as having an uncaring boss with unrealistic expectations. Clearly exasperated, she tells you, “If only I could get rid of my boss, everything would be just fine.”
Ms. D’s situation is common. When confronted with overbearing and demanding supervisors, the natural inclination for some employees is to flee. Symptoms of burnout (eg, emotional exhaustion, depersonalization, and decreased personal accomplishment) often occur, sometimes with more serious symptoms of adjustment disorder or even major depressive disorder or generalized anxiety disorder. To help patients such as Ms. D who are experiencing difficulties with their boss, you can use a simple approach aimed at helping them make the decision to stay at the job or leave for other opportunities, while supporting them along the way.
Clarify, then support and explore
A critical addition to the typical evaluation is a full social history, including prior employment and formative relationships, that may inform current workplace dynamics. Does the patient have a pattern of similar circumstances, or is this unusual for her? How does she view the supervisor-employee relationship, and how do power differentials, potential job loss, and subsequent financial impacts further amplify emotional friction?
Once the dynamics are clarified, support and validate her emotional reaction before exploring potential cognitive distortions and her own contributions to the relationship dysfunction. If her tendency is to lash out in anger, she could fan the flames and risk being fired. If her tendency is to cower or freeze, you can help to gradually empower her. Regardless of relationship dynamics, be careful not to medicalize what may simply be a difficult situation.1 Perhaps she is a perfectionist and minimizes her supervisor’s behaviors that affirm her work and value as a person. In such cases, you can use cognitive-behavioral therapy techniques to help her consider different points of view and nuance. Rarely are people all good or all bad.
Perhaps her perceptions are accurate, and her boss really is a jerk. If this is the case, she likely feels unfairly and helplessly persecuted. She may be suffering from demoralization, or feelings of impotence, isolation, and despair in which her self-esteem is damaged and she feels rejected because of her failure to meet her boss’s and her own expectations.2 In cases of demoralization, oddly enough, hospice literature lends some tools to help her. The Table3 provides some common terms associated with demoralization and discussion points you can use to help her move toward “remoralization.”

Regardless of the full story, it’s common for people to externalize uncomfortable emotions and attribute symptoms to an external cause. Help her develop self-efficacy by realizing she is in control of how she responds to her emotions. Have her focus on her role in the relationship with her supervisor, looking for common ground and brainstorming practical solutions. Ultimately you can help her realize that she always has choices about whether to stay at the job or look for work elsewhere. Your role is to support her regardless of her decision.
CASE CONTINUED
Over several visits, Ms. D begins to view the relationship with her supervisor in a different light. She has a conversation with him about what she needs to do personally and what she needs professionally from him to be successful at work. Her supervisor acknowledges he has been demanding and could be more supportive. Together they vow to communicate more clearly and regularly assess progress, including celebrating clear victories. Ms. D ultimately decides to stay at the job, and her symptoms resolve without a “bossectomy.”
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
1. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125-e131.
2. Frank JD. Psychotherapy: the restoration of morale. Am J Psychiatry. 1974;131(3):271-274.
3. Griffith JL, Gaby L. Brief psychotherapy at the bedside: countering demoralization from medical illness. Psychosomatics. 2005;46(2):109-116.
Challenges in Treating Patients with Tardive Dyskinesia
In this supplement to Current Psychiatry, Greg Mattingly, MD and Manish K. Jha, MBBS review dilemmas and treatment options for patients with tardive dyskinesia.
Click here to read the supplement and earn free CME/CE credits.
In this supplement to Current Psychiatry, Greg Mattingly, MD and Manish K. Jha, MBBS review dilemmas and treatment options for patients with tardive dyskinesia.
Click here to read the supplement and earn free CME/CE credits.
In this supplement to Current Psychiatry, Greg Mattingly, MD and Manish K. Jha, MBBS review dilemmas and treatment options for patients with tardive dyskinesia.
Click here to read the supplement and earn free CME/CE credits.
Medical transition in transgender patients
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Medical transition in transgender patients
I just read the article “Writing letters for transgender patients undergoing medical transition” by Dr. Amy Riese (Pearls,
I would like to use her piece as an opportunity to highlight what has become a chasm in psychiatric care. Dr. Riese’s article was on the letter itself and not the assessment of a patient with possible gender dysphoria, but assessment is barely mentioned, and is the single most important part of a gender transition process. Assessment has become the huge chasm in treatment. In my community, both personally and professionally, I have witnessed very little assessment taking place, yet a lot of transitioning is happening.
Concerned and caring family members take their child (or self-present if the patient is an adult) to gender specialists for their expertise. What is happening during these evaluations are brief conversations during which the gender specialist accepts a patient’s (sometimes a minor’s) self-diagnosis of gender dysphoria. There is discussion of the importance of being gender-affirming, and the beginning of a discussion of hormone therapy. During these discussions of hormone therapy, there is very little disclosure of some of the untoward effects. I understand this is a generalization, and there are some gender specialists who are doing excellent, thorough assessments. But this is what I am seeing in my community, to the point that I have no local specialists to whom I feel comfortable sending my patients who may have gender dysphoria.
During discussions, some of the significant medical outcomes of hormone therapy (immunosuppression, loss of bone density, sterility, increased risk of certain types of cancer, etc.) are not mentioned, or are mentioned in passing. Clinicians have begun using euphemisms such as “top surgery” or “upper body surgery,” as used in Dr. Riese’s article, rather than the medically accurate term, which is “bilateral mastectomy.” These behaviors are being manifested by mostly well-meaning clinicians, and start the process of ushering a patient down a one-way street toward a medical transition.
In April of this year, a prestigious institution in my state did a training on aspects of treating transgender and nonbinary youth. The training advocated giving less information to transgender youth regarding the effects of treatment on fertility, arguing that giving adequate information would disrupt the normal course of development. However, we are allowing these same youth to consent for treatment.
This is a very destructive phenomenon, and only time will tell what the psychiatric outcomes will be for patients who medically transition who did not have an adequate assessment. After so much loss under the auspices of treatment, one would hope that at the very least, these children and young adults would be in a better place psychologically, that they would finally be happy and fulfilled in their new reality, that their mental anguish would evaporate, and with it, their risk of suicide. And this may be true if the patient had gender dysphoria.
But what about the patients who did not have an adequate assessment, whose self-diagnosis was accepted without question, the gender-affirming model immediately implemented, and referrals quickly made for medical treatment? For those patients, once everything has been done, every hormone taken, every surgery performed, but still not male enough, not convincingly male in every aspect, now what? Where does one go from there?
Only time will tell what the psychiatric outcomes will be for these patients, who are primarily youth and young adults at this point. What about the psychological pain that brought them to identify as transgender in the first place? Since the patient was colluded with in the diagnosis of gender dysphoria, that pain was never identified and addressed. What will the suicide rate be of these fully transitioned patients who never had gender dysphoria?
And what shall become of the clinicians who treated them without pause or careful consideration, who bypassed informed consent, treating teens as if they had the judgment and psychological maturity of an adult? What will be their defense when the malpractice lawsuits begin to mount against them, when patients and their families emerge on the other side of the medical transition to find that life, identity, intimacy, and the most basic biological functions have been altered forever based on the capricious and suggestible whims of children?
According to the DSM-5, the prevalence of gender dysphoria is very low. Even if we were to double the DSM-5 estimate, it is still very low. As psychiatrists, we are leaders in the mental health field, and need to set the tone and guide nonphysician clinicians toward extremely careful assessment of these patients.
While Dr. Riese gives excellent information about how to write a letter for a patient who needs transition, far fewer of these letters should be written. The upward trend in the numbers of patients receiving a diagnosis, and subsequently letters, is largely imposed by clinicians who disregard the DSM-5 and fail to apply critical thought to this assessments.
Service animals and emotional support animals: Should you write that letter?
For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf