User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
How do digital technologies affect young people’s mental health?
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
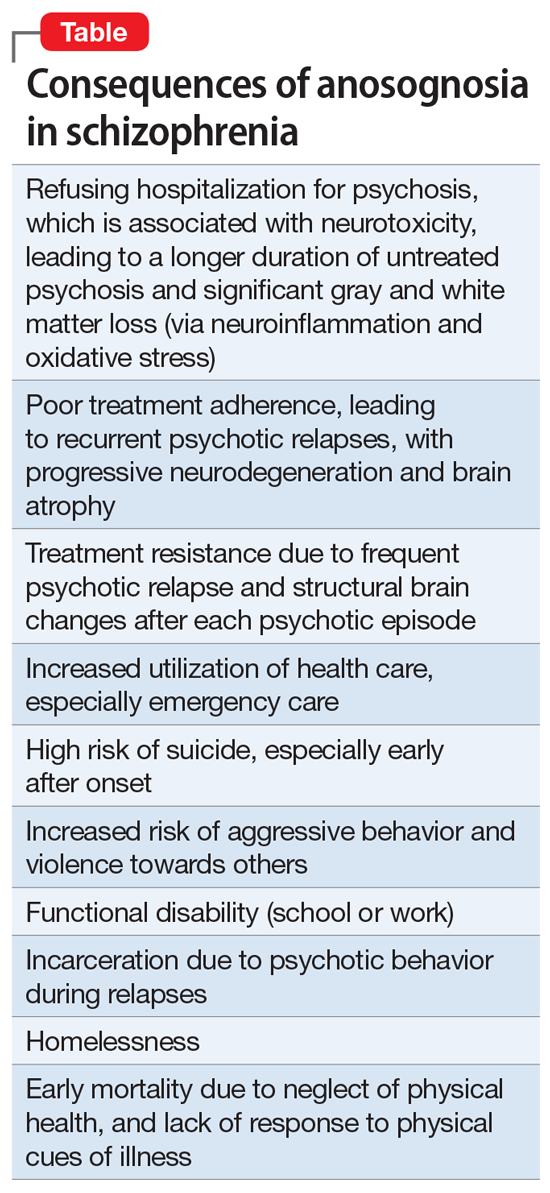
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Pediatric insomnia: Assessment and diagnosis
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
FIRST OF 2 PARTS
A thorough evaluation can identify modifiable factors and guide treatment
Sleep problems are common among children and adolescents,1 with prevalence rates of 25% to 40%.2-4 Young children most commonly exhibit what is referred to as bedtime problems and night wakenings, whereas children in middle childhood (age 4 to 12) through adolescence (age 13 to 17) report insomnia. For many children, these problems persist.3 Insufficient sleep in children and adolescents worsens inattention, daytime fatigue, and cognitive and behavioral deficits.5 Assessment and treatment of sleep problems in children and adolescents is critical because poor sleep among youth increases the risk for depression, self-harm, and suicide,6,7 increases family stress, and decreases parental well-being.1
This 2-part article describes the assessment, diagnosis, and treatment of sleep problems among children and adolescents. In part 1, we focus on:
- sleep architecture (circadian rhythms, stages of sleep)
- sleep in healthy youth (age 6 to 17) and those with attention-deficit/hyperactivity disorder (ADHD), depressive disorders, and anxiety
- how to assess sleep, and the differential diagnosis of behavioral sleep problems in pediatric patients.
In Part 2, we will cover psychotherapeutic and psychopharmacologic interventions for youth with insomnia, and describe an effective approach to consultation with pediatric sleep medicine specialists.
How much sleep do children and adolescents need?
Throughout their development, children spend 40% to 50% of their time asleep. Sleep schedules are based on circadian rhythms, which are physical, mental, and behavioral changes that follow an approximately 24-hour cycle. Human circadian rhythm varies between 24 and 25 hours and is vital in determining our sleep patterns. Exposure to sunlight drives our circadian rhythm, sending signals to our bodies to “turn on” melatonin production at night (ie, 9
Box
Sleep architecture consists of 3 states: wake; non-rapid eye movement (NREM) sleep; and rapid eye movement (REM) sleep (“dreaming” sleep).2 These stages have distinct polysomnographic features of electroencephalographic EEG patterns, eye movements, and muscle tone.2 NREM sleep can be further divided into 3 stages: stage 1 (N1), stage 2 (N2), and stage 3 (N3). Stage 1 is the lightest stage and lasts for 30 seconds to 5 minutes; it is easy to wake up from stage 1 sleep. During stage 2 sleep, the body moves into a deeper sleep stage that is considered “true” sleep. This sleep stage is characterized by bursts of rhythmic rapid EEG activity known as spindles, as well as high-amplitude slow-wave spikes called K complexes.2 Stage 2 sleep lasts for 10 to 45 minutes. Stage 3, better known as “deep sleep,” slow-wave sleep, or delta sleep, is the most restorative sleep.2 Respiration is low and parasympathetic activity is high.2 It is difficult to be awakened during deep sleep, and if aroused, the person likely will feel confused or groggy. Deep sleep is followed by a return to lighter stage of sleep before the first REM sleep period begins.
REM sleep is the active stage of sleep. Breathing and heart rate become irregular, and the body experiences muscle atonia, or temporary paralysis, of arms and legs. When in REM sleep, individuals have the highest brain metabolic rates, and periodic bursts of eye movements.2 Most individuals move through stages of NREM and REM sleep in predicable ways, meaning they experience NREM sleep, return to a lighter stage of sleep after deep sleep, then move into REM sleep before the cycle repeats. It takes approximately 90 minutes for most adults to complete the NREM sleep cycle, and then REM sleep occurs before returning to NREM sleep.
In children, especially in infants and babies, sleep cycles are closer to 50 to 60 minutes. Newborns spend approximately 50% of their sleep in REM sleep, whereas adults spend 20% to 25% of their sleep in REM sleep. Children will spend more time in REM sleep until the third and fourth years of life, at which point REM gradually decreases to 20% to 25% by adulthood.
Sleep needs also change predictably throughout the lifespan. The National Sleep Foundation guidelines for sleep duration provide clinicians and parents with a range of recommended sleep for each stage of development. Infants require 14 to 17 hours of sleep, whereas adolescents need 8 to 10 hours by age 14 to 17.8 The key for clinicians is to determine if the child is within the recommended range, and how they are functioning on the number of hours of sleep they report. This allows for variation in how much sleep an individual child might need while acknowledging that some children within a specific age group might need more or less sleep than other children of the same age.
Sleep in healthy youth: Middle childhood
School-age children (age 6 to 12) typically need 9 to 10 hours of sleep over a 24-hour period.2 This developmental period is especially important for children to develop healthy sleep habits; however, developmentally appropriate cognitive and social/emotional factors might interfere with the quality and quantity of sleep. Middle childhood is a time when children can understand the dangers of the outside world (ie, violence, health problems) and resulting anxiety can disrupt sleep. Parents usually are less involved in bedtime as children approach adolescence, which leads to later bedtimes. At this stage, many children begin to take on more serious roles in their academics and extracurricular activities, peer relationships become more important, and use of electronics (eg, television, video games, internet, and handheld devices) increases—all of which compete with sleep.9 Frequent sleep issues during middle childhood include:
- irregular sleep-wake schedules
- later bedtimes
- decreased nighttime sleep
- increased caffeine intake
- reduced parental presence at bedtime
- daytime sleepiness.3
In school-age children, regular napping, falling asleep during short car rides, and daytime fatigue at school or home are cause for concern. When these symptoms are present, an evaluation is warranted.
Sleep in healthy youth: Adolescence
The National Sleep Foundation recommends adolescents obtain 8 to 10 hours of sleep per night; for some adolescents, as much as 11 hours of sleep per night might be appropriate.8 However, this contrasts with findings from the National Sleep Foundation’s Sleep in America Poll, which revealed that 75% of 12th graders report <8 hours of sleep nightly.10 Many adolescents experience delayed sleep phase syndrome or delayed sleep-wake phase disorder, which involves a persistent phase shift of >2 hours in the sleep-wake schedule that conflicts with the adolescent’s school, work, or lifestyle demands.11 Such circadian rhythm disorders typically result from a poor match between the sleep-wake schedule and the demands of the adolescent’s life, or a failure to synchronize their internal clock with a 24-hour circadian clock.12 Children typically become tired after sunset, but puberty is associated with reduced slow-wave sleep and changes in circadian rhythms. As a result, a 3-hour delay (delayed phase preference) is common among adolescents. At approximately age 20, people start to become tired after sunset and awaken earlier in the morning—a pattern driven by sunlight and the timing of melatonin release that will remain stable until the sixth decade of life.
Continue to: Effects of chronic sleep deprivation...
Effects of chronic sleep deprivation
Most older studies of sleep loss examined the impact of total sleep loss (sleep deprivation) rather than the effect of partial sleep loss or sleep restriction, a more commonly experienced phenomenon. More recent research shows that a cumulative sleep deficit could cause the body to override voluntary wakefulness and a sleep-deprived individual can experience brief “microsleeps” where they are unaware and lose attention/wakefulness for several seconds.2 This can be deadly if a sleep-deprived adolescent experiences microsleeps while driving.13
There is a well-studied correlation between chronic sleep deprivation and increased body mass index in children.14 This might be caused by reduction in physical activity as well as alterations in the “hunger hormones”—ghrelin and leptin—that have been observed with sleep deprivation.15-17 Other studies have noted decreased glucose tolerance, reduced insulin sensitivity, and catecholamine and cortisol secretion abnormalities, which place children at higher risk for metabolic syndrome and hypertension.13,18 Sleep deprivation also is associated with mood and anxiety disorders and is an independent risk factor for substance use and suicidal ideation among adolescents.19 Sleep deprivation increases impairments in impulse control, concentration, and attention, which could be especially problematic in school-age children.
How sleep is assessed
The sleep history is the first step in evaluating a child or adolescent for a sleep disorder. The sleep history includes exploring the chief complaint, sleep patterns and schedules, bedtime routines, and nocturnal and daytime behaviors (Table).

Chief complaint
Behavioral sleep specialists will assess the primary problem with everyone involved in the child’s bedtime.20 This might include parents (custodial and noncustodial), grandparents, or stepparents as well as the child/adolescent. This important step can reveal a sleep disorder or an inappropriately early bedtime relative to the child’s development. During this assessment, ask detailed questions about how long the sleep problem has persisted, the frequency of sleep problems, and any precipitating stressors. Parents and caregivers can review strategies they have tried, and for how long and to what extent interventions were implemented consistently to result in change.
Sleep patterns and schedules
Review the child/adolescent’s typical sleep patterns and behaviors. Ask parents and caregivers, as well as the patient, about general sleep schedules for the past few weeks or a typical 2-week time period.2 A behavioral assessment of sleep should include asking families about how the child/adolescent sleeps during the week and over the weekend, and if school-year sleep differs from summer or holiday sleep schedules. These questions can illuminate how long a sleep problem has been occurring and what sleep habits might be contributing to the problem. Bedtime
Determine if there is a set bedtime or if the child goes to bed when they wish. It is important to ascertain if the bedtime is age-appropriate, if weekday and weekend bedtimes differ, and to what extent extracurricular activities or school demands impact bedtime. Assess the consistency of the bedtime, the nature of bedtime routines (eg, is the child engaging in stimulating activities before bed), where the bedtime routine occurs (eg, sibling’s room, parents’ room, child’s room), and what role (if any) electronic devices play.2
Nocturnal behaviors
Assessment should include a series of questions and age-specific questionnaires to focus on what behaviors occur at night, including awakenings. Parents should be asked how frequent night awakenings occur, how long arousals last, and how the child signals for the parent (eg, calling out, climbing into parents’ bed).2 Additionally, ask how parents respond and what is required to help the child fall back asleep (eg, rocking, soothing, feeding). The presence of nightmares, night terrors, parasomnias, and sleep-related breathing disorders also must be assessed.20
Daytime behaviors
A sleep history should include assessment of daytime functioning, including daytime sleepiness, fatigue, morning waking, and functioning during school, extracurriculars, and homework. For children and teens, falling asleep in the car, while in school, or during passive activities (meals, conversation) suggests insufficient sleep, sleep disruption, or excessive daytime sleepiness.2
Continue to: Sleep disruption in youth with psychiatric disorders...
Sleep disruption in youth with psychiatric disorders
Disordered sleep is common across psychiatric disorders. The National Comorbidity Survey Adolescent Supplement—a nationally representative cross-sectional survey of adolescents (N = 10,123)—found that a later weeknight bedtime, shorter weeknight sleep duration, and greater weekend bedtime delay increased the risk of developing a mood, anxiety, or substance use (including nicotine) disorder, and suicidality. These risk factors also were associated with lower “perceived mental and physical health.”21 Clinicians should routinely obtain a sleep history in children and adolescents with these disorders. Consider using the sleep screening tool BEARS:
- Bedtime issues
- Excessive daytime sleepiness
- Awakenings
- Regularity and duration of sleep
- Snoring.
ADHD
Up to one-half of children and adolescents with ADHD experience sleep problems,22,23 including delayed sleep onset, bedtime resistance, daytime fatigue, and feeling groggy in the morning beyond what is typical (>20 minutes). Pharmacotherapy for ADHD contributes to sleep disturbances24,25 while sleep deprivation exacerbates inattention and hyperactivity. In youth with ADHD, restless leg syndrome, periodic limb movement disorder, and sleep-disordered breathing disorder are more common than in the general population.
Depressive disorders
Up to three-quarters of depressed children and 90% of depressed adolescents report sleep disturbances, including initial, middle, and terminal insomnia as well as hypersomnia.26 Disrupted sleep in pediatric patients with major depressive disorder could be moderated by the patient’s age, with depressive symptoms more common among adolescents (age 12 to 17) than among younger children (age 6 to 11).27 Successful treatment of depression fails to relieve dyssomnia in 10% of children. Sleep problems that persist after successfully treating a depressive episode could increase the risk of another depressive episode.28
Anxiety disorders
Sleep problems are common among children and adolescents with anxiety disorders.29 Longitudinal data from >900 children found that symptoms of sleep disturbance in early childhood were correlated with experiencing an anxiety disorder 20 years later.30 Fears related to the dark or monsters under the bed that are developmentally appropriate for younger children may interfere with sleep. However, in anxious children, fears might also be related to separation, sleeping alone, worry about the loss of a loved one, concerns about personal safety, fear of frightening dreams, or concerns about academics and social relationships. Anxious individuals ruminate about their worries, and this might be especially true for children at bedtime, when there are limited distractions from ruminative fears.31 Bedtime resistance, parental involvement in bedtime rituals, and cultural factors related to sleep also could play a role for children with anxiety symptoms and sleep problems.
Having an anxiety disorder is significantly associated with an increased risk of insomnia; however, 73% of the time anxiety symptoms precede an insomnia diagnosis.29 Sleep problems and anxiety symptoms might have a reciprocal influence on one another; tiredness that results from sleep problems could exacerbate anxiety, which further worsens sleep problems.
A bridge to treatment
A thorough assessment can help identify modifiable factors and guide treatment selections. In Part 2 of this article, we will describe healthy sleep practices, cognitive-behavioral therapy for insomnia, when pharmacotherapy might be indicated, and the evidence supporting several medications commonly used to treat pediatric insomnia. We also will discuss factors to consider when seeking consultation with a pediatric behavioral sleep specialist.
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
1. Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932-948. doi:10.1093/jpepsy/jsu041
2. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569. doi:10.1016/j.pcl.2011.03.011
3. Meltzer LJ, Plaufcan MR, Thomas JH, et al. Sleep problems and sleep disorders in pediatric primary care: treatment recommendations, persistence, and health care utilization. J Clin Sleep Med. 2014;10(4):421-426. doi:10.5664/jcsm.3620
4. Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Prim Care. 2008;35(3):569-581, viii. doi:10.1016/j.pop.2008.06.002
5. Williamson AA, Mindell JA, Hiscock H, et al. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J Child Psychol Psychiatry. 2020;61(10):1092-1103. doi:10.1111/jcpp.13303
6. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002; 53(1):561-569. doi:10.1016/s0022-3999(02)00446-4
7. Singareddy R, Krishnamurthy VB, Vgontzas AN, et al. Subjective and objective sleep and self-harm behaviors in young children: a general population study. Psychiatry Res. 2013;209(3):549-553. doi:10.1016/j.psychres.2013.03.036
8. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi:10.1016/j.sleh.2015.10.004
9. Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005-1010. doi:10.1542/peds.2008-3641
10. Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8(4):695-725.
11. Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9(2):114-120. doi:10.1016/j.prrv.2008.01.001
12. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602-612. doi:10.1016/j.sleep.2006.12.002
13. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115(6):1774-1786. doi:10.1542/peds.2005-0772
14. Kaczor M, Skalski M. Prevalence and consequences of insomnia in pediatric population. Psychiatr Pol. 2016;50(3):555-569. doi:10.12740/PP/61226
15. Gomes TN, Dos Santos FK, Santos D, et al. Correlates of sedentary time in children: a multilevel modelling approach. BMC Public Health. 2014;14:890. doi:10.1186/1471-2458-14-890
16. Stone MR, Stevens D, Faulkner GEJ. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56(2):112-117. doi:10.1016/j.ypmed.2012.11.015
17. Hart CN, Fava JL, Subak LL, et al. Time in bed is associated with decreased physical activity and higher BMI in women seeking weight loss treatment. ISRN Obes. 2012;2012:320157. doi:10.5402/2012/320157
18. Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044-1049. doi:10.1073/pnas.0706446105
19. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi:10.1016/j.smrv.2017.06.009
20. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. 3rd ed. Lippincott Williams & Wilkins; 2015.
21. Zhang J, Paksarian D, Lamers F, et al. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137-143. doi:10.1016/j.jpeds.2016.11.007
22. Gregory AM, Agnew-Blais JC, Matthews T, et al. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46(2):284-294. doi:10.1080/15374416.2016.1183499
23. Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: Defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811-828. doi:10.2165/11538990-000000000-00000
24. Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. J Sleep Res. 2010;19(2):366-373. doi:10.1111/j.1365-2869.2009.00795.x
25. Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37(5):395-404. doi:10.1097/DBP.0000000000000285
26. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148(1):66-71. doi:10.1016/j.jad.2012.11.049
27. Emslie GJ, Rush AJ, Weinberg WA, et al. Sleep EEG features of adolescents with major depression. Biol Psychiatry. 1994;36(9):573-581. doi:10.1016/0006-3223(94)90067-1
28. Alfano CA, Zakem AH, Costa NM, et al. Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depress Anxiety. 2009;26(6):503-512. doi:10.1002/da.20443
29. Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):224-232. doi:10.1097/01.chi.0000242233.06011.8e
30. Gregory AM, Caspi A, Eley TC, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157-163. doi: 10.1007/s10802-005-1824-0
31. Chorney DB, Detweiler MF, Morris TL, et al. The interplay of sleep disturbance, anxiety, and depression in children. J Pediatr Psychol. 2008;33(4):339-348. doi:10.1093/jpepsy/jsm105
32. Sadeh A. Stress, trauma, and sleep in children. Child Adolesc Psychiatr Clin N Am. 1996;5(3):685-700. doi:10.1016/S1056-4993(18)30356-0

33. Glod CA, Teicher MH, Hartman CR, et al. Increased nocturnal activity and impaired sleep maintenance in abused children. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1236-1243. doi:10.1097/00004583-199709000-00016
34. Strawn JR, Lu L, Peris TS, et al. Research review: pediatric anxiety disorders: what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114-139. doi:10.1111/jcpp.13262
35. Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi:10.1007/s11920-015-0591-z
36. Hamill Skoch S, Mills JA, Ramsey L, et al. Letter to editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder— a bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387-388. doi:10.1089/cap.2020.0169
Using measurement-based care to improve outcomes for patients with depression
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.
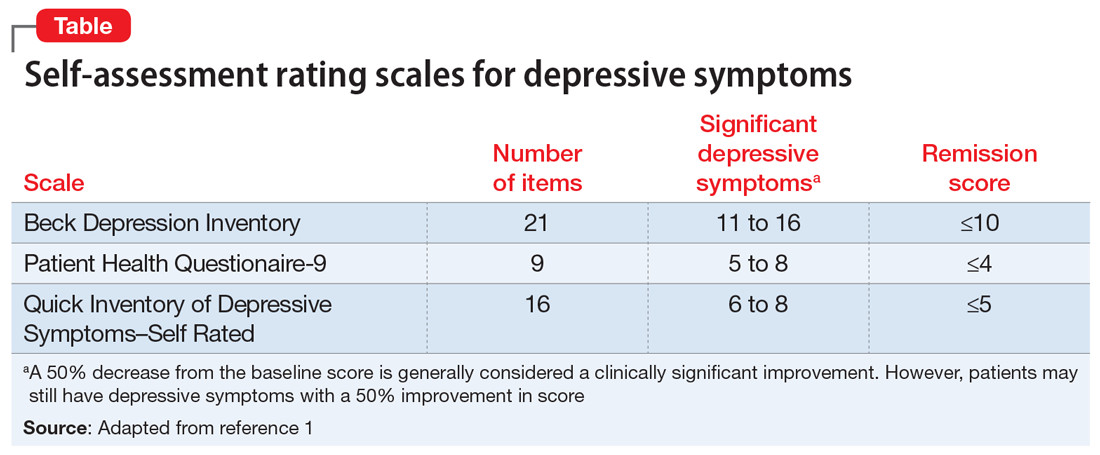
The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
Ms. H, age 42, is being treated by her family physician for her second episode of major depressive disorder (MDD). When she was 35, Ms. H experienced her first episode of MDD, which was successfully treated with
At the 8-week follow-up appointment, the physician notes how much better Ms. H seems to be doing. He says that because she has had such a good response, she should continue the fluoxetine and come back in 3 months. Later that evening, Ms. H reflects on her visit. Although she feels better, she still does not feel normal. In fact, she is not sure that she has really felt normal since before her first depressive episode. Ms. H decides to see a psychiatrist.
At her first appointment, the psychiatrist asks Ms. H to complete the Quick Inventory of Depressive Symptoms–Self Rated (QIDS-SR) scale. Her QIDS-SR score is 6, which is consistent with mild residual symptoms of depression.1 The psychiatrist increases the fluoxetine dosage to 40 mg/d and recommends that she complete a course of cognitive-behavioral therapy (CBT).
Although psychiatry currently does not have tests that provide continuous data such as blood pressure or HbA1c, well-validated rating scales can help clinicians in getting their patients to achieve symptom remission. Measurement-based care is the “systematic use of measurement tools to monitor progress and guide treatment choices.”1 Originally, psychometric rating scales were designed for research; typically, they were administered by the clinician, and were too long to be used in routine outpatient clinical practice. Subsequently, it was determined that patients without psychotic symptoms or cognitive deficits can accurately assess their own symptoms, and this led to the development of short self-assessment scales that have a high level of reliability when compared with longer, clinician-administered instruments. Despite the availability of several validated, brief rating scales, it is estimated that only approximately 18% of psychiatrists use them in clinical practice.2
Self-rated scales for depression have been shown to be as valid as clinician-rated scales. For depression, the Patient Health Questionaire-9 (PHQ-9), based on the 9 symptom criteria associated with a diagnosis of MDD, is likely the most commonly used self-assessment scale.1 However, the QIDS-SR and the Beck Depression Inventory are both well-validated.1 In particular, QIDS-SR scores and score changes have been shown to be comparable with those on the QIDS-Clinician Rating (QIDS-C) scale.3 A 50% decrease in score typically is defined as a clinical response. Remission of symptoms is often defined as a score ≤4 on the PHQ-9 or ≤5 on the QIDS-SR (Table1). Similar to laboratory tests, rating scales are not diagnostic, but are a piece of information for the clinician to use in making diagnostic and treatment decisions.

The use of brief rating scales can help identify symptoms that may not come up in discussion with the patient, and it provides a systematic method of reviewing symptoms. Patients may be encouraged when they see a decrease in their scores after beginning treatment.2 Patients with depression need to complete rating scales frequently, just as a patient with hypertension would need their blood pressure frequently monitored.2 Frequent measurement with rating scales may help identify residual depressive symptoms that indicate the need for additional intervention. Residual depressive symptoms are the best predictor of the recurrence of depression, and treatment to remission is essential in preventing recurrence. In fact, recurrence is 2 to 3 times more likely in patients who do not achieve remission.1
Continue to: Optimizing the use of self-rating scales...
Optimizing the use of self-rating scales
To save time, patients can complete a rating scale before seeing the clinician, and the use of computerized applications can automatically sum scores and plot response graphs.4 Some researchers have suggested that some patients may be more honest in completing a self-assessment than in their verbal responses to the clinician.4 It is important to discuss the rating scale results with the patient.2 With a newly diagnosed patient, goals for treatment and the treatment plan can be outlined. During follow-up visits, clinicians should note areas of improvement and provide encouragement. If the patient’s symptoms are not improving appropriately, the clinician should discuss treatment options and offer the patient hope. This may improve the patient’s engagement in care and their understanding of how symptoms are associated with their illness.2 Studies have suggested that the use of validated rating tools (along with other interventions) can result in faster improvement in symptoms and higher response rates, and can assist in achieving remission.1,2,5
CASE CONTINUED
After 6 weeks of CBT and the increased fluoxetine dose, Ms. H returns to her psychiatrist for a follow-up visit. Her QIDS-SR score is 4, which is down from her initial score of 6. Ms. H is elated when she sees that her symptoms score has decreased since the previous visit. To confirm this finding, the psychiatrist completes the QIDS-C, and records a score of 3. The psychiatrist discusses the appropriate continuation of fluoxetine and CBT.
In this case, the use of a brief clinical rating scale helped Ms. H’s psychiatrist identify residual depressive symptoms and modify treatment so that she achieved remission. Using patient-reported outcomes also helps facilitate meaningful conversations between the patient and clinician and helps identify symptoms suggestive of relapse.2 Although this case focused on the use of measurement-based care in depression, brief symptom rating scales for most major psychiatric disorders—many of them self-assessments—also are available, as are brief rating scales to assess medication adverse effects and adherence.5
Just as clinicians in other areas of medicine use assessments such as laboratory tests and blood pressure monitoring for initial assessment and in following response to treatment, measurement-based care allows for a quasi-objective evaluation of patients with psychiatric disorders. Improved response rates, time to response, and patient engagement are all positive results of measurement-based care
Related Resources
- Martin-Cook K, Palmer L, Thornton L, et al. Setting measurement-based care in motion: practical lessons in the implementation and integration of measurement-based care in psychiatry clinical practice. Neuropsychiatric Disease & Treatment. 2021;17:1621-1631.
- Aboraya A, Nasrallah HA, Elswick DE, et al. Measurementbased care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Drug Brand Names
Fluoxetine • Prozac
- Self-rated scales are believed to be as reliable as clinician-rated scales in assessing symptoms in patients who are not cognitively impaired.
- The use of rating scales can enhance engagement of the patient with the clinician.
- Utilizing computer- or smartphone appbased rating scales allows for automatic scoring and graphing.
- The use of rating scales in the pharmacotherapy of depression has been associated with more rapid symptoms improvement, greater response rates, and a greater likelihood of achieving remission.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70(suppl 6):26-31. doi:10.4088/ JCP.8133su1c.04
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324-335.
- Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82.
- Trivedi MH, Papakostas GI, Jackson WC, et al. Implementing measurement-based care to determine and treat inadequate response. J Clin Psychiatry 2020;81(3):OT19037BR1. doi: 10.4088/JCP.OT19037BR1
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446-458.
Let’s talk about ‘chemsex’: Sexualized drug use among men who have sex with men
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).
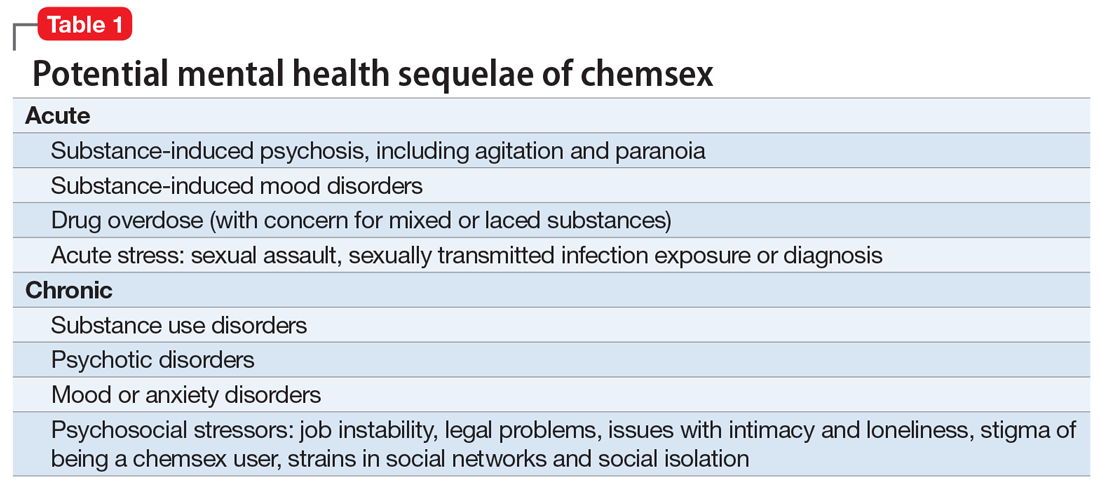
What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Comments & Controversies
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
The perils of hubris
Dr. Nasrallah’s fascinating editorial on the psychiatric aspects of prominent individuals’ fall from grace (“From famous to infamous: Psychiatric aspects of the fall from grace,” From the Editor,
Perhaps fittingly, the phenomenon of self-destruction as a byproduct of success was most prominently “diagnosed” by business school professors, not physicians. The propensity for ethical failure at the apex of achievement was coined the “Bathsheba Syndrome,” in reference to the biblical tale of King David’s degenerative sequence of temptation, infidelity, deceit, and treachery while at the height of his power.2 David’s transgressions are enabled by the very success he has achieved.3
One of my valued mentors had an interesting, albeit unscientific, method of mitigating hubris. When he was a senior military lawyer, or judge advocate (JAG), and I was a junior one, my mentor took me to a briefing in which he provided a legal overview to newly minted colonels assuming command billets. One of the functions of JAGs is to provide counsel and advice to commanders. As Dr. Nasrallah noted in his editorial, military leaders are by no means immune from the proverbial fall from grace, and arguably particularly susceptible to it. In beginning his remarks, my mentor offered his heartfelt congratulations to the attendees on their promotion and then proceeded to hand out a pocket mirror for them to pass around. He asked each officer to look in the mirror and personally confirm for him that they were just as unattractive today as they were yesterday.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
The views expressed in this letter are those of the author and do not necessarily reflect those of any government agency.
1. Wolfe T. Bonfire of the vanities. Farrar, Straus and Giroux; 1987.
2. Ludwig DC, Longenecker CO. The Bathsheba syndrome: the ethical failure of successful leaders. J Bus Ethics. 1993;12:265-273.
3. 2 Samuel 11-12.
I enjoyed Dr. Nasrallah’s editorial and his discussion of the dangers of hubris. This brought to mind the role of the auriga in ancient Rome: "the auriga was a slave with gladiator status, whose duty it was to drive a biga, the light vehicle powered by two horses, to transport some important Romans, mainly duces (military commanders). An auriga was a sort of “chauffeur” for important men and was carefully selected from among trustworthy slaves only. It has been supposed also that this name was given to the slave who held a laurel crown, during Roman Triumphs, over the head of the dux, standing at his back but continuously whispering in his ears “Memento Mori” (“remember you are mortal”) to prevent the celebrated commander from losing his sense of proportion in the excesses of the celebrations.”1
Continue to: Mark S. Komrad, MD...
Mark S. Komrad, MD
Faculty of Psychiatry
Johns Hopkins Hospital
University of Maryland
Tulane University
Towson, Maryland
Reference
1. Auriga (slave). Accessed November 9, 2021. https://en.wikipedia.org/wiki/Auriga_(slave)
Barriers to care faced by African American patients
According to the US Department of Health and Human Services, the 5 domains of social determinants of health are Economic Stability, Education Access and Quality, Health Care Access and Quality, Neighborhood and Built Environment, and Social and Community Context.1 Patients who are African American face many socioeconomic barriers to access to psychiatric care, including economic inequality, inadequate knowledge about mental health, and deficient social environments. These barriers have a significant impact on the accessibility of psychiatric health care within this community, and they need to be addressed.
Jegede et al2 discussed how financial woes and insecurity within the African American community contribute to health care inequalities and adverse health outcomes. According to the US Census Bureau,in 2020, compared to other ethnic groups, African American individuals had the lowest median income.3 Alang4 discussed how the stigma of mental health was a barrier among younger, college-educated individuals who are African American, and that those with higher education were more likely to minimize and report low treatment effectiveness. As clinicians, we often fail to discuss the effects the perceived social and cultural stigma of being diagnosed with a substance use or mental health disorder has on seeking care, treatment, and therapy by African American patients. The stigma of being judged by family members or the community and being seen as “weak” for seeking treatment has a detrimental impact on access to psychiatric care.2 It is our duty as clinicians to understand these kinds of stigmas and seek ways to mitigate them within this community.
Also, we must not underestimate the importance of patients having access to transportation to treatment. We know that social support is integral to treatment, recovery, and relapse prevention. Chronic cycles of treatment and relapse can occur due to inadequate social support. Having access to a reliable driver—especially one who is a family member or member of the community—can be vital to establishing social support. Jegede et al2 found that access to adequate transportation has proven therapeutic benefits and lessens the risk of relapse with decreased exposure to risky environments. We need to devise solutions to help patients find adequate and reliable transportation.
Clinicians should be culturally mindful and aware of the barriers to psychiatric care faced by patients who are African American. They should understand the importance of removing these barriers, and work to improve this population’s access to psychiatric care. Though this may be a daunting task that requires considerable time and resources, as health care providers, we can start the process by communicating and working with local politicians and community leaders. By working together, we can develop a plan to combat these socioeconomic barriers and provide access to psychiatric care within the African American community.
Craig Perry, MD
Elohor Otite, MD
Stacy Doumas, MD
Jersey Shore University Medical Center
Neptune, New Jersey
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
- Healthy People 2030, US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed November 9, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
2. Jegede O, Muvvala S, Katehis E, et al. Perceived barriers to access care, anticipated discrimination and structural vulnerability among African Americans with substance use disorders. Int J Soc Psychiatry. 2021;67(2):136-143.
3. Shrider EA, Kollar M, Chen F, et al. US Census Bureau, Current Population Reports, P60-273, Income and Poverty in the United States: 2020. US Government Publishing Office; 2021.
Lithium: An underutilized element
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.
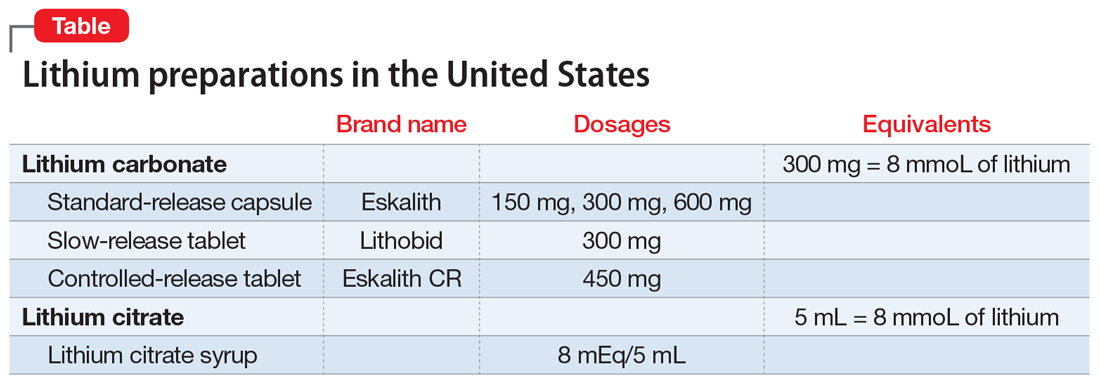
Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
In clinicians and patients alike, lithium triggers reactions ranging from apprehension and fear about adverse effects and toxicity to confusion over lithium’s usefulness compared with other mood stabilizers that do not require blood monitoring. Research from the 1950s to the 1970s demonstrated that lithium is effective for prophylaxis of mood episodes in patients with bipolar disorder and could reduce the frequency of hospitalization in patients who are depressed.1 For years, lithium was commonly prescribed to treat bipolar disorder, but in recent years its use has fallen out of favor due to concerns about its risks, and the availability of newer medications. This article reviews lithium’s origins (Box1-4), pharmacology, risks, and benefits, and makes a case for why it should remain a first-line therapy for bipolar disorder.
Box
Lithium was initially used in the 1840s to treat gout. William Hammond became the first physician to prescribe lithium bromide for acute mania in 1871, and in 1894, Danish psychiatrist Frederik Lange first used lithium carbonate to treat “melancholic depression.”1 In the 20th century, lithium-containing products were used to treat rheumatologic conditions such as renal calculi and other uric acid diatheses.
Lithium experienced a revival in 1949 when John Cade expanded upon Archibald Garrod’s theory regarding uric acid and gout. As a physician during WWII, Cade observed manic and depressive behaviors among prisoners.2 Theorizing that this was caused by either an excess or lack of a metabolite, he injected urine from patients with mania, depression, and schizophrenia and from healthy individuals into guinea pigs.3 Animals who received urine from patients with mania died faster than those injected with urine from a patient with schizophrenia.2 Concluding that urea was the culprit, Cade substituted the relatively water insoluble uric acid for “the most soluble of urates,” which was lithium urate.2,3 Rather than succumbing to a quicker death, guinea pigs injected with lithium urate became placid, tranquilized, lost their natural timidity, and generally did not respond to stimulation.3
Cade administered lithium carbonate and lithium citrate to himself and, because he did not experience any unwanted effects, began testing the medication on patients. Cade’s landmark 1949 paper4 notes improvement in all 10 patients with mania but little change in 6 patients with schizophrenia and 3 with chronic depression.2
In the United States, interest in lithium did not begin until the 1960s, when Samuel Gershon introduced the medication to a psychiatric hospital in Michigan. Financed by the National Institute of Mental Health, this program bought bulk lithium from a chemical supply store, and a local pharmacy formed it into capsules. Analysis of 4 controlled studies from 1963 to 1971 showed an average response rate to lithium of 78% in 116 patients with mania.1
By the end of the 1960s, many psychiatrists were prescribing lithium. At that time, lithium was not FDA-approved, but it could be prescribed as an investigational new drug by obtaining a special permit. In 1970, the FDA approved lithium for acute mania, and for prophylaxis of mania in 1975. Lithium has not yet been approved for prophylaxis of depression, despite substantial evidence indicating efficacy.1
How lithium works
Lithium has effects on neurotransmitters implicated in mania, such as glutamate, dopamine, and gamma-aminobutyric acid.5 Quiroz et al6 provide a detailed description of lithium’s effects, which can be summarized as modulating neuronal signaling pathways, including B-cell lymphoma 2 (BCL2), cAMP-response element binding protein (CREB), and glycogen synthase kinase-3 (GSK-3). Through these signaling cascades, lithium can curtail progression of neuronal apoptosis caused by the biochemical stress commonly seen in bipolar disorder pathogenesis.6
A wide range of potential adverse effects
Lithium can cause adverse effects in several organ systems. Clinicians must be aware of these effects before prescribing lithium or continuing long-term use. The most commonly documented adverse effects and symptoms of toxicity are:
- tremor
- renal dysfunction, including renal insufficiency and polyuria or polydipsia
- hypothyroidism
- hyperparathyroidism (with subsequent hypercalcemia)
- weight gain
- gastrointestinal (GI) symptoms.
These symptoms tend to occur when lithium serum levels are outside the reference range of 0.6 to 1.2 mEq/L, typically once blood levels reach ≥1.5 mEq/L.7 However, thyroid and renal abnormalities can occur at levels below this value, and might be related to cumulative lithium exposure.7 Adverse effects usually are precipitated by inadequate water intake or inadvertently taking an extra dose. Symptoms of lithium toxicity can be mild, moderate (GI complaints, tremor, weakness, fatigue), or severe (agitation, seizures, autonomic dysregulation, confusion, coma, death).
Lithium adverse effects and toxicity are infrequent. An analysis of 17 years of data in Sweden showed the incidence of moderate to severe lithium intoxication (serum level ≥1.5 mEq/L) was .01 patients per year.8 A recently published US analysis found the prevalence rate of lithium toxicity was 2.2%.9 Results from both groups show that drug interactions were an important cause of increased lithium levels, and specifically that initiating a medication that could interact with lithium was associated with 30-fold higher risk of needing acute care for lithium toxicity.9 Possible drug interactions include nonsteroidal anti-inflammatory drugs, diuretics, and renin-angiotensin-aldosterone system inhibitors.9 Because lithium is eliminated exclusively by the kidneys, impaired or altered renal function can increase the risk of lithium retention, leading to intoxication. Other risk factors include older age, alteration of water-salt homeostasis (fever, diarrhea, vomiting), higher number of treated chronic diseases as measured by Chronic Disease Score (range: 0 to 35; higher scores denotes higher number of treated chronic diseases and increased hospitalization risk), and higher total daily lithium dosage.9
Presentation of lithium intoxication often is mild or nonspecific, and physicians should have a low threshold for checking lithium blood levels.8 Lithium intoxication can be safely managed with volume expansion, forced diuresis, and hemodialysis.
Continue to: Lithium use during pregnancy...
Lithium use during pregnancy
When considering lithium for a woman who is pregnant, it is important to weigh the potential teratogenic risks against the benefit of successful management of the mood disorder. Ebstein’s anomaly (abnormal tricuspid valve leaflets) is the most well-known teratogenic risk associated with lithium, with an estimated absolute risk of 1 in 1,000 in patients treated with lithium compared with 1 in 20,000 in controls.10,11 The risk of congenital anomalies is increased in infants exposed to lithium in utero (4% to 12% vs 2% to 4% in controls)12; exposure during the first trimester of pregnancy is associated with increased risk. Lithium levels must be adjusted during pregnancy. Pregnant patients are at higher risk of relapse to mania because renal lithium clearance increases by 30% to 50% during pregnancy, and normalizes shortly after delivery.13
Lithium exposure during pregnancy has been linked to increased risk of miscarriage and preterm delivery; however, more research is needed to define the true risk of noncardiac teratogenicity associated with lithium.11 Because there is a lack of definitive data regarding teratogenicity, and because of lithium’s well-documented effectiveness in mood disorders, lithium should be considered a first-line therapy for pregnant patients with bipolar disorder.10
Prescribing trends
Despite data showing the efficacy and benefits of lithium, there has been a paradoxical decrease in lithium prescribing. This is the result of multiple factors, including fear of adverse effects and lithium toxicity and a shift toward newer medications, such as anticonvulsants and antipsychotics, for treatment and prophylaxis of mania.
A 2011 study examined prescribing trends for bipolar disorder in the United Kingdom.14 Overall, it found increased usage of valproate, carbamazepine, and lamotrigine from 1995 to 2009. During that time, lithium prescribing mostly remained steady at approximately 30%, whereas valproate use increased from 0% to 22.7%. Overall, antipsychotic and valproate prescribing increased relative to lithium.14 A literature review15 analyzed 6 studies of lithium prescribing trends from 1950 to 2010. Four of these studies (2 in the United States, 1 in Canada, and 1 in German-Swiss-Austrian hospitals) found lithium use was declining. The increased use found in Italy and Spain was attributed to multiple factors, including a broader definition of bipolar disorders and the unavailability of valproate in Spain, lithium’s low cost, and mental health reforms in both countries that resulted in overall increased psychotropic prescribing. Decreased lithium use was attributed to increased use of valproate and second-generation antipsychotics, lack of clinician training in lithium therapy, and aggressive marketing of brand-name medications.15
Reduced suicides, possible protection against dementia
A 2013 meta-analysis of 48 randomized controlled trials (RCTs) that included a total of 6,674 patients with mood disorders indicated that compared with placebo, lithium was more effective in reducing suicides and deaths from any cause.16
Large retrospective studies have demonstrated that compared with valproate, lithium has superior anti-suicide properties.17 Researchers found that risk of suicide attempt or completion was 1.5 to 3 times higher during periods of valproate treatment compared with lithium.18 Both short- and long-term lithium use was associated with decreased non-suicide mortality compared with valproate.19 In Denmark, compared with valproate, lithium was associated with fewer psychiatric hospital admissions.19 One RCT, the BALANCE trial, showed that lithium (alone or in combination with valproate) is more likely to prevent relapse in persons with bipolar I disorder than valproate monotherapy.20
Recent research in Denmark suggests that long-term doses of naturally occurring lithium in drinking water might confer some level of protection against dementia.21 Researchers examined the Danish National Patient Register to determine where participants lived and their local water supply. Drinking water lithium levels were assessed, and the mean lithium level for each municipality was calculated. This case-control study selected patients with dementia and 10 age- and sex-matched controls.21
Researchers found that the incidence rate ratio of Alzheimer disease, vascular dementia, and dementia overall was significantly lower among individuals whose drinking water contained lithium, 15.1 to 27.0 µg/L, compared with those whose water had lithium levels 2.0 to 5.0 µg/L.21 Although this study does not prove causality, it opens the door for continued research on lithium as a neuroprotective agent involved in pathways beyond mood stabilization.
Why should you prescribe lithium?
Lithium, which is available in several formulations (Table), should continue to be first-line pharmacotherapy for treating acute mood episodes, prophylaxis, and suicide prevention in bipolar disorder. Although there are many effective medications for treating bipolar disorder—such as second-generation antipsychotics that are available as a long-acting injectable formulation or can be combined with a mood stabilizer—lithium is a thoroughly researched medication with a long history of effectiveness for managing bipolar disorder. As is the case with all psychotropic medications, lithium has adverse effects and necessary precautions, but these are outweighed by its neuroprotective benefits and efficacy. Research has demonstrated that lithium outperforms medications that have largely replaced it, specifically valproate.

Related Resources
- Ali ZA, El-Mallakh RS. Lithium and kidney disease: Understand the risks. Current Psychiatry. 2021;20(6):34- 38,50. doi:10.12788/cp.0130
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord. 2017;217: 266-280. doi:10.1016/j.jad.2017.03.052
Drug Brand Names
Carbamazepine • Tegretol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Valproate • Depacon, Depakote, Depakene
Bottom Line
Lithium is a well-researched first-line pharmacotherapy for bipolar disorder, with efficacy equivalent to—or superior to—newer pharmacotherapies such as valproate and second-generation antipsychotics. When prescribing lithium, carefully monitor patients for symptoms of adverse effects or toxicity. Despite teratogenic risks, lithium can be considered for pregnant patients with bipolar disorder.
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
1. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11 suppl 2(suppl 2):4-9. doi: 10.1111/j.1399-5618.2009.00706.x
2. Cole N, Parker G. Cade’s identification of lithium for manic-depressive illness—the prospector who found a gold nugget. J Nerv Ment Dis. 2012;200(12):1101-1104. doi:10.1097/NMD.0b013e318275d3cb
3. Johnson FN. Lithium research and therapy. Academic Press; 1975.
4. Cade J. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):518-520. doi:10.1080/j.1440-1614.1999.06241.x
5. Malhi GS, Tanious M, Das P, et al. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012;46(3):192-211. doi:10.1177/0004867412437346
6. Quiroz JA, Machado-Vieira R, Zarate CA Jr, et al. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60. doi:10.1159/000314310
7. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. doi:10.1186/s40345-016-0068-y
8. Ott M, Stegmayr B, Salander Renberg E, et al. Lithium intoxication: incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol. 2016;30(10):1008-1019. doi:10.1177/0269881116652577
9. Heath LJ, Billups SJ, Gaughan KM, et al. Risk factors for utilization of acute care services for lithium toxicity. Psychiatr Serv. 2018;69(6):671-676. doi:10.1176/appi.ps.201700346
10. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi: 10.1016/j.clp.2019.02.004
11. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728. doi:10.1016/S0140-6736(11)61516-X
12. Mohandas E, Rajmohan V. Lithium use in special populations. Indian J Psychiatry. 2007;49(3):211-8. doi: 10.4103/0019-5545.37325
13. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244-55. doi: 10.1097/JCP.0000000000000087
14. Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. doi:10.1371/journal.pone.0028725
15. Netto I, Patil R, Kamble P, et al. Lithium prescribing trends: review. International Journal of Healthcare and Biomedical Research. 2014;2(2):95-103.
16. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646
17. Meyer J. Lithium is regaining favor over anticonvulsants. Psychiatric News. October 2, 2015. Accessed October 12, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.PP10a6
18. Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290(11):1467-1473. doi:10.1001/jama.290.11.1467
19. Smith EG, Austin KL, Kim HM, et al. Mortality associated with lithium and valproate treatment of US Veterans Health Administration patients with mental disorders. Br J Psychiatry. 2015;207(1):55-63. doi:10.1192/bjp.bp.113.138685
20. Geddes JR, Goodwin GM, Rendell J, et al; BALANCE investigators and collaborators. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385-395. doi:10.1016/S0140-6736(09)61828-6
21. Kessing LV, Gerds TA, Knudsen NN, et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry. 2017;74(10):1005-1010. doi:10.1001/jamapsychiatry.2017.2362
Is it bipolar disorder, or a complex form of PTSD?
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.

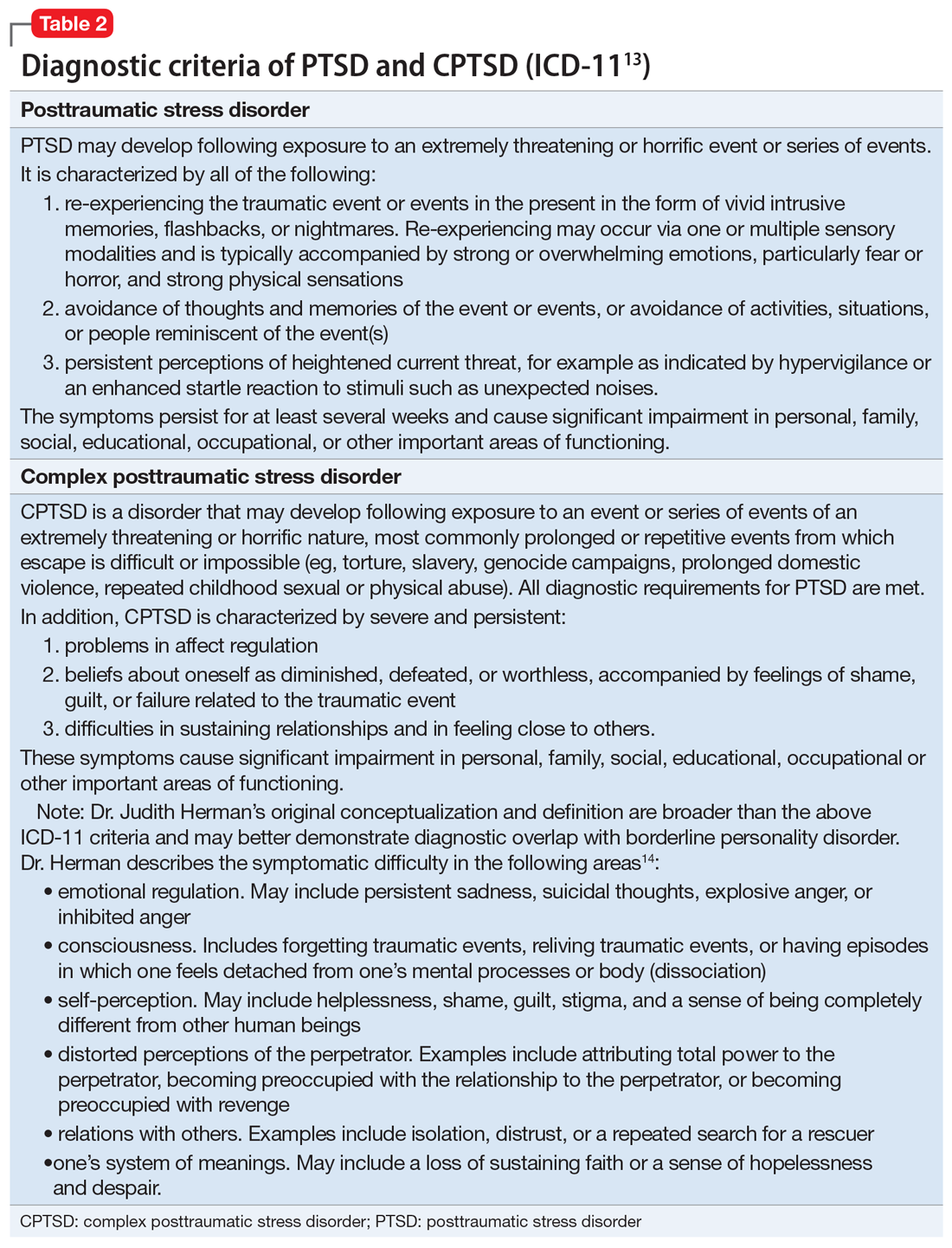
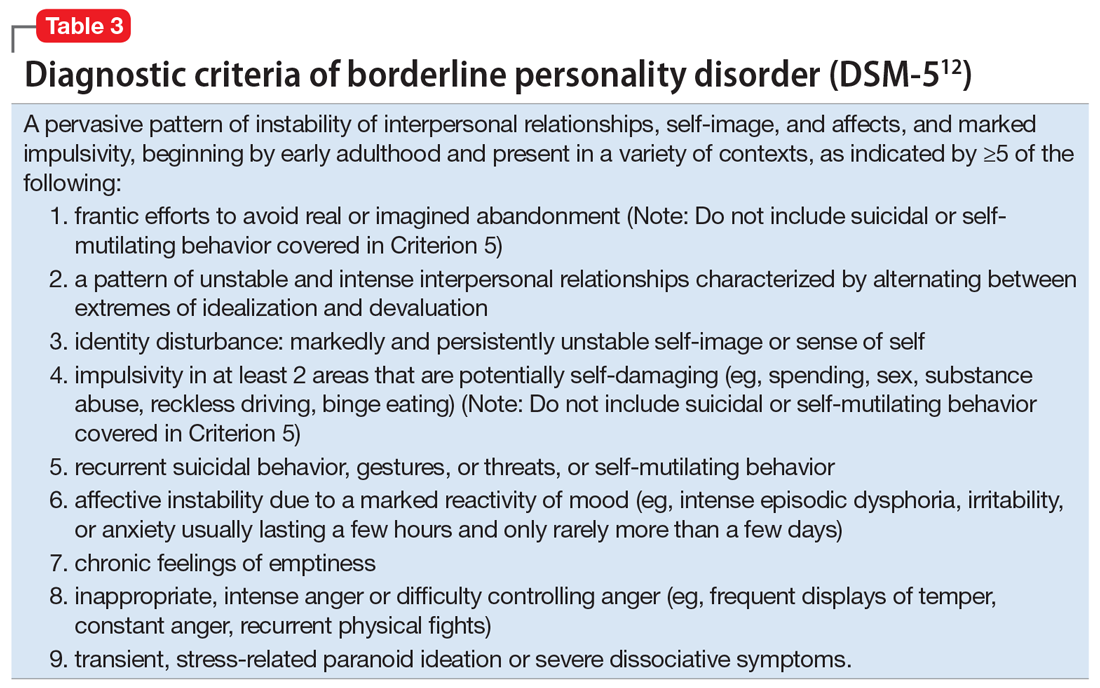
Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
Could stem cells have a role in treating mental illnesses?
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
While laboratory studies move forward at full speed, the clinical use of stem cells—undifferentiated cells that can develop into many different types of specialized cells—remains controversial. Presently, only unadulterated stem cells are allowed to be used in patients, and only on an experimental and investigational basis. Stem cells that have been expanded, modified, or enhanced outside of the body are not allowed to be used for clinical application in the United States at this time. In June 2021, the FDA strengthened the language of stem cell regulation, further limiting their clinical application (see https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies). Yet some applications, such as treatment of lymphoma or restorative knee injections, are covered by some health insurance plans, and the acceptance of stem cell treatment is growing.
In this article, I describe the basics of stem cells, and explore the potential therapeutic use of stem cells for treating various mental illnesses.
Stem cells: A primer
Human embryonic stem cells were initially investigated for their healing properties. However, the need to harvest these cells from embryos drew much criticism, and many found the process to be ethically and religiously unacceptable. This was resolved by the Nobel prize–winning discovery that adult somatic cells can be reprogrammed into cells with embryonic stem cell properties by introducing specific transcription factors. These cells have been termed “induced pluripotent stem cells” (iPSCs).1 The use of adult stem cells and stem cells from the umbilical cords of healthy newborns has allowed for wider acceptance of stem cell research and treatment.
Stem cells may be collected from the patient himself or herself; these are autologous stem cells. They may also be harvested from healthy newborn waste, such as the umbilical cord blood and wall; these are allogenic stem cells. Autologous stem cells are present in almost any tissue but are usually collected from the patient’s adipose tissue or from bone marrow. Understandably, younger stem cells possess higher healing properties. Stem cells may be mesenchymal, producing primarily connective and nervous tissue, or hematopoietic, influencing the immune system and blood cell production, though there is a considerable overlap in the function of these types of cells.
Adult somatic stem cells may be turned into stem cells (iPSCs) and then become any tissue, including neurons. This ability of stem cells to physically regenerate the CNS is directly relevant to psychiatry.
In addition to neurogenesis, stem cell transplants can assist in immune and vascular restoration as well as in suppressing inflammation. The ability of stem cells to replace mutated genes may be useful for addressing inheritable neuropsychiatric conditions.
Both autoimmune and inflammatory mechanisms play an important role in most psychiatric illnesses. The more we learn, the more it is clear that brain function is profoundly dependent on more than just its structure, and that structure depends on more than blood supply. Stem cells influence the vascular, nutritional, functional, inflammatory, and immune environment of the brain, potentially assisting in cognitive and emotional rehabilitation.
Stem cells operate in 2 fundamental ways: via direct cell-to-cell interaction, and via the production and release of growth, immune-regulating, and anti-inflammatory factors. Such factors are produced within the cells and then released in the extracellular environment as a content of exosomes. The route of administration is important in the delivery of the stem cells to the target tissue. Unlike their direct introduction into a joint, muscle, or intervertebral disk, injection of stem cells into the brain is more complicated and not routinely feasible. Intrathecal injections may bring stem cells into the CNS, but cerebrospinal fluid does not easily carry stem cells into the brain, and certainly cannot deliver them to an identified target within the brain. Existing technology can allow stem cells to be packaged in such a way that they can penetrate the blood-brain barrier, but this requires stem cell modification, which presently is not permitted in clinical practice in the United States. Alternatively, there is a way to weaken the blood-brain barrier to allow stem cells to travel through the “opened doors,” so to speak, but this allows everything to have access to the CNS, which may be unsafe. IV administration is technologically easy, and it grants stem cells the environment to multiply and produce extracellular factors that can cross the blood-brain barrier, while large cells cannot.
Continue to: Stem cells as a treatment for mental illness...
Stem cells as a treatment for mental illness
Based on our understanding of the function of stem cells, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions can be influenced by stem cell treatment. Here I review the potential therapeutic role of stem cells in the treatment of several psychiatric disorders.
Alzheimer’s dementia
Alzheimer’s dementia (AD) is a progressive neurodegenerative pathology based on neuronal and synaptic loss. Repopulation and regeneration of depleted neuronal circuitry by exogenous stem cells may be a rational therapeutic strategy.2 The regeneration of lost neurons has the potential to restore cognitive function. Multiple growth factors that regulate neurogenesis are abundant during child development but dramatically decline with age. The introduction of stem cells—especially those derived from newborn waste—seem to promote recovery from neurodegenerative disease or injury.3
There currently is no cure for AD. Cellular therapy promises new advances in treatment.4 Neurogenesis occurs not only during fetal development but in the adult brain. Neural stem cells reside in the adult CNS of all mammals.5 They are intimately involved in continuous restoration, but age just like the rest of the animal tissue, providing ever-decreasing restorative potential.
The number of studies of stem cells in AD has increased since the early 2000 s,6,7 and research continues to demonstrate robust CNS neurogenesis. In a 2020 study, Zappa Villar et al8 evaluated stem cells as a treatment for rats in which an AD model was induced by the intracerebroventricular injection of streptozotocin (STZ). The STZ-treated rats displayed poor performance in all behavioral tests. Stem cell therapy increased exploratory behavior, decreased anxiety, and improved spatial memory and marble-burying behavior; the latter was representative of daily life activities. Importantly, stem cell therapy ameliorated and restored hippocampal atrophy and some presynaptic protein levels in the rats with AD.8 Animal models cannot be automatically applied to humans, but they shine a light on the areas that need further exploration.
In humans, elevated cortisol levels during aging predict hippocampal atrophy and memory deficits,9 and this deficiency may be positively influenced by stem cell treatment.
Schizophrenia
Recent research indicates that schizophrenia may begin with abnormal neurogenesis from neural stem cells inside the embryo, and that this process may be particularly vulnerable to numerous genetic and/or environmental disturbances of early brain development.10 Because neurogenesis is not confined to the womb but is a protracted process that continues into postnatal life, adolescence and beyond, influencing this process may be a way to add to the schizophrenia treatment armamentarium.10 Sacco et al11 described links between the alteration of intrauterine and adult neurogenesis and the causes of neuropsychiatric disorders, including schizophrenia. Immune and inflammatory mechanisms are important in the etiology of schizophrenia. By their core function, stem cells address both mechanisms, and may directly modulate this devastating disease.
In addition to clinical hopes, advances in research tools hold the promise of new discoveries. With the advent of iPSC technology, it is possible to generate live neurons in vitro from somatic tissue of patients with schizophrenia. Despite its many limitations, this revolutionary technology has already helped to advance our understanding of schizophrenia.11
Bipolar disorder
Many of the fundamental neurobiological mechanisms of schizophrenia are mirrored in bipolar disorder.12 Though we are not ready to bring stem cells into the day-to-day treatment of this condition, several groups are starting to apply iPSC technology to the study of bipolar disorder.13
Neurodevelopmental factors—particularly pathways related to nervous system development, cell migration, extracellular matrix, methylation, and calcium signaling—have been identified in large gene expression studies as altered in bipolar disorder.14 Stem cell technology opens doorways to reverse engineering of human neurodegenerative disease.15
Continue to: Autism spectrum disorders...
Autism spectrum disorders
Autism spectrum disorders (ASDs) are multiple heterogeneous neurodevelopmental disorders.16 Neuroinflammation and immune dysregulation influence the origin of ASDs. Due to the neurobiologic changes underlying ASD development, cell-based therapies, including the use of mesenchymal stem cells (MSCs), have been applied to ASDs.16 Stem cells show specific immunologic properties that make them promising candidates for treating ASDs.17
The exact mechanisms of action of MSCs to restore function in patients with ASDs are largely unknown, but proposed mechanisms include:
- synthesizing and releasing anti-inflammatory cytokines and survival-promoting growth factors
- integrating into the existing neural and synaptic network
- restoring plasticity.18
In a study of transplantation of human cord blood cells and umbilical cord–derived MSCs for patients with ASDs, Bradstreet et al19 found a statistically significant difference on scores for domains of speech, sociability, sensory, and overall health, as well as reductions in the total scores, in those who received transplants compared to their pretreatment values.
In another study of stem cell therapy for ASDs, Lv et al20 demonstrated the safety and efficacy of combined transplantation of human cord blood cells and umbilical cord–derived MSCs in treating children with ASDs. The transplantations included 4 stem cell IV infusions and intrathecal injections once a week. Statistically significant differences were shown at 24 weeks post-treatment. Although this nonrandomized, open-label, single-center Phase I/II trial cannot be relied on for any definitive conclusions, it suggests an important area of investigation.20
The vascular aspects of ASDs’ pathogenesis should not be overlooked. For example, specific temporal lobe areas associated with facial recognition, social interaction, and language comprehension have been demonstrated to be hypoperfused in children with ASDs, but not in controls. The degree of hypoperfusion and resulting hypoxia correlates with the severity of ASD symptoms. The damage causing hypoperfusion of temporal areas was associated with the onset of autism-like disorders. Damage of the amygdala, hippocampus, or other temporal structures induces permanent or transient autistic-like characteristics, such as unexpressive faces, little eye contact, and motor stereotypes. Clinically, temporal lobe damage by viral and other means has been implicated in the development of ASD in children and adults. Hypoperfusion may contribute to defects, not only by inducing hypoxia, but also by allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons glutamate toxicity has been implicated in ASD. The augmentation of perfusion through stimulation of angiogenesis by stem cells should allow for metabolite clearance and restoration of functionality. Vargas et al21 compared brain autopsy samples from 11 children with ASDs to those of 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and cerebellum of patients with ASDs, both by immunohistochemistry and morphology.21
Multiple studies have confirmed that the systemic administration of cord blood cells is sufficient to induce neuroregeneration.22,23 Angiogenesis has been experimentally demonstrated in peripheral artery disease, myocardial ischemia, and stroke, and has direct implications on brain repair.24 Immune dysregulation25,26 and immune modulation27 also are addressed by stem cell treatment, which provides a promising avenue for battling ASDs.
Like attention-deficit/hyperactivity disorder and obsessive-compulsive disorder, ASDs are neurodevelopmental conditions. Advances based on the use of stem cells hold great promise for understanding, diagnosing and, possibly, treating these psychiatric disorders.28,29
Depression
Neuropsychiatric disorders arise from deviations from the regular differentiation process of the CNS, leading to altered neuronal connectivity. Relatively subtle abnormalities in the size and number of cells in the prefrontal cortex and basal ganglia have been observed in patients with depressive disorder and Tourette syndrome.30 Fibroblast-derived iPSCs generate serotonergic neurons through the exposure of the cells to growth factors and modulators of signaling pathways. If these serotonergic neurons are made from the patients’ own cells, they can be used to screen for new therapeutics and elucidate the unknown mechanisms through which current medications may function.31 This development could lead to the discovery of new medication targets and new insights into the molecular biology of depression.32
Deficiencies of brain-derived neurotrophic factor (BDNF) have a role in depression, anxiety, and other neuropsychiatric illnesses. The acute behavioral effects of selective serotonin reuptake inhibitors and tricyclic antidepressants seem to require BDNF signaling, which suggests that BDNF holds great potential as a therapeutic agent. Cell therapies focused on correcting BDNF deficiencies in mice have had some success.33
Dysregulation of GABAergic neurons has also been implicated in depression and anxiety. Patients with major depressive disorder have reduced gamma aminobutyric acid (GABA) receptors in the parahippocampal and lateral temporal lobes.34
Ultimately, the development of differentiation protocols for serotonergic and GABAergic neuronal populations will pave the way for examining the role of these populations in the pathogenesis of depression and anxiety, and may eventually open the door for cell-based therapies in humans.35
Studies have demonstrated a reduction in the density of pyramidal and nonpyramidal neurons in the anterior cingulate cortex of patients with schizophrenia and bipolar disorder,36 glial reduction in the subgenual prefrontal cortex in mood disorders,37 and morphometric evidence for neuronal and glial prefrontal cell pathology in major depressive disorder.38 The potential for stem cells to repair such pathology may be of clinical benefit to many patients.
Aside from their other suggested clinical uses, iPSCs may be utilized in new pathways for research on the biology and pharmacology of major depressive disorder.39
Continue to: Obsessive-compulsive disorder...
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is often characterized by excessive behaviors related to cleanliness, including grooming, which is represented across most animal species. In mice, behaviors such as compulsive grooming and hair removal—similar to behaviors in humans with OCD or trichotillomania—are associated with a specific mutation. Chen et al40 reported that the transplantation of bone marrow stem cells into mice with this mutation (bone marrow–derived microglia specifically home to the brain) rescues their pathological phenotype by repairing native neurons.
The autoimmune, inflammatory, and neurodegenerative changes that are prevalent in OCD may be remedied by stem cell treatment in a fashion described throughout this article.
Other conditions
The Box41-50 describes a possible role for stem cells in the treatment or prevention of several types of substance use disorders.
Box
Researchers have begun to explore stem cells as a potential treatment for several substance use disorders, including those involving alcohol, cocaine, and opioids, as well as their interactions with cannabinoids.
Alcohol use disorder. In a 2017 study, Israel et al41 gave intra-cerebral injections of mesenchymal stem cells (MSCs) to rats that were bred to have a high alcohol intake. The MSC injections resulted in drastic reductions in the rats’ alcohol consumption. A single intracerebroventricular MSC administration inhibited relapse-like drinking by up to 85% for 40 days.
It is beyond unlikely that direct brain injections would be used to treat alcohol use disorder in humans. To address this problem, researchers aggregated MSCs into smaller spheroid shapes, which reduced their size up to 75% and allowed them to be injected intravenously to reach the brain in a study conducted in rats.42 Within 48 hours of a single treatment, the rats had reduced their intake of alcohol by 90%. The IV administration of antiinflammatory MSCs in human trials will be the next step to verify these results.
Alcohol research using human stem cells is also being conducted as a model system to understand the neural mechanisms of alcohol use disorder.43
Cocaine use disorder. In a grant proposal, Yadid and Popovtzer44 suggested that cocaine addiction affects neurogenesis, especially in the dentate gyrus, ventral tegmental area, nucleus accumbens, and prefrontal cortex; it damages mitochondrial RNA, brain-derived neurotrophic factor (BDNF), glutamate transporter (excitatory amino acid transporter; EAAT), and interleukin-10. MSCs have a predilection to these areas and influence neurogenesis. Currently, there are no FDAapproved medications for the safe and effective treatment of cocaine addiction. MSCs can home to pathological areas in the brain, release growth factors, and serve as cellular delivery tools in various brain disorders. Moreover, restoration of basal glutamate levels via the EAAT has been proposed as a promising target for treating cocaine dependence. Therefore, MSCs differentiated to express EAATs may have a combined long-term effect that can attenuate cocaine craving and relapse.44
Neural stem cells undergo a series of developmental processes before giving rise to newborn neurons, astrocytes, and oligodendrocytes in adult neurogenesis. During the past decade, studies of adult neurogenesis modulated by addictive drugs have highlighted the role of stem cells. These drugs have been shown to regulate the proliferation, differentiation, and survival of adult cells in different manners, which results in the varying consequences of adult neurogenesis.45 Reversal of these influences by healthy stem cells can be a worthy goal to pursue.
Opioid use disorder. Opiate medications cause a loss of newly born neural progenitors in the subgranular zone of the dentate gyrus by either modulating proliferation or interfering with differentiation and maturation.46 Opiates were the first medications shown to negatively impact neurogenesis in the adult mammalian hippocampus.47,48 The restoration of hippocampal function may positively affect the prognosis of a patient who is addicted.
Cannabinoids. Cannabinoids’ influence on the brain and on stem cells is controversial. On one hand, deteriorated neurogenesis results in reduced long-term potentiation in hippocampal formation. These cellular and physiological alterations lead to decreased short-term spatial memory and increased depressionlike behaviors.49 On the other hand, there is emerging evidence that cannabinoids improve neurogenesis and CNS plasticity, at least in the adult mouse.50 Through normalization of immune function, and restoration of the brain and the body, stem cells may assist in better health and in treatment of cannabis use disorder.
Chronic pain is a neuropsychiatric condition that involves the immune system, inflammation, vascularization, trophic changes, and other aspects of the CNS function in addition to peripheral factors and somatic pain generators. Treatment of painful conditions with the aid of stem cells represents a large and ever-developing field that lies outside of the scope of this article.51
Experimental, but promising
It is not easy to accept revolutionary new approaches in medicine. Endless research and due diligence are needed to prove a concept and then to work out specific applications, safeguards, and limitations for any novel treatments. The stem cell terrain is poorly explored, and one needs to be careful when venturing there. Presently, the FDA appropriately sees treatment with stem cells as experimental and investigational, particularly in the mental health arena. Stem cells are not approved for treatment of any specific condition. At the same time, research and clinical practice suggest stem cell treatment may someday play a more prominent role in health care. Undoubtedly, psychiatry will eventually benefit from the knowledge and application of stem cell research and practice.
Related Resources
- De Los Angeles A, Fernando MB, Hall NAL, et al. Induced pluripotent stem cells in psychiatry: an overview and critical perspective. Biol Psychiatry. 2021;90(6):362-372.
- Heider J, Vogel S, Volkmer H, et al. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22(19):10254.
Drug Brand Name
Streptozotocin • Zanosar
Bottom Line
Treatment with stem cell transplantation is experimental and not approved for any medical or psychiatric illness. However, based on our growing understanding of the function of stem cells, and preliminary research conducted mainly in animals, many neurodegenerative-, vascular-, immune-, and inflammation-based psychiatric conditions might be beneficially influenced by stem cell treatment.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
- Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111.
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185-190.
- Taupin P. Adult neurogenesis, neural stem cells, and Alzheimer’s disease: developments, limitations, problems, and promises. Curr Alzheimer Res. 2009;6(6):461-470.
- Taupin P. Neurogenesis, NSCs, pathogenesis, and therapies for Alzheimer’s disease. Front Biosci (Schol Ed). 2011;3:178-90.
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54(3):879-889.
- Li M, Guo K, Ikehara S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238.
- Zappa Villar MF, López Hanotte J, Pardo J, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020;57(2):600-615.
- Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69-73.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414-442.
- Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr Opin Neurobiol. 2018; 48:131-138.
- Miller ND, Kelsoe JR. Unraveling the biology of bipolar disorder using induced pluripotent stem-derived neurons. Bipolar Disord. 2017;19(7):544-551.
- O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2016;73:63-83.
- Jacobs BM. A dangerous method? The use of induced pluripotent stem cells as a model for schizophrenia. Schizophr Res. 2015;168(1-2):563-568.
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2016;1638(Pt A):30-41.
- Siniscalco D, Kannan S, Semprún-Hernández N, et al. Stem cell therapy in autism: recent insights. Stem Cells Cloning. 2018;11:55-67.
- Siniscalco D, Bradstreet JJ, Sych N, et al. Mesenchymal stem cells in treating autism: novel insights. World J Stem Cells. 2014;6(2):173-178.
- Siniscalco D, Sapone A, Cirillo A, et al. Autism spectrum disorders: is mesenchymal stem cell personalized therapy the future? J Biomed Biotechnol. 2012; 2012:480289.
- Bradstreet JJ, Sych N, Antonucci N, et al. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105-S112.
- Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cordderived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67-81.
- Wei L, Keogh CL, Whitaker VR, et al. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12(1): 47-62.
- Newman MB, Willing AE, Manresa JJ, et al. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201-218.
- Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114(3):312-314.
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317-341.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3(7-8):557-562.
- Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679.
- Vaccarino FM, Urban AE, Stevens HE, et al. Annual Research Review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504-516.
- Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev Rep. 2014;10(2):145-150.
- Richardson-Jones JW, Craige CP, Guiard BP, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40-52.
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349-357.
- Klumpers UM, Veltman DJ, Drent ML, et al. Reduced parahippocampal and lateral temporal GABAA-[11C] flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3): 565-574.
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973-981.
- Vincent SL, Todtenkopf MS, Benes FM. A comparison of the density of pyramidal and non-pyramidal neurons in the anterior cingulate cortex of schizophrenics and manic depressives. Soc Neurosci Abstr. 1997;23:2199.
- Benes FM, Kwok EW, Vincent SL, et al. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2): 88-97.
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9): 1085-1098.
- Licinio J, Wong ML. Serotonergic neurons derived from induced pluripotent stem cells (iPSCs): a new pathway for research on the biology and pharmacology of major depression. Mol Psychiatry. 2016;21(1):1-2.
- Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775-785.
- Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral stem cell administration inhibits relapse-like alcohol drinking in rats. Alcohol Alcohol. 2017;52(1):1-4.
- Ezquer F, Morales P, Quintanilla ME, et al. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8(1):4325.
- Scarnati MS, Halikere A, Pang ZP. Using human stem cells as a model system to understand the neural mechanisms of alcohol use disorders: current status and outlook. Alcohol. 2019;74:83-93.
- Yadid GM, Popovtzer R. Nanoparticle-mesenchymal stem cell conjugates for cell therapy in drug addiction. NIH grant application. 2017.
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73(2):327-348.
- Dholakiya SL, Aliberti A, Barile FA. Morphine sulfate concomitantly decreases neuronal differentiation and opioid receptor expression in mouse embryonic stem cells. Toxicol Lett. 2016;247:45-55.
- Zhang Y, Loh HH, Law PY. Effect of opioid on adult hippocampal neurogenesis. Scientific World Journal. 2016;2016:2601264.
- Bortolotto V, Grilli M. Opiate analgesics as negative modulators of adult hippocampal neurogenesis: potential implications in clinical practice. Front Pharmacol. 2017; 8:254.
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, et al. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013; 52(4):633-650.
- Zimmermann T, Maroso M, Beer A, et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb Cortex. 2018;28(12):4454-4471.
- Ren J, Liu N, Sun N, et al. Mesenchymal stem cells and their exosomes: promising therapies for chronic pain. Curr Stem Cell Res Ther. 2019;14(8):644-653.