User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Too close for comfort: When the psychiatrist is stalked
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
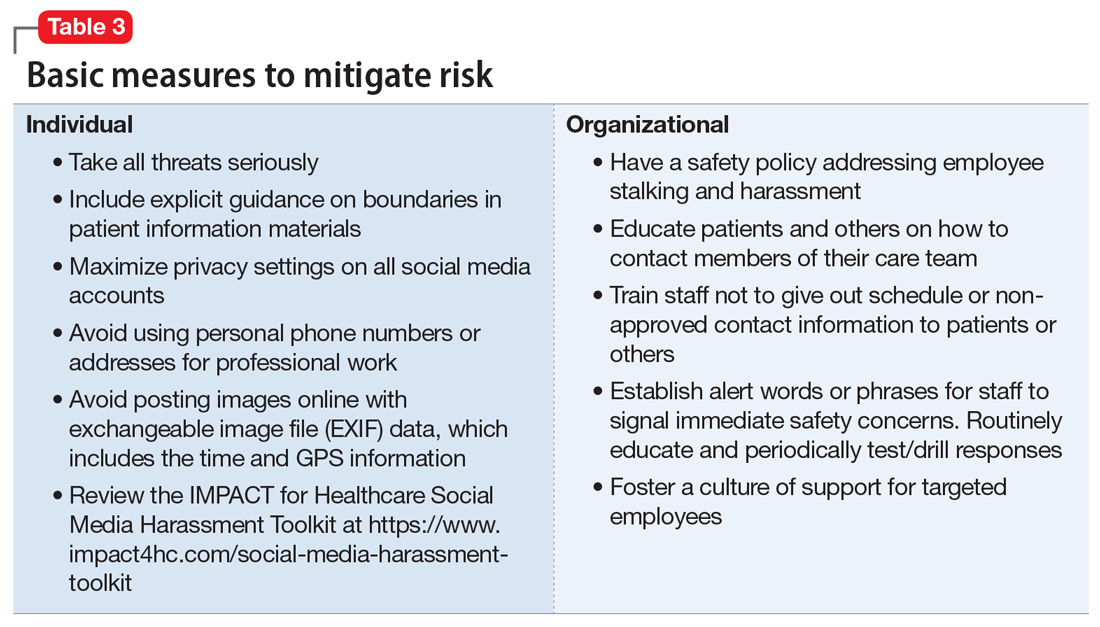
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.
What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
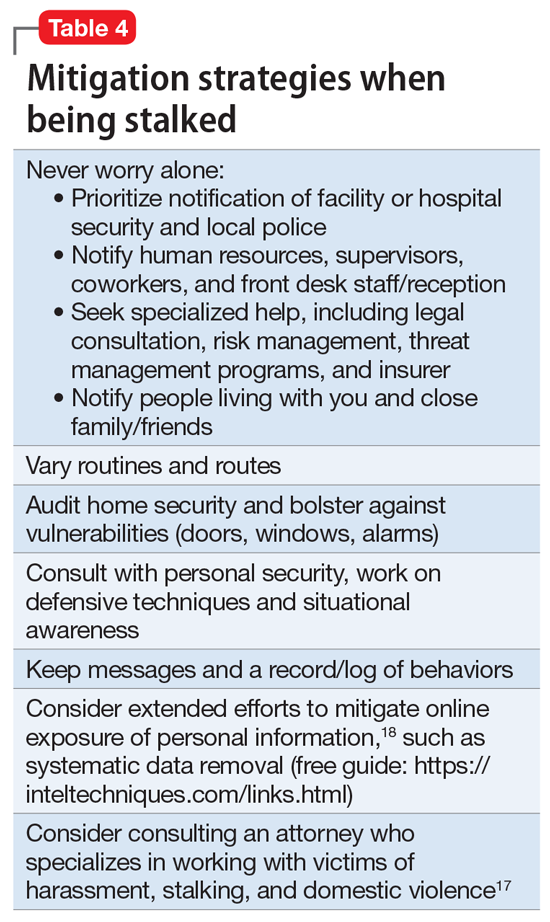
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.
While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Pediatric insomnia: Treatment
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
Children and adolescents who do not receive sufficient sleep can experience worsening inattention, daytime fatigue, and cognitive and behavioral difficulties. Assessment and treatment of insomnia and other sleep difficulties in young patients is critical as poor sleep increases their risk for depression, self-harm, and suicide.
In Part 1 of this article (Pediatric insomnia: Assessment and diagnosis,
Psychotherapeutic interventions
Regardless of the source of a child’s insomnia or co-occurring disorders, healthy sleep practices are the first line behavioral treatment, including for youth with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders, obsessive-compulsive disorder, and depressive disorders.
Healthy sleep practices/sleep hygiene
Developmentally appropriate bedtimes and routines (Table). Helping children establish a regular, consistent bedtime is key in promoting healthy sleep. Ideally, the bedtime routine involves 3 to 4 activities each night in the same order, and these activities should be relaxing and soothing (eg, taking a bath, putting on pajamas, reading books). Setting age-appropriate bedtimes also is important. If an older child is asked to go to bed at 8 pm but cannot fall asleep for an hour, they may not have insomnia but instead a developmentally inappropriate bedtime. Several studies found that children younger than age 10 should go to bed no later than 9 pm. Bedtimes later than 9 pm for young children are correlated with shorter sleep duration.1

Consistent sleep schedules. Another important aspect of healthy sleep is working with parents to enforce a consistent bedtime and wake-up time, including weekdays and weekends. Ideally, bedtime on weekdays and weekends should not vary by more than 1 hour. Helping children wake up at the same time each day helps to set and regulate their circadian rhythm. Keeping these schedules consistent on vacations and school holidays also is helpful. For adolescents, the weekday/weekend bedtimes can vary by up to 2 hours because adolescents have a delayed circadian rhythm and wake-up times for high school can be early.
Environmental factors. An important piece of parental education is stimulus control and the ingredients of healthy sleep. Healthy sleep ingredients include a dark, quiet, consistent, and cool bedroom; a comfortable bed, the child feeling safe, and limited environmental stimuli.
Continue to: Cognitive-behavioral therapy for insomnia...
Cognitive-behavioral therapy for insomnia
Relaxation. Pediatric patients can be taught relaxation, mindfulness, meditation, and progressive muscle relaxation techniques to help lower overall stress. This can be especially helpful for youth with sleep disorders or anxiety. Guided relaxation apps are popular among children and teens, and various apps offer soothing sounds, deep breathing, progressive muscle relaxation, and guided imagery. This can be taught in psychotherapy sessions and used at home to promote gains in between sessions.
Stimulus control. Stimulus control involves using the bed exclusively for sleep and avoiding nonsleep activities in bed (eg, reading, watching television, using a computer, worrying). These activities promote wakefulness and insomnia. This may mean the child does not get into bed until they cannot keep their eyes open, even if that delays bedtime. If the child is still awake within 15 to 20 minutes, they should be encouraged to get out of bed and engage in a nonstimulating activity such as meditation, reading, or sitting quietly in the dark or low light. This recommendation can run counter to parents’ intuition that children with sleep problems should go to bed earlier. Using the bed only for sleep conditions the child to falling asleep or being asleep when in bed.
Sleep restriction. Sleep restriction involves restricting sleep to a set number of hours in order to increase their sleep efficiency (time slept in bed divided by total time spent in bed x 100). Restricting sleep to 6 to 7 hours increases sleep efficiency, consolidates sleep, and extinguishes the association of being awake in bed. For older adolescents, sleep restriction may help to limit their time in bed to either falling asleep or being asleep. This is intended to be used as a short-term strategy and only after other sleep hygiene measures (bedtime routine, environmental factors, etc) have been put into place for several weeks. While this strategy sounds unappealing to most individuals with insomnia, it can lead to lasting change due to the use of behavioral conditioning in bed. Because sleep restriction can lead to significant daytime sleepiness and impairment during the day, sleep should not be restricted to <6 hours a day for children and adolescents. Once the adolescent is sleeping more consistently and sleep efficiency reaches 85% or higher, time in bed for sleep is increased.2
Cognitive restructuring. Some children and adolescents develop maladaptive thoughts about sleep that further promote insomnia. These thoughts might include “I will never get to sleep,” “I am going to have a terrible day if I cannot fall asleep,” or “I will fail my test tomorrow if I am unable to sleep.” Such maladaptive thoughts are often untrue but promote wakefulness in the early or middle part of the night. Cognitive restructuring involves helping the child identify each problematic thought, challenge how accurate each thought is with evidence, and replace the problematic thought with a more helpful thought. For instance, an adolescent can recognize that even if they have a sleepless night, their catastrophic outcome (eg, “I will not be able to function”) is likely untrue. A psychologist can help review evidence for this, including previous times when the adolescent has not slept well and managed to get through the next day.
When is pharmacologic treatment needed?
Pharmacologic treatment may be indicated if a child does not show significant improvement following behavioral intervention (Figure). However, it is critical to exclude other primary causes of dyssomnia (eg, obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy, because pharmacotherapy could mask an underlying disorder. Moreover, while there is relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children and adolescents, a large survey of child and adolescent psychiatrists (N = 1,273) suggested that medications were considered for one-quarter of pediatric patients with insomnia.3 Further, patients with specific comorbidities such as neurodevelopmental disorders may be more likely to be prescribed soporifics.4

Continue to: What is the evidence for pharmacotherapy?...
What is the evidence for pharmacotherapy?
Antihistamines. Histamine antagonists—which promote sleep by blocking the wakefulness-promoting and circadian-related effects of histamine—are the most commonly used medications to treat pediatric insomnia, despite a dearth of data from prospective trials.5,6 In 1 small study, Russo et al7 found diphenhydramine, 1 mg/kg at bedtime, reduced sleep latency and nighttime awakenings, and increased sleep duration in patients ages 2 to 12; similar effects have been observed in pediatric burn patients.8 There are some limited data for other H1 antagonists (eg, hydroxyzine) in pediatric insomnia.9-11
Alpha-2 agonists increase rapid eye movement sleep via dose-dependent downregulation of noradrenergic signaling12 and thus have been commonly prescribed for insomnia in children and adolescents. In fact, the nonselective alpha-2 agonist clonidine is among the most prescribed medications for youth with insomnia, and may be efficacious in youth with neurodevelopmental disorders and ADHD.13 In small retrospective studies, clonidine decreased sleep latency and nighttime awakenings in addition to increasing sleep duration.14 Also, clonidine was well tolerated but associated with daytime somnolence. Guanfacine—a selective alpha-2 agonist—is also commonly prescribed for insomnia in youth, although results of trials have been equivocal.15 Given the more rapid absorption and shorter Tmax of clonidine relative to guanfacine, the former may be preferred as a soporific.
Melatonin and melatonin agonists. The primary regulator of the sleep-wake cycle is melatonin, an endogenous hormone produced by the pineal gland in response to changes in retinal light perception. Exogenous melatonin supplementation may be the preferred initial pharmacotherapy for sleep-onset insomnia due to its chronobiotic properties.16 In clinical studies, both immediate-release17,18 and extended-release19 melatonin reduced sleep-onset latency and increased total sleep duration in pediatric patients, although the increase in total duration of sleep was greater with extended-release preparations. Additionally, tolerability data for melatonin in pediatric patients are encouraging. A 2-year randomized trial of prolonged-release melatonin for insomnia in pediatric patients found no adverse effects with regard to growth, body mass index, or pubertal development.20 Additionally, significant improvements in sleep quality, sleep patterns, and caregiver satisfaction were maintained throughout the trial, and no withdrawal symptoms were observed upon discontinuation.
Melatonin may have a particularly important role in circadian rhythm sleep disorders. In this regard, low-dose melatonin (0.5 mg), when timed relative to the endogenous dim light melatonin onset (DLMO), is more effective in shifting sleep phase than higher doses, which suggests that timing may have greater impact than dosage.21 Data regarding melatonin administration with respect to DLMO suggest that the optimal administration time is 4 to 6 hours before a child’s preferred bedtime, and doses of 0.5 to 1 mg have been effective when given in this window.22 Variation across studies has contributed to a lack of consensus regarding pediatric melatonin dosing. For example, .05 mg/kg may be a minimal effective dose when given 1 to 2 hours before bedtime18; however, in surveys doses vary considerably, with typical doses of 2.5 to 3 mg for prepubertal children and 5 mg for adolescents.5 Of note, in patients with decreased cytochrome P450 (CYP) 1A2 activity, lack of diurnal variation in melatonin serum concentration may decrease the effectiveness of melatonin.16Ramelteon is a potent agonist of the melatonin MT1 and MT2 receptors, with a significantly higher binding affinity than melatonin in vitro. In case reports, ramelteon was well-tolerated, improved delayed sleep onset, and decreased nighttime awakenings.23
Zolpidem, eszopiclone and zaleplon. Studies of selective GABAergic modulators and benzodiazepines have not produced positive results in prospective trials of youth with insomnia. Zolpidem was studied in children and adolescents (N = 201) with ADHD; although sleep latency did not differ between zolpidem and placebo, some significant improvements were observed in adolescents.24 Zolpidem was generally well tolerated, with approximately 10% of youth discontinuing due to adverse effects. Additionally, eszopiclone—which has a longer duration of action compared with zolpidem—has been studied in children and adolescents with ADHD (N = 486) who were also evaluated with a sleep study. No differences were observed between placebo and eszopiclone in terms of sleep latency and approximately 10% of patients discontinued treatment as a result of adverse events.25 We were unable to locate any prospective trials of zaleplon or benzodiazepine receptor agonists for insomnia in youth, although some reports suggest that clonazepam may have a possible role for specific parasomnias.26,27Dual orexin receptor antagonists. Suvorexant, an antagonist of the wakefulness-promoting neuropeptide orexin, improved subjective sleep quality in a prospective trial of adolescents with insomnia (N = 30), although dropout was high (44%).28 Of those patients, reasons for discontinuation included loss to follow-up, lack of effectiveness, and abnormal dreams. We were unable to locate any trials of lemborexant in pediatric patients.
Atypical antidepressants. Trazodone is commonly prescribed for insomnia in pediatric patients with comorbid mood or anxiety disorders. In open-label studies of children and toddlers, trazodone may be well-tolerated and improve sleep.29 Additionally, development of a physiologically based pharmacokinetic model to inform trazodone dosing for youth with insomnia is underway.30 Some studies in adolescents with depression suggest that caution should be used when combining trazodone with medications that inhibit CYP2D6. In the Treatment of SSRI-Resistant Depression in Adolescents study, none of the patients who were treated with trazodone (vs other soporifics) improved.31 This may relate to CYP2D6 interactions and accumulation of methyl-chloro-piperazine (mCPP), a trazodone metabolite that is associated with dysphoria, irritability, and depression.31 This finding has been replicated in a separate cohort of depressed adolescents.32
Because of its antihistaminergic effects, mirtazapine has been used to treat insomnia in adults. One open-label study of mirtazapine in children and young adults ages 3 to 23 with neurodevelopmental disorders suggested that mirtazapine improved behavioral symptoms and insomnia, and was associated with few treatment-limiting adverse effects.33
Tricyclic antidepressants. In a retrospective study of youth with insomnia who failed behavioral interventions and melatonin (N = 29), doxepin, a potent H1 antagonist, improved subjective sleep in one-half of patients.34
Continue to: Consultation with pediatric sleep medicine specialists...
Consultation with pediatric sleep medicine specialists
It often will behoove the psychiatric clinician to review their concerns with a behavioral sleep medicine specialist or a psychologist with specific expertise in the psychotherapeutic treatment of sleep who can provide important guidance regarding the key aspects of treatment. When discussing a particular patient’s presentation with the pediatric behavioral sleep psychologist/specialist, consider the following questions:
- Is the child’s sleep disorder the primary problem, or is the child’s insomnia secondary to another diagnosis (psychiatric or nonpsychiatric)?
- What are the primary sleep-related problems the child/family presents with? How long have the symptoms been present?
- Is the sleep disorder interfering with the child’s functioning, either academically or socially? Does the child’s sleep problem interfere with other family members’ sleep?
- Does the child have a comorbid psychological conditions such as ADHD, depression, or anxiety, and/or is undergoing treatment for these disorders, which may play a role in the sleep problem?
- Is a sleep study warranted?
A sleep study, also known as polysomnography (PSG), is a diagnostic test in which physiologic parameters are continuously recorded during a period of sleep via electroencephalography, electromyography, electrooculogram, electrocardiogram, airflow sensors, pulse oximeter, body position monitors, and video. In 2012, the American Academy of Sleep Medicine published evidenced-based practice parameters for respiratory and nonrespiratory indications for PSG.35 It is most commonly indicated to rule out obstructive sleep apnea in pediatric patients who exhibit snoring, gasping, irregular breathing, witnessed apneic events, unusual head positioning, or other signs of obstructive breathing during sleep. Nonrespiratory indications for PSG include children suspected of having periodic limb movement disorder and suspected narcolepsy. Children with frequent parasomnias, epilepsy, or nocturnal enuresis should be clinically screened for presence of comorbid sleep disorders, and PSG would be indicated if there are concerns about a possible sleep-disordered breathing disorder. PSG is also recommended for children with hypersomnia, to differentiate a parasomnia from sleep-related epilepsy, and for children suspected of having restless leg syndrome.36 PSG is not typically indicated in the initial evaluation of insomnia (unless there is evidence of a comorbid sleep disorder), circadian rhythm disorders (ie, delayed sleep phase syndrome), or for evaluation of children with sleep-related bruxism.3 Special considerations for PSG in children include ensuring a parent or guardian is always with the child, providing developmentally appropriate sleeping arrangements, arranging family tours of the sleep lab prior to the study, and accommodating for earlier bedtimes.37
Bottom Line
Techniques to promote healthy sleep in pediatric patients include behavioral interventions such as setting a developmentally appropriate bedtime and a consistent wake time, establishing bedtime routines, and encouraging relaxation/ wind-down period before bed. Cognitive-behavioral therapy for insomnia (CBT-I) may include cognitive restructuring of anxious thoughts, relaxation training, stimulus control, and sleep restriction. Use of medications may be indicated for children and teens who have not responded to CBT-I or soporific dosing of melatonin.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
1. Mindell JA, Li AM, Sadeh A, et al. Bedtime routines for young children: a dose-dependent association with sleep outcomes. Sleep. 2015;38(5):717-722.
2. Kansagra S. Sleep disorders in adolescents. Pediatrics. 2020;145(Suppl 2):S204-S209.
3. Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555-569.
4. Bruni O, Angriman M, Melegari MG, et al. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin Pharmacother. 2019;20(18):2257-2271.
5. Owens JA, Rosen CL, Mindell JA, et al. Use of pharmacotherapy for insomnia in child psychiatry practice: a national survey. Sleep Med. 2010;11(7):692-700.
6. Schnoes CJ, Kuhn BR, Workman EF, et al. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin Pediatr (Phila). 2006;45(3):229-238.
7. Russo RM, Gururaj VJ, Allen JE. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J Clin Pharmacol. 1976;16(5-6):284-288.
8. Yangzom N, Gottschlich MM, Ossege J, et al. The effect of diphenhydramine on sleep in pediatric burn patients: a secondary analysis. J Burn Care Res. 2015;36(2):266-271.
9. Ghanizadeh A, Zare S. A preliminary randomised double-blind placebo-controlled clinical trial of hydroxyzine for treating sleep bruxism in children. J Oral Rehabil. 2013;40(6):413-417.
10. Bektas O, Arıca B, Teber S, et al. Chloral hydrate and/or hydroxyzine for sedation in pediatric EEG recording. Brain Dev. 2014;36(2):130-136.
11. Ottaviano S, Giannotti F, Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Childs Nerv Syst. 1991;7(6):332-335.
12. Nguyen M, Tharani S, Rahmani M, et al. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin Pediatr (Phila). 2014;53(3):211-216.
13. Prince JB, Wilens TE, Biederman J, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599-605.

14. Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders--a case series. Eur Child Adolesc Psychiatry. 2005;14(1):34-40.
15. Politte LC, Scahill L, Figueroa J, et al. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology. 2018;43(8):1772-1778.
16. Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122-1233.
17. Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637-644.
18. van Geijlswijk IM, van der Heijden KB, Egberts AC, et al. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCT. Psychopharmacology (Berl). 2010;212(3):379-391.
19. Gringras P, Nir T, Breddy J, et al. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957.e4.
20. Malow BA, Findling RL, Schroder CM, et al. Sleep, growth, and puberty after two years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252-261.e3.
21. Burgess HJ, Emens JS. Drugs used in circadian sleep-wake rhythm disturbances. Sleep Med Clin. 2020;15(2):301-310.
22. Arns M, Kooij JJS, Coogan AN. Review: identification and management of circadian rhythm sleep disorders as a transdiagnostic feature in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1085-1095.
23. Kawabe K, Horiuchi F, Oka Y, et al. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep Psychiatry. 2014;2014:561071.
24. Blumer JL, Findling RL, Shih WJ, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770-e776.
25. Sangal RB, Blumer JL, Lankford DA, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095-e1103.
26. Arens R, Wright B, Elliott J, et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133(5):670-674.
27. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173-178.
28. Kawabe K, Horiuchi F, Ochi M, et al. Suvorexant for the treatment of insomnia in adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792-795.
29. Pranzatelli MR, Tate ED, Dukart WS, et al. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J Pediatr. 2005;147(3):372-378.
30. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone-atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77-86.
31. Shamseddeen W, Clarke G, Keller MB, et al. Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. J Child Adolesc Psychopharmacol. 2012;22(1):29-36.
32. Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233-240.
33. Posey DJ, Guenin KD, Kohn AE, et al. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267-277.
34. Shah YD, Stringel V, Pavkovic I, et al. Doxepin in children and adolescents with symptoms of insomnia: a single-center experience. J Clin Sleep Med. 2020;16(5):743-747.
35. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473.
36. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24.
37. Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393-406.
Olanzapine-samidorphan combination for schizophrenia or bipolar I disorder
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Alan F. Schatzberg, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Alan F. Schatzberg, MD. Dr. Schatzberg is the Kenneth T. Norris, Jr., Professor of Psychiatry and Behavioral Sciences at Stanford University. He served as the Chair of the Department at Stanford until 2010 and currently directs the Stanford Mood Disorders Center. He was the 136th president of the American Psychiatric Association (APA) (2009-2010). He has been an active investigator in the biology and psychopharmacology of depressive disorders, and has authored more than 700 publications and abstracts, including Schatzberg’s Manual of Clinical Psychopharmacology. Dr. Schatzberg is also the coeditor of the Textbook of Psychopharmacology with Charles B. Nemeroff, MD, PhD. He is a Past President of the American College of Neuropsychopharmacology (ACNP) and the Society of Biological Psychiatry, and was also the Secretary-General of the International Society of Psychoneuroendocrinology (ISPNE). In 2003, he was elected to the Institute of Medicine of the National Academy of Sciences (National Academy of Medicine). He has received numerous prestigious awards, including the 2005 Distinguished Service in Psychiatry Award from the American College of Psychiatrists, the 2005 Falcone Award from the National Alliance for Research in Schizophrenia and Affective Disorders, the 2014 Kraepelin Gold Medal from the Max Planck Institute of Psychiatry, the 2015 Gold Medal from the Society of Biological Psychiatry, the 2015 Lifetime Achievement Award of the ISPNE, the 2017 Julius Axelrod Mentorship Award from the ACNP, the 2018 Donald Klein, MD, Lifetime Achievement Award from the American Society of Clinical Psychopharmacology, and the 2018 Jules Marmor, MD, Award for Biopsychosocial Research from the APA.
Dr. Aftab: You have devoted much of your career to the development of psychopharmacology. What is your perspective on where the field of psychopharmacology stands at present, especially amid the widespread recognition of “treatment resistance” as a pervasive phenomenon and the scarcity of validated neurobiologic etiological models for psychiatric disorders?
Dr. Schatzberg: We have made considerable progress in the development of new classes of agents for major depression, but as we develop new agents, we still see a large percentage of patients who do not seem to demonstrate adequate responses, particularly in major depressive disorder. This has driven us to look for agents that work differently than previous ones. Although we have some new agents with seeming efficacy and newer mechanisms of action, eg, esketamine, these have largely been derived from clinical, often serendipitous, observations of antidepressant effects rather than from prospective development based on a known pharmacological effect or a biological construct of the disorder. Another intriguing and possibly effective anxiolytic and antidepressive agent is psilocybin, whose potential use is largely derived from clinicians who found it helpful in their practices in combination with psychotherapy. These 2 demonstrate how as we branch out into new territory, we find ourselves moving more and more toward drugs of known clinical risk; eg, mind-altering agents or drugs of abuse. These agents may offer risk-benefit ratios that can ultimately prove to be less attractive than what we might have wanted when we ventured on the journey. Unfortunately, there has been little dialogue about the limitations of several of these agents.
In the case of esketamine, the notion has been that the drug is a blocker of the N-methyl-
One approach that has been applied recently is target validation that purports to use functional MRI to assess behavioral and cognitive effects of drugs to allow inferences regarding efficacy in specific disorders. As we have discussed in a recent paper published in the American Journal of Psychiatry,4 this can be quite misleading and may provide both false positive and negative information. From my perspective, these tests do not appear sensitive enough to screen for patients having a disorder, nor for assessing possible drug effects in those patients. Thus, it is unclear if they can provide answers today that we can be confident in.
Continue to: Dr. Aftab...
Dr. Aftab: What do you see as some of the strengths of psychiatry as a profession?
Dr. Schatzberg: Psychiatry as a specialty combines 2 major perspectives—psychological processes and psychobiology—to develop methods for treating patients who suffer from disorders of the mind/brain. It is the most challenging of our specialties because we cannot study the brain directly. We cannot do procedures as we do in cardiology and pulmonology because they may prove dangerously invasive. That hands-off approach limits us, but for the curious it provides an opportunity to begin to unravel the processes that underlie brain functioning. Fortunately, we have therapies—both psychosocial and somatic—that can provide great relief to patients. These can be shown to be effective in sufficient numbers of patients to help many.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Schatzberg: We need to train our residents in a host of approaches, and not just medications and psychotherapy. They need to understand the basis of brain stimulation approaches (such as repetitive transcranial magnetic stimulation) as well as know how to apply them. We need to train residents more in substance abuse problems and the biology of addiction if they are to better understand the risks of certain new classes of medication. Lastly, we need to train residents in the application of genomics, proteomics, and brain imaging to somatic treatment development.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Schatzberg: The biggest threats come from ourselves. We need to do better with our classification approaches, such as the Diagnostic and Statistical Manual of Mental Disorders or the Research Domain Criteria. They need to become more rapidly adaptive to research in the field. We need to be more open to looking at what is a potentially dangerous trend in developing drugs of abuse and mind-altering drugs as therapeutics. We need to be able to demonstrate that telepsychiatry can be as effective as face-to-face treatment and should be reimbursed. Lastly, we need to develop better models for taking care of the psychiatric patient. We have too many patients and not enough psychiatrists.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Schatzberg: I see the future as bright. Over the past 10 years, led by efforts at the APA, some while I was President, reimbursement has increased dramatically. Over the past 10 years, we have done well developing some new drugs and somatic therapies, and these will continue. Less than a decade ago, large pharmaceutical had abandoned psychiatric drug development and investment into biotech start-ups had waned to near zero. However, the last year few years have seen a dramatic surge in investment, and these should yield novel agents and ones that may be combined with innovative biomarkers as companions.
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
1. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215. doi:10.1176/appi.ajp.2018.18020138
2. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786. doi:10.1038/s41380-019-0503-4
3. Bonaventura J, Lam S, Carlton M, et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 2021;10.1038/s41380-021-01093-2. doi:10.1038/s41380-021-01093-2
4. Schatzberg AF. Can target engagement studies miss their targets and mislead drug development? Am J Psychiatry. 2021;178(5):372-374. doi:10.1176/appi.ajp.2020.21030247
Inpatient violence: Take steps to reduce your risk
Inpatient violence is a significant problem for psychiatric facilities because it can have serious physical and psychological consequences for both staff and patients.1 Victimized staff can experience decreased productivity and emotional distress, while victimized patients can experience disrupted treatment and delayed discharge.1 Twenty-five to 35% of psychiatric inpatients display violent behavior during their hospitalization.1 A subset are extreme offenders.1,2 This small group of violent patients accounts for the majority of inpatient violence and the most serious injuries.1,2
Reducing inpatient violence starts with conducting a targeted violence risk assessment to identify patients who are at elevated risk of being violent. Although conducting a targeted violence risk assessment is beyond the scope of this article, here I outline practical steps that clinicians can take to reduce the risk of inpatient violence. These steps complement and overlap with those I described in “Workplace violence: Enhance your safety in outpatient settings” (Pearls,
Identify underlying motives. Inpatient violence is often a result of 3 primary psychiatric etiologies: difficulty with impulse control, symptoms of psychosis, or predatory traits.1 Impulsivity drives most of the violence on inpatient units, followed by predatory violence and symptoms of psychosis.1 Once you identify the psychiatric motive, you can develop an individualized, tailored treatment plan to reduce the risk of violence. The treatment plan can include using de-escalation techniques, administering scheduled and as-needed medications to target underlying symptoms, having patients assume responsibility for their behaviors, holding patients accountable for their behaviors, and other psychosocial interventions.1 Use seclusion and restraint only when it is the least restrictive means of providing safety.1,4
Develop plans and policies. As you would do in an outpatient setting, assess for hazards within the inpatient unit. Plan for the possible types of violence that may occur on the unit (eg, physical violence against hospital personnel and/or other patients, verbal harassment, etc).3 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors (eg, posting a safety board where staff can record aggressive behaviors and other safety issues).3,4 When developing these plans and policies, include patients by creating patient/staff workgroups to develop expectations for civil behavior that apply to both patients and staff, as well as training patients to co-lead groups dealing with accepting responsibility for their own recovery.5 These plans and policies should include informing patients that threats and violence will not be tolerated. Frequently review these plans and policies with patients and staff.
Provide communication and education. Maintain strong psychiatric leadership on the unit that encourages open lines of communication. Encourage staff to promptly report incidents. Frequently ask staff if they have any safety concerns, and solicit their opinions on how to reduce risks.4 Include discussions about safety during staff and community meetings. Communicate patients’ behaviors that are distressing or undesired (eg, threats, harassment, etc) to all unit personnel.3 Notify staff when you plan to interact with a patient who is at risk for violence or is acutely agitated.4 Teach staff how to recognize the nonverbal warning signs of behavior escalation and provide training on proper de-escalation and response.3,4 Also train staff on how to develop strong therapeutic alliances with patients.1 After a violent incident, use the postincident debriefing session to gather information that can be used to develop additional interventions and reduce the risk of subsequent violence.1
Implement common-sense strategies. Ensure that there are adequate numbers of nursing staff during each shift.1 Avoid overcrowded units, hallways, and common areas. Consider additional monitoring during unit transition times, such as during shift changes, meals, and medication administration.1 Avoid excessive noise.1 Employ one-to-one staff observation as clinically indicated.1 Avoid taking an authoritarian stance when explaining to patients why their requests have been denied4; if possible, when you are unable to meet a patient’s demands, offer them choices.1,4 If feasible, accompany patients to a calmer space where they can de-escalate.1 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.3 Install panic buttons at the nursing station and other areas (eg, restrooms).3
Ensure your personal safety. As mentioned previously, do not interact with a patient who has recently been aggressive or has voiced threats without adequate staff support.4 During the patient encounter, leave space between you and the patient.1 Avoid having your back to the exit of the room,3,4 and make sure the patient is not blocking the exit and that you can leave the room quickly if needed. Don’t wear anything that could be used as a weapon against you (eg, ties or necklaces) or could impede your escape.4 Avoid wearing valuables that might be damaged during a “take down.”4 If feasible, wear an audible alarm.3
1. Fisher K. Inpatient violence. Psychiatr Clin North Am. 2016;39(4):567-577.
2. Kraus JE, Sheitman BB. Characteristics of violent behavior in a large state psychiatric hospital. Psychiatr Serv. 2004;55(2):183-185.
3. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
4. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
5. Hardy DW, Patel M. Reduce inpatient violence: 6 strategies. Current Psychiatry. 2011;10(5):80-81.
Inpatient violence is a significant problem for psychiatric facilities because it can have serious physical and psychological consequences for both staff and patients.1 Victimized staff can experience decreased productivity and emotional distress, while victimized patients can experience disrupted treatment and delayed discharge.1 Twenty-five to 35% of psychiatric inpatients display violent behavior during their hospitalization.1 A subset are extreme offenders.1,2 This small group of violent patients accounts for the majority of inpatient violence and the most serious injuries.1,2
Reducing inpatient violence starts with conducting a targeted violence risk assessment to identify patients who are at elevated risk of being violent. Although conducting a targeted violence risk assessment is beyond the scope of this article, here I outline practical steps that clinicians can take to reduce the risk of inpatient violence. These steps complement and overlap with those I described in “Workplace violence: Enhance your safety in outpatient settings” (Pearls,
Identify underlying motives. Inpatient violence is often a result of 3 primary psychiatric etiologies: difficulty with impulse control, symptoms of psychosis, or predatory traits.1 Impulsivity drives most of the violence on inpatient units, followed by predatory violence and symptoms of psychosis.1 Once you identify the psychiatric motive, you can develop an individualized, tailored treatment plan to reduce the risk of violence. The treatment plan can include using de-escalation techniques, administering scheduled and as-needed medications to target underlying symptoms, having patients assume responsibility for their behaviors, holding patients accountable for their behaviors, and other psychosocial interventions.1 Use seclusion and restraint only when it is the least restrictive means of providing safety.1,4
Develop plans and policies. As you would do in an outpatient setting, assess for hazards within the inpatient unit. Plan for the possible types of violence that may occur on the unit (eg, physical violence against hospital personnel and/or other patients, verbal harassment, etc).3 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors (eg, posting a safety board where staff can record aggressive behaviors and other safety issues).3,4 When developing these plans and policies, include patients by creating patient/staff workgroups to develop expectations for civil behavior that apply to both patients and staff, as well as training patients to co-lead groups dealing with accepting responsibility for their own recovery.5 These plans and policies should include informing patients that threats and violence will not be tolerated. Frequently review these plans and policies with patients and staff.
Provide communication and education. Maintain strong psychiatric leadership on the unit that encourages open lines of communication. Encourage staff to promptly report incidents. Frequently ask staff if they have any safety concerns, and solicit their opinions on how to reduce risks.4 Include discussions about safety during staff and community meetings. Communicate patients’ behaviors that are distressing or undesired (eg, threats, harassment, etc) to all unit personnel.3 Notify staff when you plan to interact with a patient who is at risk for violence or is acutely agitated.4 Teach staff how to recognize the nonverbal warning signs of behavior escalation and provide training on proper de-escalation and response.3,4 Also train staff on how to develop strong therapeutic alliances with patients.1 After a violent incident, use the postincident debriefing session to gather information that can be used to develop additional interventions and reduce the risk of subsequent violence.1
Implement common-sense strategies. Ensure that there are adequate numbers of nursing staff during each shift.1 Avoid overcrowded units, hallways, and common areas. Consider additional monitoring during unit transition times, such as during shift changes, meals, and medication administration.1 Avoid excessive noise.1 Employ one-to-one staff observation as clinically indicated.1 Avoid taking an authoritarian stance when explaining to patients why their requests have been denied4; if possible, when you are unable to meet a patient’s demands, offer them choices.1,4 If feasible, accompany patients to a calmer space where they can de-escalate.1 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.3 Install panic buttons at the nursing station and other areas (eg, restrooms).3
Ensure your personal safety. As mentioned previously, do not interact with a patient who has recently been aggressive or has voiced threats without adequate staff support.4 During the patient encounter, leave space between you and the patient.1 Avoid having your back to the exit of the room,3,4 and make sure the patient is not blocking the exit and that you can leave the room quickly if needed. Don’t wear anything that could be used as a weapon against you (eg, ties or necklaces) or could impede your escape.4 Avoid wearing valuables that might be damaged during a “take down.”4 If feasible, wear an audible alarm.3
Inpatient violence is a significant problem for psychiatric facilities because it can have serious physical and psychological consequences for both staff and patients.1 Victimized staff can experience decreased productivity and emotional distress, while victimized patients can experience disrupted treatment and delayed discharge.1 Twenty-five to 35% of psychiatric inpatients display violent behavior during their hospitalization.1 A subset are extreme offenders.1,2 This small group of violent patients accounts for the majority of inpatient violence and the most serious injuries.1,2
Reducing inpatient violence starts with conducting a targeted violence risk assessment to identify patients who are at elevated risk of being violent. Although conducting a targeted violence risk assessment is beyond the scope of this article, here I outline practical steps that clinicians can take to reduce the risk of inpatient violence. These steps complement and overlap with those I described in “Workplace violence: Enhance your safety in outpatient settings” (Pearls,
Identify underlying motives. Inpatient violence is often a result of 3 primary psychiatric etiologies: difficulty with impulse control, symptoms of psychosis, or predatory traits.1 Impulsivity drives most of the violence on inpatient units, followed by predatory violence and symptoms of psychosis.1 Once you identify the psychiatric motive, you can develop an individualized, tailored treatment plan to reduce the risk of violence. The treatment plan can include using de-escalation techniques, administering scheduled and as-needed medications to target underlying symptoms, having patients assume responsibility for their behaviors, holding patients accountable for their behaviors, and other psychosocial interventions.1 Use seclusion and restraint only when it is the least restrictive means of providing safety.1,4
Develop plans and policies. As you would do in an outpatient setting, assess for hazards within the inpatient unit. Plan for the possible types of violence that may occur on the unit (eg, physical violence against hospital personnel and/or other patients, verbal harassment, etc).3 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors (eg, posting a safety board where staff can record aggressive behaviors and other safety issues).3,4 When developing these plans and policies, include patients by creating patient/staff workgroups to develop expectations for civil behavior that apply to both patients and staff, as well as training patients to co-lead groups dealing with accepting responsibility for their own recovery.5 These plans and policies should include informing patients that threats and violence will not be tolerated. Frequently review these plans and policies with patients and staff.
Provide communication and education. Maintain strong psychiatric leadership on the unit that encourages open lines of communication. Encourage staff to promptly report incidents. Frequently ask staff if they have any safety concerns, and solicit their opinions on how to reduce risks.4 Include discussions about safety during staff and community meetings. Communicate patients’ behaviors that are distressing or undesired (eg, threats, harassment, etc) to all unit personnel.3 Notify staff when you plan to interact with a patient who is at risk for violence or is acutely agitated.4 Teach staff how to recognize the nonverbal warning signs of behavior escalation and provide training on proper de-escalation and response.3,4 Also train staff on how to develop strong therapeutic alliances with patients.1 After a violent incident, use the postincident debriefing session to gather information that can be used to develop additional interventions and reduce the risk of subsequent violence.1
Implement common-sense strategies. Ensure that there are adequate numbers of nursing staff during each shift.1 Avoid overcrowded units, hallways, and common areas. Consider additional monitoring during unit transition times, such as during shift changes, meals, and medication administration.1 Avoid excessive noise.1 Employ one-to-one staff observation as clinically indicated.1 Avoid taking an authoritarian stance when explaining to patients why their requests have been denied4; if possible, when you are unable to meet a patient’s demands, offer them choices.1,4 If feasible, accompany patients to a calmer space where they can de-escalate.1 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.3 Install panic buttons at the nursing station and other areas (eg, restrooms).3
Ensure your personal safety. As mentioned previously, do not interact with a patient who has recently been aggressive or has voiced threats without adequate staff support.4 During the patient encounter, leave space between you and the patient.1 Avoid having your back to the exit of the room,3,4 and make sure the patient is not blocking the exit and that you can leave the room quickly if needed. Don’t wear anything that could be used as a weapon against you (eg, ties or necklaces) or could impede your escape.4 Avoid wearing valuables that might be damaged during a “take down.”4 If feasible, wear an audible alarm.3
1. Fisher K. Inpatient violence. Psychiatr Clin North Am. 2016;39(4):567-577.
2. Kraus JE, Sheitman BB. Characteristics of violent behavior in a large state psychiatric hospital. Psychiatr Serv. 2004;55(2):183-185.
3. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
4. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
5. Hardy DW, Patel M. Reduce inpatient violence: 6 strategies. Current Psychiatry. 2011;10(5):80-81.
1. Fisher K. Inpatient violence. Psychiatr Clin North Am. 2016;39(4):567-577.
2. Kraus JE, Sheitman BB. Characteristics of violent behavior in a large state psychiatric hospital. Psychiatr Serv. 2004;55(2):183-185.
3. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
4. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
5. Hardy DW, Patel M. Reduce inpatient violence: 6 strategies. Current Psychiatry. 2011;10(5):80-81.
Racial disparities in perinatal mental health care during COVID-19
Perinatal mental health disorders such as perinatal depression are common complications of pregnancy1 and cause significant disability in mothers and children.2 Yet despite facing higher 12-month rates of depression than White women,3 Black and Hispanic women are less likely than White women to be diagnosed with and receive treatment for postpartum depression.4
In addition to leading to >800,000 deaths in the United States alone (as of mid-December 2021),5 COVID-19 has disrupted health care delivery, including perinatal mental health services.6 Emerging data also describe neuropsychiatric effects of COVID-19 on both infected and uninfected individuals.7 Because Black and Hispanic individuals bear a disproportionate burden of COVID-19,8 compared to White women, women of color stand to be more adversely impacted by the direct effects of the disease as well as by related disruptions in perinatal psychiatry services.
Reasons for perinatal health disparities are multifactorial, complex, and interrelated. Disparities, which can be seen as proportionate differences in access by members of minority groups compared with groups in the majority, are related to differences in mental health screening, health care accessibility, and decisions to initiate treatment. In this commentary, we define “women of color” as non-White women, and focus on how traditional barriers to perinatal mental health treatment in women of color are exacerbated in the era of COVID-19. We focus primarily on postpartum depression because it is the peripartum mental health disorder with the highest likelihood of uptake in screening and treatment practices; however, disparities may be present in other mental health disorders during this period.
Gaps in screening and identification
Postpartum depression is a source of mitigatable risk for mother and neonate in the peripartum period, and the topic of screening for its presence arises in educational and best practices materials for primary care, OB-GYN, and pediatric care clinicians. Despite considerable evidence demonstrating better outcomes (for mother and child) with early detection and treatment of perinatal mental health disorders, racial and ethnic disparities persist in the screening process. At baseline, Black, Asian, and American Indian and Alaska Native women are less likely than White women to be screened for depression.9 Research shows that screening practices differ based on type of clinic, with one study noting that patients of family physicians were more likely to be screened for perinatal depression than were patients of OB-GYNs or nursing midwives.9 Even after adjusting for clinic type, racial differences in screening persist, with fewer women of color screened than their White counterparts.9 The literature consistently shows that within the same care settings, physicians deliver less information, less supportive talk, and less evidence-based treatment to Black and Hispanic patients and patients of lower economic status.10-12 Patient-clinician ethnic concordance is shown to positively impact the therapeutic relationship; at present, depressive symptoms are underrecognized in people of color, for whom referral to psychiatric care may be further compounded by inadequate knowledge of psychiatric resources.10-13
Data from Medicaid programs reveal that compared to White women, Black women are less likely to attend postpartum visits, which leads to a downstream effect on the ability to identify Black women with mental health disorders during the postpartum period.14 In addition to experiencing fewer opportunities for detection, women of color are more likely to report somatic symptoms of depression, which may not be detected in routinely employed perinatal depression screening tools.15
Continue to: Disparities in accessibility and treatment...
Disparities in accessibility and treatment
Black women are more likely to present in crisis and, hence, to acute care settings, which is likely related to disparities in screening and early detection.16,17 In a recent study investigating racial and ethnic differences in postpartum depression care, Chan et al16 found that Black women experience higher rates of hospital-based care compared with other racial groups. This study highlights the unavailability or inaccessibility of primary preventive measures to women in racial minority groups, which supports earlier studies that reported a correlation between access to care and severity of illness.16 Women in crisis may experience magnified disparities in access to high-quality care as they encounter institutional racism, potential loss of parental rights, and barriers due to insurance status.17,18 Furthermore, access to care for patients who are members of racial minority groups is limited in settings where culturally competent practices are absent or diminished, or discriminatory procedures are implicitly accepted and prevalent.12,19-22 The adverse impact of language constraints on accessibility of care is also well-documented, with recommendations such as ready access to interpreters to mitigate against miscommunications.23
Black and Hispanic women also experience significant delays between the time of delivery and treatment initiation.4 Studies of postpartum depression detection and treatment in specialty and primary care clinics show that, even when they desire treatment, women of color are less likely than White women to be offered treatment for postpartum depression.24 In terms of treatment options, research suggests women of color prefer psychotherapy over medication management.25,26 However, studies show that White women are more likely to be referred to psychotherapy.27 Research also reveals that Black and Hispanic women who are receptive to psychotropic medications have reduced rates of medication refills,4 which suggests that in these patients, counseling and monitoring adverse effects is suboptimal. In terms of treatment for substance use disorders (SUDs), after adjusting for maternal characteristics, Black and Hispanic women are significantly less likely to receive medication-assisted treatment (MAT) in pregnancy,28 and MAT is significantly less likely to be available in neighborhoods more densely populated by individuals of color.29,30
Several studies have explored possible explanations for discrepancies in treatment, including cultural expectations, differences in socioeconomic class, and racism. The stigma associated with psychiatric illness, misinformation about psychiatric treatments, and financial limitations have a substantial bearing on a patient’s willingness or ability to engage in psychiatric care.25 Regarding SUDs, a fear of legal reprisal is likely to deter women of color from seeking care.31 Such fears are not unfounded; research has demonstrated that interactions with Child Protective Services are increased among women of color compared to White women in similar situations.32
Furthermore, there is evidence that women of color receive less practical support, such as childcare, breastfeeding support, and transportation, during the postpartum period. Despite the preponderance of literature demonstrating the psychological benefits of breastfeeding,33,34 structural and psychosocial barriers appear to disproportionately affect breastfeeding rates in Hispanic, Black, American Indian, and Native women, with Black women experiencing the lowest rates of breastfeeding overall.35 Women in minority groups additionally experience disproportionate uncertainty about employment-based breastfeeding regulations.35,36 Specifically, many low-income jobs are not covered under the Family and Medical Leave Act, and compared to White women, Black women return to work on average 2 weeks earlier to jobs that are less welcoming to breastfeeding.35 In addition, insufficient education and support from health care settings and counselors play significant roles in disincentivizing women in minority groups from engaging in recommended breastfeeding and childcare practices.37,38
Continue to: COVID-19’s influence on these disparities...
COVID-19’s influence on these disparities
The COVID-19 pandemic has disproportionately impacted individuals of color. Black communities have experienced a higher rate of COVID-19 infection and a higher rate of death attributed to COVID-19, even after adjusting for age, poverty, medical comorbidities, and epidemic duration.39 The reasons for the disproportionate effects of the pandemic are complex and deeply ingrained in society.39 Emerging data indicate that COVID-19 might also lead to increased levels of psychological distress, anxiety, and depression in pregnant women33,40,41 and in Black women in particular.42 A survey of 913 pregnant women in Philadelphia conducted in May 2020 found significantly higher rates of anxiety and depression among Black women compared with White women, even after controlling for maternal age, gestational age, socioeconomic status, and marital status.42 A cross-sectional study of 163 women found that during the perinatal period, women of color were more likely than their White counterparts to experience negative changes in their mental health.43 These differences are concerning because pregnant women who experience high levels of stress during the pandemic are at high risk for preterm delivery and perinatal complications.44
Women of color may be disproportionately excluded by models of care that have become commonplace during the pandemic. Remote obstetric care became more common during the COVID-19 pandemic45; however, Black and Hispanic patients have been less likely than White patients to use telehealth services.46 Whether the differences are related to a lower likelihood of having a usual source of care, less access to digital resources, decreased awareness of the availability of telehealth, or less familiarity with digital technology, the common factor in all of the hypothesized reasons is structural racism.46 This is despite the fact that pregnant Black women report higher rates of concern than their White peers regarding the quality of their prenatal care during the pandemic.42 In a small study that surveyed 100 women about their preference for obstetric care, a significantly higher proportion of White women preferred virtual visits, with non-White women preferring in-person visits.47 Reasons cited for preferring virtual visits included convenience, safety with respect to viral transmission, compatibility with working from home, and less time waiting for the clinician; reasons cited for preferring in-person visits included a feeling of missing out on important parts of care, receiving less clinician attention, and having less of a connection with their clinician during virtual visits.47 Women of color have lower rates of perinatal depression screening than their White counterparts,9 and less frequent telehealth visits might lead to a further reduction in the detection and treatment of depression and other mental health conditions in this population.
Along with increasing telehealth services during the pandemic, many hospitals implemented stricter visitation policies for patients, including women giving birth, with the potential for greater detrimental impact on women of color. Before the pandemic, a survey of >2,500 women found that up to 10% of Black women reported experiencing racism during hospitalization for obstetrics-related care.48 These women also reported barriers to open and supportive communication with their clinicians.48 A recent study by Gur et al42 found that pregnant Black women reported more worries about the birthing experience during the pandemic than White women. In a setting with restricted visitors, all women are at risk for having a lonelier birth experience, but women of color who are already concerned about barriers to communication and racist care practices also must contend with their lived experience of systemic inequity, barriers to communication, and concerns about frank racism, without the support and potential advocacy they may usually rely upon to get them through medical experiences. Furthermore, pregnant women with mental illness are at greater risk for pregnancy complications. Together, these data suggest that women in minority groups who are pregnant and have mental illness are particularly vulnerable and are at greater risk without social support and advocacy during hospitalization.
The postpartum period is accompanied by unique concerns in terms of breastfeeding and social support for women of color. Women in minority groups had lower breastfeeding rates before the pandemic. Several studies looked at the impact of COVID-19 and associated restrictions on breastfeeding. In the United Kingdom, women in minority groups were more likely to stop breastfeeding due to the challenges of COVID-19–related restrictions.49 Compared with White women, these women were also more likely to report less practical support for breastfeeding during the pandemic.49 Other factors associated with low breastfeeding rates include lower levels of education and stressful living conditions.49 Though these factors were present before COVID-19, the pandemic has exacerbated these differences. Taken together, the evidence points to a role of long-standing structural and systemic inequity and racism in the health and wellbeing of women in minority groups.
A look towards solutions
Although perinatal mental health racial disparities predate the COVID-19 pandemic, differences in access to screening, identification, and treatment for mental health disorders place pregnant women of color and their children at heightened risk for poor health outcomes compared to their White counterparts during and after the pandemic. Despite the advent and progression of telehealth, existing race-based differences appear to have been maintained or exacerbated. The reasons for disparities are multifactorial and interrelated, and some of the outcomes perpetuate certain drivers of racism, which in turn drive continued inequity. Given the symptoms of depression, it is especially worrisome that clinicians may expect vulnerable women with illness-induced amotivation, anhedonia, and apathy to advocate for their own care.
Overall, the evidence confirms an imperative need—before, during, and after the COVID-19 pandemic—to provide education in mental health and cultural competency to clinicians such as obstetricians and pediatricians, who are more likely to have the first contact with women with perinatal depression. Health systems and government agencies also bear a responsibility to provide avenues for perinatal care clinicians to receive training and to increase access to culturally appropriate treatments through policy and structural changes.
Bottom Line
Racial disparities in perinatal mental health care persist despite widespread incorporation of telehealth into psychiatric services. Until causal factors are appropriately addressed through education, implementation, and structural changes, the benefits that have accompanied expanded psychiatric services via telehealth may only serve to exacerbate these differences.
1. Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86-92.
2. Slomian J, Honvo G, Emonts P, et al. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). 2019;15:174550651984404.
3. Kurz B, Hesselbrock M. Ethnic differences in mental health symptomatology and mental health care utilization among WIC mothers. Social Work in Mental Health. 2006;4(3):1-21.
4. Kozhimannil KB, Trinacty CM, Busch AB, et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011;62(6):619-625.
5. COVID-19 global cases. Coronavirus Resource Center for Systems Science and Engineering. Johns Hopkins University. Accessed December 10, 2021. https://coronavirus.jhu.edu/map.html
6. Gressier F, Mezzacappa A, Lasica PA, et al. COVID outbreak is changing our practices of perinatal psychiatry. Arch Womens Ment Health. 2020;23(6):791-792.
7. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
8. COVID-19: Data. NYC Health. Accessed February 3, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
9. Sidebottom A, Vacquier M, LaRusso E, et al. Perinatal depression screening practices in a large health system: identifying current state and assessing opportunities to provide more equitable care. Arch Womens Ment Health. 2021;24(1):133-144.
10. Ma A, Sanchez A, Ma M. The impact of patient-provider race/ethnicity concordance on provider visits: updated evidence from the medical expenditure panel survey. J Racial Ethn Health Disparities. 2019;6(5):1011-1020.
11. Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci USA. 2020;117(35):21194-21200.
12. Chaudron LH, Kitzman HJ, Peifer KL, et al. Self-recognition of and provider response to maternal depressive symptoms in low-income Hispanic women. J Womens Health (Larchmt). 2005;14(4):331-338.
13. Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. The National Academies Press; 2003. Accessed December 7, 2021. https://www.nap.edu/catalog/12875/unequal-treatment-confronting-racial-and-ethnic-disparities-in-health-care
14. Thiel de Bocanegra H, Braughton M, Bradsberry M, et al. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. Am J Obstet Gynecol. 2017;217(1):47.e1-47.e7.
15. Nadeem E, Lange JM, Miranda J. Perceived need for care among low-income immigrant and U.S.-born Black and Latina women with depression. J Womens Health (Larchmt). 2009;18(3):369-375.
16. Chan AL, Guo N, Popat R, et al. Racial and ethnic disparities in hospital-based care associated with postpartum depression. J Racial Ethn Health Disparities. 2021;8(1):220-229.
17. Kopelman R, Moel J, Mertens C, et al. Barriers to care for antenatal depression. Psychiatr Serv. 2008;59(4):429-432.
18. Kimerling R, Baumrind N. Access to specialty mental health services among women in California. Psychiatr Serv. 2005;56(6):729-734.
19. Ta Park V, Goyal D, Nguyen T, et al. Postpartum traditions, mental health, and help-seeking considerations among Vietnamese American women: a mixed-methods pilot study. J Behav Health Serv Res. 2017;44(3):428-441.
20. Chen F, Fryer GE Jr, Phillips RL Jr, et al. Patients’ beliefs about racism, preferences for physician race, and satisfaction with care. Ann Fam Med. 2005;3(2):138-143.
21. Holopainen D. The experience of seeking help for postnatal depression. Aust J Adv Nurs. 2002;19(3):39-44.
22. Alvidrez J, Azocar F. Distressed women’s clinic patients: preferences for mental health treatments and perceived obstacles. Gen Hosp Psychiatry. 1999;21(5):340-347.
23. Lara-Cinisomo S, Clark CT, Wood J. Increasing diagnosis and treatment of perinatal depression in Latinas and African American women: addressing stigma is not enough. Womens Health Issues. 2018;28(3):201-204.
24. Zittel-Palamara K, Rockmaker JR, Schwabel KM, et al. Desired assistance versus care received for postpartum depression: access to care differences by race. Arch Womens Ment Health. 2008;11(2):81-92.
25. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323-331.
26. Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African American, Hispanic, and white primary care patients. Med Care. 2003;41(4):479-489.
27. House TS, Alnajjar E, Mulekar M, et al. Mommy meltdown: understanding racial differences between black and white women in attitudes about postpartum depression and treatment modalities. J Clin Gynecol Obstet. 2020;9(3):37-42.
28. Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Netw Open. 2020;3(5):e205734.
29. Hansen H, Siegel C, Wanderling J, et al. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity, and race of neighborhoods in New York City. Drug Alcohol Depend. 2016;164:14-21.
30. Goedel WC, Shapiro A, Cerdá M, et al. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711.
31. Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Health Justice. 2015;3:2.
32. Roberts SC, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39(1):3-16.

33. Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(8):977-985.
34. Evans K, Labbok M, Abrahams SW. WIC and breastfeeding support services: does the mix of services offered vary with race and ethnicity? Breastfeed Med. 2011;6(6):401-406.
35. Jones KM, Power ML, Queenan JT, et al. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186-196.
36. Hohl S, Thompson B, Escareño M, et al. Cultural norms in conflict: breastfeeding among Hispanic immigrants in rural Washington state. Matern Child Health J. 2016;20(7):1549-1557.
37. McKinney CO, Hahn-Holbrook J, Chase-Lansdale PL, et al. Racial and ethnic differences in breastfeeding. Pediatrics. 2016;138(2):e20152388.
38. Louis-Jacques A, Deubel TF, Taylor M, et al. Racial and ethnic disparities in U.S. breastfeeding and implications for maternal and child health outcomes. Semin Perinatol. 2017;41(5):299-307.
39. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37-44.
40. Fan S, Guan J, Cao L, et al. Psychological effects caused by COVID-19 pandemic on pregnant women: a systematic review with meta-analysis. Asian J Psychiatr. 2021;56:102533.
41. Robinson GE, Benders-Hadi N, Conteh N, et al. Psychological impact of COVID-19 on pregnancy. J Nerv Ment Dis. 2021;209(6):396-397.
42. Gur RE, White LK, Waller R, et al. The disproportionate burden of the COVID-19 pandemic among pregnant Black women. Psychiatry Res. 2020;293:113475.
43. Masters GA, Asipenko E, Bergman AL, et al. Impact of the COVID-19 pandemic on mental health, access to care, and health disparities in the perinatal period. J Psychiatr Res. 2021;137:126-130.
44. Preis H, Mahaffey B, Pati S, et al. Adverse perinatal outcomes predicted by prenatal maternal stress among U.S. women at the COVID-19 pandemic onset. Ann Behav Med. 2021;55(3):179-191.
45. Fryer K, Delgado A, Foti T, et al. Implementation of obstetric telehealth during COVID-19 and beyond. Matern Child Health J. 2020;24(9):1104-1110.
46. Weber E, Miller SJ, Astha V, et al. Characteristics of telehealth users in NYC for COVID-related care during the coronavirus pandemic. J Am Med Inform Assoc. 2020;27(12):1949-1954.
47. Sullivan MW, Kanbergs AN, Burdette ER, et al. Acceptability of virtual prenatal care: thinking beyond the pandemic. J Matern Fetal Neonatal Med. 2021:1-4.
48. National Partnership for Women & Families. Listening to Black mothers in California. Issue Brief. September 2018. Accessed December 7, 2021. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-black-mothers-in-california.pdf
49. Brown A, Shenker N. Experiences of breastfeeding during COVID-19: lessons for future practical and emotional support. Matern Child Nutr. 2021;17(1):e13088.
Perinatal mental health disorders such as perinatal depression are common complications of pregnancy1 and cause significant disability in mothers and children.2 Yet despite facing higher 12-month rates of depression than White women,3 Black and Hispanic women are less likely than White women to be diagnosed with and receive treatment for postpartum depression.4
In addition to leading to >800,000 deaths in the United States alone (as of mid-December 2021),5 COVID-19 has disrupted health care delivery, including perinatal mental health services.6 Emerging data also describe neuropsychiatric effects of COVID-19 on both infected and uninfected individuals.7 Because Black and Hispanic individuals bear a disproportionate burden of COVID-19,8 compared to White women, women of color stand to be more adversely impacted by the direct effects of the disease as well as by related disruptions in perinatal psychiatry services.
Reasons for perinatal health disparities are multifactorial, complex, and interrelated. Disparities, which can be seen as proportionate differences in access by members of minority groups compared with groups in the majority, are related to differences in mental health screening, health care accessibility, and decisions to initiate treatment. In this commentary, we define “women of color” as non-White women, and focus on how traditional barriers to perinatal mental health treatment in women of color are exacerbated in the era of COVID-19. We focus primarily on postpartum depression because it is the peripartum mental health disorder with the highest likelihood of uptake in screening and treatment practices; however, disparities may be present in other mental health disorders during this period.
Gaps in screening and identification
Postpartum depression is a source of mitigatable risk for mother and neonate in the peripartum period, and the topic of screening for its presence arises in educational and best practices materials for primary care, OB-GYN, and pediatric care clinicians. Despite considerable evidence demonstrating better outcomes (for mother and child) with early detection and treatment of perinatal mental health disorders, racial and ethnic disparities persist in the screening process. At baseline, Black, Asian, and American Indian and Alaska Native women are less likely than White women to be screened for depression.9 Research shows that screening practices differ based on type of clinic, with one study noting that patients of family physicians were more likely to be screened for perinatal depression than were patients of OB-GYNs or nursing midwives.9 Even after adjusting for clinic type, racial differences in screening persist, with fewer women of color screened than their White counterparts.9 The literature consistently shows that within the same care settings, physicians deliver less information, less supportive talk, and less evidence-based treatment to Black and Hispanic patients and patients of lower economic status.10-12 Patient-clinician ethnic concordance is shown to positively impact the therapeutic relationship; at present, depressive symptoms are underrecognized in people of color, for whom referral to psychiatric care may be further compounded by inadequate knowledge of psychiatric resources.10-13
Data from Medicaid programs reveal that compared to White women, Black women are less likely to attend postpartum visits, which leads to a downstream effect on the ability to identify Black women with mental health disorders during the postpartum period.14 In addition to experiencing fewer opportunities for detection, women of color are more likely to report somatic symptoms of depression, which may not be detected in routinely employed perinatal depression screening tools.15
Continue to: Disparities in accessibility and treatment...
Disparities in accessibility and treatment
Black women are more likely to present in crisis and, hence, to acute care settings, which is likely related to disparities in screening and early detection.16,17 In a recent study investigating racial and ethnic differences in postpartum depression care, Chan et al16 found that Black women experience higher rates of hospital-based care compared with other racial groups. This study highlights the unavailability or inaccessibility of primary preventive measures to women in racial minority groups, which supports earlier studies that reported a correlation between access to care and severity of illness.16 Women in crisis may experience magnified disparities in access to high-quality care as they encounter institutional racism, potential loss of parental rights, and barriers due to insurance status.17,18 Furthermore, access to care for patients who are members of racial minority groups is limited in settings where culturally competent practices are absent or diminished, or discriminatory procedures are implicitly accepted and prevalent.12,19-22 The adverse impact of language constraints on accessibility of care is also well-documented, with recommendations such as ready access to interpreters to mitigate against miscommunications.23
Black and Hispanic women also experience significant delays between the time of delivery and treatment initiation.4 Studies of postpartum depression detection and treatment in specialty and primary care clinics show that, even when they desire treatment, women of color are less likely than White women to be offered treatment for postpartum depression.24 In terms of treatment options, research suggests women of color prefer psychotherapy over medication management.25,26 However, studies show that White women are more likely to be referred to psychotherapy.27 Research also reveals that Black and Hispanic women who are receptive to psychotropic medications have reduced rates of medication refills,4 which suggests that in these patients, counseling and monitoring adverse effects is suboptimal. In terms of treatment for substance use disorders (SUDs), after adjusting for maternal characteristics, Black and Hispanic women are significantly less likely to receive medication-assisted treatment (MAT) in pregnancy,28 and MAT is significantly less likely to be available in neighborhoods more densely populated by individuals of color.29,30
Several studies have explored possible explanations for discrepancies in treatment, including cultural expectations, differences in socioeconomic class, and racism. The stigma associated with psychiatric illness, misinformation about psychiatric treatments, and financial limitations have a substantial bearing on a patient’s willingness or ability to engage in psychiatric care.25 Regarding SUDs, a fear of legal reprisal is likely to deter women of color from seeking care.31 Such fears are not unfounded; research has demonstrated that interactions with Child Protective Services are increased among women of color compared to White women in similar situations.32
Furthermore, there is evidence that women of color receive less practical support, such as childcare, breastfeeding support, and transportation, during the postpartum period. Despite the preponderance of literature demonstrating the psychological benefits of breastfeeding,33,34 structural and psychosocial barriers appear to disproportionately affect breastfeeding rates in Hispanic, Black, American Indian, and Native women, with Black women experiencing the lowest rates of breastfeeding overall.35 Women in minority groups additionally experience disproportionate uncertainty about employment-based breastfeeding regulations.35,36 Specifically, many low-income jobs are not covered under the Family and Medical Leave Act, and compared to White women, Black women return to work on average 2 weeks earlier to jobs that are less welcoming to breastfeeding.35 In addition, insufficient education and support from health care settings and counselors play significant roles in disincentivizing women in minority groups from engaging in recommended breastfeeding and childcare practices.37,38
Continue to: COVID-19’s influence on these disparities...
COVID-19’s influence on these disparities
The COVID-19 pandemic has disproportionately impacted individuals of color. Black communities have experienced a higher rate of COVID-19 infection and a higher rate of death attributed to COVID-19, even after adjusting for age, poverty, medical comorbidities, and epidemic duration.39 The reasons for the disproportionate effects of the pandemic are complex and deeply ingrained in society.39 Emerging data indicate that COVID-19 might also lead to increased levels of psychological distress, anxiety, and depression in pregnant women33,40,41 and in Black women in particular.42 A survey of 913 pregnant women in Philadelphia conducted in May 2020 found significantly higher rates of anxiety and depression among Black women compared with White women, even after controlling for maternal age, gestational age, socioeconomic status, and marital status.42 A cross-sectional study of 163 women found that during the perinatal period, women of color were more likely than their White counterparts to experience negative changes in their mental health.43 These differences are concerning because pregnant women who experience high levels of stress during the pandemic are at high risk for preterm delivery and perinatal complications.44
Women of color may be disproportionately excluded by models of care that have become commonplace during the pandemic. Remote obstetric care became more common during the COVID-19 pandemic45; however, Black and Hispanic patients have been less likely than White patients to use telehealth services.46 Whether the differences are related to a lower likelihood of having a usual source of care, less access to digital resources, decreased awareness of the availability of telehealth, or less familiarity with digital technology, the common factor in all of the hypothesized reasons is structural racism.46 This is despite the fact that pregnant Black women report higher rates of concern than their White peers regarding the quality of their prenatal care during the pandemic.42 In a small study that surveyed 100 women about their preference for obstetric care, a significantly higher proportion of White women preferred virtual visits, with non-White women preferring in-person visits.47 Reasons cited for preferring virtual visits included convenience, safety with respect to viral transmission, compatibility with working from home, and less time waiting for the clinician; reasons cited for preferring in-person visits included a feeling of missing out on important parts of care, receiving less clinician attention, and having less of a connection with their clinician during virtual visits.47 Women of color have lower rates of perinatal depression screening than their White counterparts,9 and less frequent telehealth visits might lead to a further reduction in the detection and treatment of depression and other mental health conditions in this population.
Along with increasing telehealth services during the pandemic, many hospitals implemented stricter visitation policies for patients, including women giving birth, with the potential for greater detrimental impact on women of color. Before the pandemic, a survey of >2,500 women found that up to 10% of Black women reported experiencing racism during hospitalization for obstetrics-related care.48 These women also reported barriers to open and supportive communication with their clinicians.48 A recent study by Gur et al42 found that pregnant Black women reported more worries about the birthing experience during the pandemic than White women. In a setting with restricted visitors, all women are at risk for having a lonelier birth experience, but women of color who are already concerned about barriers to communication and racist care practices also must contend with their lived experience of systemic inequity, barriers to communication, and concerns about frank racism, without the support and potential advocacy they may usually rely upon to get them through medical experiences. Furthermore, pregnant women with mental illness are at greater risk for pregnancy complications. Together, these data suggest that women in minority groups who are pregnant and have mental illness are particularly vulnerable and are at greater risk without social support and advocacy during hospitalization.
The postpartum period is accompanied by unique concerns in terms of breastfeeding and social support for women of color. Women in minority groups had lower breastfeeding rates before the pandemic. Several studies looked at the impact of COVID-19 and associated restrictions on breastfeeding. In the United Kingdom, women in minority groups were more likely to stop breastfeeding due to the challenges of COVID-19–related restrictions.49 Compared with White women, these women were also more likely to report less practical support for breastfeeding during the pandemic.49 Other factors associated with low breastfeeding rates include lower levels of education and stressful living conditions.49 Though these factors were present before COVID-19, the pandemic has exacerbated these differences. Taken together, the evidence points to a role of long-standing structural and systemic inequity and racism in the health and wellbeing of women in minority groups.
A look towards solutions
Although perinatal mental health racial disparities predate the COVID-19 pandemic, differences in access to screening, identification, and treatment for mental health disorders place pregnant women of color and their children at heightened risk for poor health outcomes compared to their White counterparts during and after the pandemic. Despite the advent and progression of telehealth, existing race-based differences appear to have been maintained or exacerbated. The reasons for disparities are multifactorial and interrelated, and some of the outcomes perpetuate certain drivers of racism, which in turn drive continued inequity. Given the symptoms of depression, it is especially worrisome that clinicians may expect vulnerable women with illness-induced amotivation, anhedonia, and apathy to advocate for their own care.
Overall, the evidence confirms an imperative need—before, during, and after the COVID-19 pandemic—to provide education in mental health and cultural competency to clinicians such as obstetricians and pediatricians, who are more likely to have the first contact with women with perinatal depression. Health systems and government agencies also bear a responsibility to provide avenues for perinatal care clinicians to receive training and to increase access to culturally appropriate treatments through policy and structural changes.
Bottom Line
Racial disparities in perinatal mental health care persist despite widespread incorporation of telehealth into psychiatric services. Until causal factors are appropriately addressed through education, implementation, and structural changes, the benefits that have accompanied expanded psychiatric services via telehealth may only serve to exacerbate these differences.
Perinatal mental health disorders such as perinatal depression are common complications of pregnancy1 and cause significant disability in mothers and children.2 Yet despite facing higher 12-month rates of depression than White women,3 Black and Hispanic women are less likely than White women to be diagnosed with and receive treatment for postpartum depression.4
In addition to leading to >800,000 deaths in the United States alone (as of mid-December 2021),5 COVID-19 has disrupted health care delivery, including perinatal mental health services.6 Emerging data also describe neuropsychiatric effects of COVID-19 on both infected and uninfected individuals.7 Because Black and Hispanic individuals bear a disproportionate burden of COVID-19,8 compared to White women, women of color stand to be more adversely impacted by the direct effects of the disease as well as by related disruptions in perinatal psychiatry services.
Reasons for perinatal health disparities are multifactorial, complex, and interrelated. Disparities, which can be seen as proportionate differences in access by members of minority groups compared with groups in the majority, are related to differences in mental health screening, health care accessibility, and decisions to initiate treatment. In this commentary, we define “women of color” as non-White women, and focus on how traditional barriers to perinatal mental health treatment in women of color are exacerbated in the era of COVID-19. We focus primarily on postpartum depression because it is the peripartum mental health disorder with the highest likelihood of uptake in screening and treatment practices; however, disparities may be present in other mental health disorders during this period.
Gaps in screening and identification
Postpartum depression is a source of mitigatable risk for mother and neonate in the peripartum period, and the topic of screening for its presence arises in educational and best practices materials for primary care, OB-GYN, and pediatric care clinicians. Despite considerable evidence demonstrating better outcomes (for mother and child) with early detection and treatment of perinatal mental health disorders, racial and ethnic disparities persist in the screening process. At baseline, Black, Asian, and American Indian and Alaska Native women are less likely than White women to be screened for depression.9 Research shows that screening practices differ based on type of clinic, with one study noting that patients of family physicians were more likely to be screened for perinatal depression than were patients of OB-GYNs or nursing midwives.9 Even after adjusting for clinic type, racial differences in screening persist, with fewer women of color screened than their White counterparts.9 The literature consistently shows that within the same care settings, physicians deliver less information, less supportive talk, and less evidence-based treatment to Black and Hispanic patients and patients of lower economic status.10-12 Patient-clinician ethnic concordance is shown to positively impact the therapeutic relationship; at present, depressive symptoms are underrecognized in people of color, for whom referral to psychiatric care may be further compounded by inadequate knowledge of psychiatric resources.10-13
Data from Medicaid programs reveal that compared to White women, Black women are less likely to attend postpartum visits, which leads to a downstream effect on the ability to identify Black women with mental health disorders during the postpartum period.14 In addition to experiencing fewer opportunities for detection, women of color are more likely to report somatic symptoms of depression, which may not be detected in routinely employed perinatal depression screening tools.15
Continue to: Disparities in accessibility and treatment...
Disparities in accessibility and treatment
Black women are more likely to present in crisis and, hence, to acute care settings, which is likely related to disparities in screening and early detection.16,17 In a recent study investigating racial and ethnic differences in postpartum depression care, Chan et al16 found that Black women experience higher rates of hospital-based care compared with other racial groups. This study highlights the unavailability or inaccessibility of primary preventive measures to women in racial minority groups, which supports earlier studies that reported a correlation between access to care and severity of illness.16 Women in crisis may experience magnified disparities in access to high-quality care as they encounter institutional racism, potential loss of parental rights, and barriers due to insurance status.17,18 Furthermore, access to care for patients who are members of racial minority groups is limited in settings where culturally competent practices are absent or diminished, or discriminatory procedures are implicitly accepted and prevalent.12,19-22 The adverse impact of language constraints on accessibility of care is also well-documented, with recommendations such as ready access to interpreters to mitigate against miscommunications.23
Black and Hispanic women also experience significant delays between the time of delivery and treatment initiation.4 Studies of postpartum depression detection and treatment in specialty and primary care clinics show that, even when they desire treatment, women of color are less likely than White women to be offered treatment for postpartum depression.24 In terms of treatment options, research suggests women of color prefer psychotherapy over medication management.25,26 However, studies show that White women are more likely to be referred to psychotherapy.27 Research also reveals that Black and Hispanic women who are receptive to psychotropic medications have reduced rates of medication refills,4 which suggests that in these patients, counseling and monitoring adverse effects is suboptimal. In terms of treatment for substance use disorders (SUDs), after adjusting for maternal characteristics, Black and Hispanic women are significantly less likely to receive medication-assisted treatment (MAT) in pregnancy,28 and MAT is significantly less likely to be available in neighborhoods more densely populated by individuals of color.29,30
Several studies have explored possible explanations for discrepancies in treatment, including cultural expectations, differences in socioeconomic class, and racism. The stigma associated with psychiatric illness, misinformation about psychiatric treatments, and financial limitations have a substantial bearing on a patient’s willingness or ability to engage in psychiatric care.25 Regarding SUDs, a fear of legal reprisal is likely to deter women of color from seeking care.31 Such fears are not unfounded; research has demonstrated that interactions with Child Protective Services are increased among women of color compared to White women in similar situations.32
Furthermore, there is evidence that women of color receive less practical support, such as childcare, breastfeeding support, and transportation, during the postpartum period. Despite the preponderance of literature demonstrating the psychological benefits of breastfeeding,33,34 structural and psychosocial barriers appear to disproportionately affect breastfeeding rates in Hispanic, Black, American Indian, and Native women, with Black women experiencing the lowest rates of breastfeeding overall.35 Women in minority groups additionally experience disproportionate uncertainty about employment-based breastfeeding regulations.35,36 Specifically, many low-income jobs are not covered under the Family and Medical Leave Act, and compared to White women, Black women return to work on average 2 weeks earlier to jobs that are less welcoming to breastfeeding.35 In addition, insufficient education and support from health care settings and counselors play significant roles in disincentivizing women in minority groups from engaging in recommended breastfeeding and childcare practices.37,38
Continue to: COVID-19’s influence on these disparities...
COVID-19’s influence on these disparities
The COVID-19 pandemic has disproportionately impacted individuals of color. Black communities have experienced a higher rate of COVID-19 infection and a higher rate of death attributed to COVID-19, even after adjusting for age, poverty, medical comorbidities, and epidemic duration.39 The reasons for the disproportionate effects of the pandemic are complex and deeply ingrained in society.39 Emerging data indicate that COVID-19 might also lead to increased levels of psychological distress, anxiety, and depression in pregnant women33,40,41 and in Black women in particular.42 A survey of 913 pregnant women in Philadelphia conducted in May 2020 found significantly higher rates of anxiety and depression among Black women compared with White women, even after controlling for maternal age, gestational age, socioeconomic status, and marital status.42 A cross-sectional study of 163 women found that during the perinatal period, women of color were more likely than their White counterparts to experience negative changes in their mental health.43 These differences are concerning because pregnant women who experience high levels of stress during the pandemic are at high risk for preterm delivery and perinatal complications.44
Women of color may be disproportionately excluded by models of care that have become commonplace during the pandemic. Remote obstetric care became more common during the COVID-19 pandemic45; however, Black and Hispanic patients have been less likely than White patients to use telehealth services.46 Whether the differences are related to a lower likelihood of having a usual source of care, less access to digital resources, decreased awareness of the availability of telehealth, or less familiarity with digital technology, the common factor in all of the hypothesized reasons is structural racism.46 This is despite the fact that pregnant Black women report higher rates of concern than their White peers regarding the quality of their prenatal care during the pandemic.42 In a small study that surveyed 100 women about their preference for obstetric care, a significantly higher proportion of White women preferred virtual visits, with non-White women preferring in-person visits.47 Reasons cited for preferring virtual visits included convenience, safety with respect to viral transmission, compatibility with working from home, and less time waiting for the clinician; reasons cited for preferring in-person visits included a feeling of missing out on important parts of care, receiving less clinician attention, and having less of a connection with their clinician during virtual visits.47 Women of color have lower rates of perinatal depression screening than their White counterparts,9 and less frequent telehealth visits might lead to a further reduction in the detection and treatment of depression and other mental health conditions in this population.
Along with increasing telehealth services during the pandemic, many hospitals implemented stricter visitation policies for patients, including women giving birth, with the potential for greater detrimental impact on women of color. Before the pandemic, a survey of >2,500 women found that up to 10% of Black women reported experiencing racism during hospitalization for obstetrics-related care.48 These women also reported barriers to open and supportive communication with their clinicians.48 A recent study by Gur et al42 found that pregnant Black women reported more worries about the birthing experience during the pandemic than White women. In a setting with restricted visitors, all women are at risk for having a lonelier birth experience, but women of color who are already concerned about barriers to communication and racist care practices also must contend with their lived experience of systemic inequity, barriers to communication, and concerns about frank racism, without the support and potential advocacy they may usually rely upon to get them through medical experiences. Furthermore, pregnant women with mental illness are at greater risk for pregnancy complications. Together, these data suggest that women in minority groups who are pregnant and have mental illness are particularly vulnerable and are at greater risk without social support and advocacy during hospitalization.
The postpartum period is accompanied by unique concerns in terms of breastfeeding and social support for women of color. Women in minority groups had lower breastfeeding rates before the pandemic. Several studies looked at the impact of COVID-19 and associated restrictions on breastfeeding. In the United Kingdom, women in minority groups were more likely to stop breastfeeding due to the challenges of COVID-19–related restrictions.49 Compared with White women, these women were also more likely to report less practical support for breastfeeding during the pandemic.49 Other factors associated with low breastfeeding rates include lower levels of education and stressful living conditions.49 Though these factors were present before COVID-19, the pandemic has exacerbated these differences. Taken together, the evidence points to a role of long-standing structural and systemic inequity and racism in the health and wellbeing of women in minority groups.
A look towards solutions
Although perinatal mental health racial disparities predate the COVID-19 pandemic, differences in access to screening, identification, and treatment for mental health disorders place pregnant women of color and their children at heightened risk for poor health outcomes compared to their White counterparts during and after the pandemic. Despite the advent and progression of telehealth, existing race-based differences appear to have been maintained or exacerbated. The reasons for disparities are multifactorial and interrelated, and some of the outcomes perpetuate certain drivers of racism, which in turn drive continued inequity. Given the symptoms of depression, it is especially worrisome that clinicians may expect vulnerable women with illness-induced amotivation, anhedonia, and apathy to advocate for their own care.
Overall, the evidence confirms an imperative need—before, during, and after the COVID-19 pandemic—to provide education in mental health and cultural competency to clinicians such as obstetricians and pediatricians, who are more likely to have the first contact with women with perinatal depression. Health systems and government agencies also bear a responsibility to provide avenues for perinatal care clinicians to receive training and to increase access to culturally appropriate treatments through policy and structural changes.
Bottom Line
Racial disparities in perinatal mental health care persist despite widespread incorporation of telehealth into psychiatric services. Until causal factors are appropriately addressed through education, implementation, and structural changes, the benefits that have accompanied expanded psychiatric services via telehealth may only serve to exacerbate these differences.
1. Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86-92.
2. Slomian J, Honvo G, Emonts P, et al. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). 2019;15:174550651984404.
3. Kurz B, Hesselbrock M. Ethnic differences in mental health symptomatology and mental health care utilization among WIC mothers. Social Work in Mental Health. 2006;4(3):1-21.
4. Kozhimannil KB, Trinacty CM, Busch AB, et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011;62(6):619-625.
5. COVID-19 global cases. Coronavirus Resource Center for Systems Science and Engineering. Johns Hopkins University. Accessed December 10, 2021. https://coronavirus.jhu.edu/map.html
6. Gressier F, Mezzacappa A, Lasica PA, et al. COVID outbreak is changing our practices of perinatal psychiatry. Arch Womens Ment Health. 2020;23(6):791-792.
7. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
8. COVID-19: Data. NYC Health. Accessed February 3, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
9. Sidebottom A, Vacquier M, LaRusso E, et al. Perinatal depression screening practices in a large health system: identifying current state and assessing opportunities to provide more equitable care. Arch Womens Ment Health. 2021;24(1):133-144.
10. Ma A, Sanchez A, Ma M. The impact of patient-provider race/ethnicity concordance on provider visits: updated evidence from the medical expenditure panel survey. J Racial Ethn Health Disparities. 2019;6(5):1011-1020.
11. Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci USA. 2020;117(35):21194-21200.
12. Chaudron LH, Kitzman HJ, Peifer KL, et al. Self-recognition of and provider response to maternal depressive symptoms in low-income Hispanic women. J Womens Health (Larchmt). 2005;14(4):331-338.
13. Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. The National Academies Press; 2003. Accessed December 7, 2021. https://www.nap.edu/catalog/12875/unequal-treatment-confronting-racial-and-ethnic-disparities-in-health-care
14. Thiel de Bocanegra H, Braughton M, Bradsberry M, et al. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. Am J Obstet Gynecol. 2017;217(1):47.e1-47.e7.
15. Nadeem E, Lange JM, Miranda J. Perceived need for care among low-income immigrant and U.S.-born Black and Latina women with depression. J Womens Health (Larchmt). 2009;18(3):369-375.
16. Chan AL, Guo N, Popat R, et al. Racial and ethnic disparities in hospital-based care associated with postpartum depression. J Racial Ethn Health Disparities. 2021;8(1):220-229.
17. Kopelman R, Moel J, Mertens C, et al. Barriers to care for antenatal depression. Psychiatr Serv. 2008;59(4):429-432.
18. Kimerling R, Baumrind N. Access to specialty mental health services among women in California. Psychiatr Serv. 2005;56(6):729-734.
19. Ta Park V, Goyal D, Nguyen T, et al. Postpartum traditions, mental health, and help-seeking considerations among Vietnamese American women: a mixed-methods pilot study. J Behav Health Serv Res. 2017;44(3):428-441.
20. Chen F, Fryer GE Jr, Phillips RL Jr, et al. Patients’ beliefs about racism, preferences for physician race, and satisfaction with care. Ann Fam Med. 2005;3(2):138-143.
21. Holopainen D. The experience of seeking help for postnatal depression. Aust J Adv Nurs. 2002;19(3):39-44.
22. Alvidrez J, Azocar F. Distressed women’s clinic patients: preferences for mental health treatments and perceived obstacles. Gen Hosp Psychiatry. 1999;21(5):340-347.
23. Lara-Cinisomo S, Clark CT, Wood J. Increasing diagnosis and treatment of perinatal depression in Latinas and African American women: addressing stigma is not enough. Womens Health Issues. 2018;28(3):201-204.
24. Zittel-Palamara K, Rockmaker JR, Schwabel KM, et al. Desired assistance versus care received for postpartum depression: access to care differences by race. Arch Womens Ment Health. 2008;11(2):81-92.
25. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323-331.
26. Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African American, Hispanic, and white primary care patients. Med Care. 2003;41(4):479-489.
27. House TS, Alnajjar E, Mulekar M, et al. Mommy meltdown: understanding racial differences between black and white women in attitudes about postpartum depression and treatment modalities. J Clin Gynecol Obstet. 2020;9(3):37-42.
28. Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Netw Open. 2020;3(5):e205734.
29. Hansen H, Siegel C, Wanderling J, et al. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity, and race of neighborhoods in New York City. Drug Alcohol Depend. 2016;164:14-21.
30. Goedel WC, Shapiro A, Cerdá M, et al. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711.
31. Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Health Justice. 2015;3:2.
32. Roberts SC, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39(1):3-16.

33. Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(8):977-985.
34. Evans K, Labbok M, Abrahams SW. WIC and breastfeeding support services: does the mix of services offered vary with race and ethnicity? Breastfeed Med. 2011;6(6):401-406.
35. Jones KM, Power ML, Queenan JT, et al. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186-196.
36. Hohl S, Thompson B, Escareño M, et al. Cultural norms in conflict: breastfeeding among Hispanic immigrants in rural Washington state. Matern Child Health J. 2016;20(7):1549-1557.
37. McKinney CO, Hahn-Holbrook J, Chase-Lansdale PL, et al. Racial and ethnic differences in breastfeeding. Pediatrics. 2016;138(2):e20152388.
38. Louis-Jacques A, Deubel TF, Taylor M, et al. Racial and ethnic disparities in U.S. breastfeeding and implications for maternal and child health outcomes. Semin Perinatol. 2017;41(5):299-307.
39. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37-44.
40. Fan S, Guan J, Cao L, et al. Psychological effects caused by COVID-19 pandemic on pregnant women: a systematic review with meta-analysis. Asian J Psychiatr. 2021;56:102533.
41. Robinson GE, Benders-Hadi N, Conteh N, et al. Psychological impact of COVID-19 on pregnancy. J Nerv Ment Dis. 2021;209(6):396-397.
42. Gur RE, White LK, Waller R, et al. The disproportionate burden of the COVID-19 pandemic among pregnant Black women. Psychiatry Res. 2020;293:113475.
43. Masters GA, Asipenko E, Bergman AL, et al. Impact of the COVID-19 pandemic on mental health, access to care, and health disparities in the perinatal period. J Psychiatr Res. 2021;137:126-130.
44. Preis H, Mahaffey B, Pati S, et al. Adverse perinatal outcomes predicted by prenatal maternal stress among U.S. women at the COVID-19 pandemic onset. Ann Behav Med. 2021;55(3):179-191.
45. Fryer K, Delgado A, Foti T, et al. Implementation of obstetric telehealth during COVID-19 and beyond. Matern Child Health J. 2020;24(9):1104-1110.
46. Weber E, Miller SJ, Astha V, et al. Characteristics of telehealth users in NYC for COVID-related care during the coronavirus pandemic. J Am Med Inform Assoc. 2020;27(12):1949-1954.
47. Sullivan MW, Kanbergs AN, Burdette ER, et al. Acceptability of virtual prenatal care: thinking beyond the pandemic. J Matern Fetal Neonatal Med. 2021:1-4.
48. National Partnership for Women & Families. Listening to Black mothers in California. Issue Brief. September 2018. Accessed December 7, 2021. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-black-mothers-in-california.pdf
49. Brown A, Shenker N. Experiences of breastfeeding during COVID-19: lessons for future practical and emotional support. Matern Child Nutr. 2021;17(1):e13088.
1. Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86-92.
2. Slomian J, Honvo G, Emonts P, et al. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). 2019;15:174550651984404.
3. Kurz B, Hesselbrock M. Ethnic differences in mental health symptomatology and mental health care utilization among WIC mothers. Social Work in Mental Health. 2006;4(3):1-21.
4. Kozhimannil KB, Trinacty CM, Busch AB, et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011;62(6):619-625.
5. COVID-19 global cases. Coronavirus Resource Center for Systems Science and Engineering. Johns Hopkins University. Accessed December 10, 2021. https://coronavirus.jhu.edu/map.html
6. Gressier F, Mezzacappa A, Lasica PA, et al. COVID outbreak is changing our practices of perinatal psychiatry. Arch Womens Ment Health. 2020;23(6):791-792.
7. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
8. COVID-19: Data. NYC Health. Accessed February 3, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
9. Sidebottom A, Vacquier M, LaRusso E, et al. Perinatal depression screening practices in a large health system: identifying current state and assessing opportunities to provide more equitable care. Arch Womens Ment Health. 2021;24(1):133-144.
10. Ma A, Sanchez A, Ma M. The impact of patient-provider race/ethnicity concordance on provider visits: updated evidence from the medical expenditure panel survey. J Racial Ethn Health Disparities. 2019;6(5):1011-1020.
11. Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci USA. 2020;117(35):21194-21200.
12. Chaudron LH, Kitzman HJ, Peifer KL, et al. Self-recognition of and provider response to maternal depressive symptoms in low-income Hispanic women. J Womens Health (Larchmt). 2005;14(4):331-338.
13. Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. The National Academies Press; 2003. Accessed December 7, 2021. https://www.nap.edu/catalog/12875/unequal-treatment-confronting-racial-and-ethnic-disparities-in-health-care
14. Thiel de Bocanegra H, Braughton M, Bradsberry M, et al. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. Am J Obstet Gynecol. 2017;217(1):47.e1-47.e7.
15. Nadeem E, Lange JM, Miranda J. Perceived need for care among low-income immigrant and U.S.-born Black and Latina women with depression. J Womens Health (Larchmt). 2009;18(3):369-375.
16. Chan AL, Guo N, Popat R, et al. Racial and ethnic disparities in hospital-based care associated with postpartum depression. J Racial Ethn Health Disparities. 2021;8(1):220-229.
17. Kopelman R, Moel J, Mertens C, et al. Barriers to care for antenatal depression. Psychiatr Serv. 2008;59(4):429-432.
18. Kimerling R, Baumrind N. Access to specialty mental health services among women in California. Psychiatr Serv. 2005;56(6):729-734.
19. Ta Park V, Goyal D, Nguyen T, et al. Postpartum traditions, mental health, and help-seeking considerations among Vietnamese American women: a mixed-methods pilot study. J Behav Health Serv Res. 2017;44(3):428-441.
20. Chen F, Fryer GE Jr, Phillips RL Jr, et al. Patients’ beliefs about racism, preferences for physician race, and satisfaction with care. Ann Fam Med. 2005;3(2):138-143.
21. Holopainen D. The experience of seeking help for postnatal depression. Aust J Adv Nurs. 2002;19(3):39-44.
22. Alvidrez J, Azocar F. Distressed women’s clinic patients: preferences for mental health treatments and perceived obstacles. Gen Hosp Psychiatry. 1999;21(5):340-347.
23. Lara-Cinisomo S, Clark CT, Wood J. Increasing diagnosis and treatment of perinatal depression in Latinas and African American women: addressing stigma is not enough. Womens Health Issues. 2018;28(3):201-204.
24. Zittel-Palamara K, Rockmaker JR, Schwabel KM, et al. Desired assistance versus care received for postpartum depression: access to care differences by race. Arch Womens Ment Health. 2008;11(2):81-92.
25. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323-331.
26. Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African American, Hispanic, and white primary care patients. Med Care. 2003;41(4):479-489.
27. House TS, Alnajjar E, Mulekar M, et al. Mommy meltdown: understanding racial differences between black and white women in attitudes about postpartum depression and treatment modalities. J Clin Gynecol Obstet. 2020;9(3):37-42.
28. Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Netw Open. 2020;3(5):e205734.
29. Hansen H, Siegel C, Wanderling J, et al. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity, and race of neighborhoods in New York City. Drug Alcohol Depend. 2016;164:14-21.
30. Goedel WC, Shapiro A, Cerdá M, et al. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711.
31. Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Health Justice. 2015;3:2.
32. Roberts SC, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39(1):3-16.

33. Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(8):977-985.
34. Evans K, Labbok M, Abrahams SW. WIC and breastfeeding support services: does the mix of services offered vary with race and ethnicity? Breastfeed Med. 2011;6(6):401-406.
35. Jones KM, Power ML, Queenan JT, et al. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186-196.
36. Hohl S, Thompson B, Escareño M, et al. Cultural norms in conflict: breastfeeding among Hispanic immigrants in rural Washington state. Matern Child Health J. 2016;20(7):1549-1557.
37. McKinney CO, Hahn-Holbrook J, Chase-Lansdale PL, et al. Racial and ethnic differences in breastfeeding. Pediatrics. 2016;138(2):e20152388.
38. Louis-Jacques A, Deubel TF, Taylor M, et al. Racial and ethnic disparities in U.S. breastfeeding and implications for maternal and child health outcomes. Semin Perinatol. 2017;41(5):299-307.
39. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37-44.
40. Fan S, Guan J, Cao L, et al. Psychological effects caused by COVID-19 pandemic on pregnant women: a systematic review with meta-analysis. Asian J Psychiatr. 2021;56:102533.
41. Robinson GE, Benders-Hadi N, Conteh N, et al. Psychological impact of COVID-19 on pregnancy. J Nerv Ment Dis. 2021;209(6):396-397.
42. Gur RE, White LK, Waller R, et al. The disproportionate burden of the COVID-19 pandemic among pregnant Black women. Psychiatry Res. 2020;293:113475.
43. Masters GA, Asipenko E, Bergman AL, et al. Impact of the COVID-19 pandemic on mental health, access to care, and health disparities in the perinatal period. J Psychiatr Res. 2021;137:126-130.
44. Preis H, Mahaffey B, Pati S, et al. Adverse perinatal outcomes predicted by prenatal maternal stress among U.S. women at the COVID-19 pandemic onset. Ann Behav Med. 2021;55(3):179-191.
45. Fryer K, Delgado A, Foti T, et al. Implementation of obstetric telehealth during COVID-19 and beyond. Matern Child Health J. 2020;24(9):1104-1110.
46. Weber E, Miller SJ, Astha V, et al. Characteristics of telehealth users in NYC for COVID-related care during the coronavirus pandemic. J Am Med Inform Assoc. 2020;27(12):1949-1954.
47. Sullivan MW, Kanbergs AN, Burdette ER, et al. Acceptability of virtual prenatal care: thinking beyond the pandemic. J Matern Fetal Neonatal Med. 2021:1-4.
48. National Partnership for Women & Families. Listening to Black mothers in California. Issue Brief. September 2018. Accessed December 7, 2021. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-black-mothers-in-california.pdf
49. Brown A, Shenker N. Experiences of breastfeeding during COVID-19: lessons for future practical and emotional support. Matern Child Nutr. 2021;17(1):e13088.
Severe GI distress: Is clozapine to blame?
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
CASE GI distress while taking clozapine
Mr. F, age 29, has a history of psychiatric hospitalizations for psychotic episodes. It took a herculean effort to get him to agree to try clozapine, to which he has experienced a modest to good response. Unfortunately, recently he has been experiencing significant upper gastrointestinal (GI) distress. He attributes this to clozapine, and asks if he can discontinue this medication.
HISTORY Nausea becomes severe
Mr. F, age 29, resides in a long-term residential setting for patients with serious mental illness who need additional support following acute hospitalization. He has treatment-refractory schizophrenia. He first developed symptoms at age 18, and experienced multiple psychotic episodes requiring psychiatric hospitalizations that lasted for months. He has had numerous antipsychotic trials and a course of electroconvulsive therapy, with limited benefit.
More recently, Mr. F’s symptoms began to stabilize on a medication regimen that includes clozapine, 350 mg/d at bedtime, and haloperidol, 2 mg/d. He has not required psychiatric hospitalization for the past year.
Within months of initiating clozapine, Mr. F starts to complain daily about symptoms of worsening abdominal pain, abdominal bloating, nausea, intermittent episodes of emesis, and heartburn. The symptoms begin when he wakes up, are worse in the morning, and persist throughout the morning. He has experienced occasional mild constipation, but no diarrhea or weight loss. There have been no major changes in his diet, addition of new medications, or significant use of nonsteroidal anti-inflammatory drugs.
Mr. F’s nausea worsens over the next several weeks, to the point he begins to significantly limit how much he eats to cope with it. His GI symptoms are also impacting his mood and daily functioning.
This is not Mr. F’s first experience with significant GI distress. A few months before his first psychotic episode, Mr. F began developing vision problems, joint and abdominal pain, and a general decline in social and academic functioning. At that time, he underwent a significant workup by both GI and integrative medicine, including stool testing, upper endoscopy, and a Cyrex panel (a complementary medicine approach to exploring for specific autoimmune conditions). Results were largely within expected parameters, though a hydrogen breath test was suggestive of possible small intestine bowel overgrowth. More recently, he has been adhering to a gluten-free diet, which his family felt may help prevent some of his physical symptoms as well as mitigate some of his psychotic symptoms. He now asks if he can stop taking clozapine.
[polldaddy:11008393]
EVALUATION Establishing the correct diagnosis
Initially, Mr. F is diagnosed with gastroesophageal reflux disease (GERD) and attempts to manage his symptoms with pharmacologic and diet-based interventions. He significantly cuts down on soda consumption, and undergoes trials of calcium carbonate, antiemetics, and a PPI. Unfortunately, no material improvements are noted, and he continued to experience significant upper GI distress, especially after meals.
The psychiatric treatment team, Mr. F, and his family seek consultation with a GI specialist, who recommends that Mr. F. undergo a nuclear medicine solid gastric emptying scintigraphy study to evaluate for gastroparesis (delayed gastric emptying).1 Results demonstrate grade 3 gastroparesis, with 56% radiotracer retainment at 4 hours. Mr. F is relieved to finally have an explanation for his persistent GI symptoms, and discusses his treatment options with the GI consultant and psychiatry team.
Continue to: The authors’ observations...
The authors’ observations
Mr. F and his family are opposed to starting a dopamine antagonist such as metoclopramide or domperidone (the latter is not FDA-approved but is available by special application to the FDA). These are first-line treatments for gastroparesis, but Mr. F and his family do not want them because of the risk of tardive dyskinesia. This is consistent with their previously expressed concerns regarding first-generation antipsychotics, and is why Mr. F has only been treated with a very low dose of haloperidol while the clozapine was titrated. Instead, Mr. F, his family, the psychiatry treatment team, and the GI specialist agree to pursue a combination of a GI hypomotility diet—which includes frequent small meals (4 to 6 per day), ideally with low fiber, low fat, and increased fluid intake—and a trial of the second line agent for gastroparesis, erythromycin, a medication with known hepatic cytochrome P450 (CYP) drug-drug interactions that impacts the clearance of clozapine.
Shared decision making is an evidence-based approach to engaging patients in medical decision making. It allows clinicians to provide education on potential treatment options and includes a discussion of risks and benefits. It also includes an assessment of the patient’s understanding of their condition, explores attitudes towards treatment, and elicits patient values specific to the desired outcome. Even in very ill patients with schizophrenia, shared decision making has been demonstrated to increase patient perception of involvement in their own care and knowledge about their condition.2 Using this framework, Mr. F and his family, as well as the GI and psychiatric teams, felt confident that the agreed-upon approach was the best one for Mr. F.
TREATMENT Erythromycin and continued clozapine
Mr. F. is started on erythromycin, 100 mg 3 times a day. Erythromycin is a prokinetic agent that acts as a motilin agonist and increases the rate of gastric emptying. The liquid formulation of the medication is a suspension typically taken in 3- to 4-week courses, with 1 week “off” to prevent tachyphylaxis.3 Compared to the tablet, the liquid suspension has higher bioavailability, allows for easier dose adjustment, and takes less time to reach peak serum concentrations, which make it the preferred formulation for gastroparesis treatment.
Per the GI consultant’s recommendation, Mr. F receives a total of 3 courses of erythromycin, with some improvement in the frequency of his nausea noted only during the third erythromycin course. His clozapine levels are closely monitored during this time, as well as symptoms of clozapine toxicity (ie, sedation, confusion, hypersalivation, seizures, myoclonic jerks), because erythromycin can directly affect clozapine levels.4,5 Case reports suggest that when these 2 medications are taken concomitantly, erythromycin inhibits the metabolism of hepatic enzyme CYP3A4, causing increased plasma concentrations of clozapine. Before starting erythromycin, Mr. F’s clozapine levels were 809 ng/mL at 350 mg/d. During the erythromycin courses, his levels are 1,043 to 1,074 ng/mL, despite reducing clozapine to 300 mg/d. However, he does not experience any adverse effects of clozapine (including seizures), which were being monitored closely.
The authors’ observations
Clozapine is the most effective medication for treatment-refractory schizophrenia.6 Compared to the other second-generation antipsychotics, it is associated with a lower risk of rehospitalization and treatment discontinuation, a significant decrease of positive symptom burden, and a reduction in suicidality.7,8 Unfortunately, clozapine use is not without significant risk. FDA black box warnings highlight severe neutropenia, myocarditis, seizures, and hypotension as potentially life-threatening adverse effects that require close monitoring.9
Recently, clinicians have increasingly focused on the underrecognized but well-established finding that clozapine can cause significant GI adverse effects. While constipation is a known adverse effect of other antipsychotics, a 2016 meta-analysis of 32 studies estimated that the pooled prevalence of clozapine-associated constipation was 31.2%, and showed that patients receiving clozapine were 3 times more likely to be constipated than patients receiving other antipsychotics (odds ratio 3.02, CI 1.91-4.77, P < .001, n = 11 studies).10 A 2012 review of 16 studies involving potentially lethal adverse effects of clozapine demonstrated that rates of agranulocytosis and GI hypomotility were nearly identical, but that mortality from constipation was 3.6 to 12.5 times higher than mortality from agranulocytosis.11
In 2020, the FDA issued an increased warning regarding severe bowel-related complications in patients receiving clozapine, ranging in severity from mild discomfort to ileus, bowel obstruction, toxic megacolon, and death.9
As exemplified by Mr. F’s case, upper GI symptoms associated with clozapine also are distressing and can have a significant impact on quality of life. Dyspepsia is a common complaint in patients with chronic psychiatric illness. A study of 79 psychiatric inpatients hospitalized long-term found that 80% reported at least 1 symptom of dyspepsia.12 There are few older studies describing the effect of clozapine on the upper GI system. We and others previously reported on significantly increased use of—not only antacids—but also H2 blockers and prokinetic agents after initiating clozapine, but sample sizes are small.13-15 These older data and newer studies suggest that GERD is a common upper GI disorder diagnosis following clozapine initiation, perhaps reflecting a knowledge gap and infrequent use of the more complex testing required to confirm a diagnosis of GI motility disorders such as gastroparesis.
In a study of 17 patients receiving clozapine, wireless motility capsules were used to measure whole gut motility, including gastric emptying time, small bowel transit time, and colonic transit time. In 82% of patients, there was demonstrated GI hypomotility in at least 1 region, and 41% of participants exhibited delayed gastric emptying, with a cut-off time of >5 hours required for a gastroparesis diagnosis.16 This is significantly higher than the prevalence of gastroparesis observed in studies of the general community.17 The Table18,19 summarizes the differences between GERD and gastroparesis.

OUTCOME Some improvement
Mr. F experiences limited improvement of some of his nausea symptoms during the third erythromycin cycle and returns to the gastroenterologist for a follow-up appointment. The GI specialist decides to discontinue erythromycin in view of potential drug-drug interactions and Mr. F’s elevated clozapine levels and the associated risks that might entail. Mr. F is again offered the D2 dopamine antagonist metoclopramide, but again refuses due to the risk for tardive dyskinesia. He is asked to continue the GI dysmotility diet. Mr. F finds some relief of nausea symptoms from an over-the-counter product for nausea (a nasal inhalant containing essential oils) and is advised to follow up with the GI specialist in 3 months. Shortly thereafter, he is discharged to live in a less restrictive supportive housing environment, and his follow-up psychiatric care is provided by an assertive community treatment team. Over the next several months, the dosage of clozapine is decreased to 250 mg/d. Mr. F initially experiences worsening psychiatric symptoms, but stabilizes thereafter. He then moves out of state to be closer to his family.
Bottom Line
In patients receiving clozapine, frequent nausea along with clustering of heartburn, abdominal pain, bloating, early satiety, and vomiting (especially after meals) may signal gastroparesis rather than gastroesophageal reflux disease. Such patients may require consultation with a gastroenterologist, a scintigraphy-based gastric emptying test, and treatment if gastroparesis is confirmed.
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi:10.1038/s41572-018-0038-z
2. Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265-273. doi: 10.1111/j.1600-0447.2006.00798.x
3. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98(2):259-263. doi:10.1111/j.1572-0241.2003.07167.x
4. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109-112. doi:10.1192/bjp.171.2.109
5. Edge SC, Markowitz JS, Devane CL. Clozapine drug-drug interactions: a review of the literature. Human Psychopharmacology: Clinical and Experimental. 1997;12(1):5-20.
6. Vanasse A, Blais L, Courteau J, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134(5):374-384. doi:10.1111/acps.12621
7. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
8. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305-1313. doi:10.1176/appi.ajp.158.8.1305
9. National Alliance on Mental Illness. Clozapine. Accessed June 13, 2021. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication/Clozapine-(Clozaril-and-FazaClo)
10. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. doi:10.3390/ijms17060863
11. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73(10):1307-1312. doi:10.4088/JCP.11r06977
12. Mookhoek EJ, Meijs VM, Loonen AJ, et al. Dyspepsia in chronic psychiatric patients. Pharmacopsychiatry. 2005;38(3):125-127. doi:10.1055/s-2005-864123
13. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry. 1995;7(3):119-125. doi: 10.3109/10401239509149038
14. Schwartz BJ, Frisolone JA. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry. 1993;150(10):1563. doi:10.1176/ajp.150.10.1563a
15. Taylor D, Olofinjana O, Rahimi T. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol. 2010;30(4):460-461. doi:10.1097/JCP.0b013e3181e5c0f7
16. Every-Palmer S, Inns SJ, Grant E, et al. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33(1):81-91. doi:10.1007/s40263-018-0587-4
17. Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225-1233. doi: 10.1053/j.gastro.2008.12.047
18. Antunes C, Aleem A, Curtis SA. Gastroesophageal reflux disease. StatPearls Publishing. Updated July 7, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK441938/
19. Reddivari AKR, Mehta P. Gastroparesis. StatPearls Publishing. Updated June 30, 2021. Accessed December 8, 2021. https://www.ncbi.nlm.nih.gov/books/NBK551528/
SCAMP: Assessing body-focused repetitive behaviors
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
Repetitive behaviors towards the body, such as hair pulling and skin picking, are common. Approximately 5% of the general population may meet criteria for trichotillomania or excoriation disorder, in which the repetitive behaviors are excessive and impairing. The category of body-focused repetitive behaviors (BFRBs) extends beyond these 2 disorders to include onychophagia (nail biting), onychotillomania (nail picking), and lip or cheek chewing, which in DSM-5 are categorized under Other Specified Obsessive-Compulsive Disorder—BFRB. Of particular concern are trichophagia or dermatophagia, the ritualizing and eating of skin or hair that can lead to gastrointestinal complications.1
The prevalence and associated distress from BFRBs have spurred increased research into psychotherapeutic interventions to remediate suffering and curb bodily damage. Under the broader umbrella of behavioral therapy or cognitive-behavioral therapy, the Expert Consensus Treatment Guidelines from the TLC Foundation2 describe habit reversal therapy, comprehensive behavioral treatment, and behavioral therapy that is enhanced by acceptance and commitment therapy or dialectical behavioral therapy (DBT) skills. (Although these guidelines also summarize possible pharmacologic interventions, medication for patients with BFRBs is not discussed in this article.)
Understanding the antecedents and consequences of these recurrent behaviors is a key aspect of psychotherapeutic treatments because diverse contingencies reinforce these repetitive behaviors. As with any comprehensive assessment, asking questions to understand the function of the behaviors guides personalized treatment recommendations or referrals. Mansueto et al3 described a systematic approach to assessing BFRBs. Asking questions based on these researchers’ SCAMP domains (Sensory, Cognitive, Affective, Motor, Place) can provide patients and clinicians with a clear picture of pulling, picking, or other repetitive behaviors.
Sensory. Start with an assessment of how sensory experiences might play into the cycle. Questions might include: Does the patient see a distinctive hair (eg, color, texture) or skin irregularity that draws them into the behavior? Do they visually inspect the hair or skin before, during, or after? Do they describe a premonitory sensation, such as an itch? Do they have a dermatologic condition that cues interoceptive hypervigilance? Do they taste or smell the scab, excoriate, or hair? Are they particularly attuned to the auditory experiences of the process (ie, hearing the pop or a pull)? Could any substances or medications be impacting the body’s restlessness?
Cognitive. Just as we assess common automatic thoughts associated with other psychopathologies, it is important to appreciate the cognitions that occur during this behavioral chain. Some thoughts involve an intolerance of imperfection: “That hair looks different. I have to remove it.” “It is important for pores to be completely clean.” Other thoughts may involve granting permission: “I’ll just pull one.” “It has been a long week so I deserve to do just this one.” Certainly, many patients may be thinking about other daily stressors, such as occupational or interpersonal difficulties. Knowing about the patient’s mental state throughout the BFRB can guide a clinician to recommend treatment focused on (for example) cognitive-behavioral therapy for perfectionism or approaches to address existing stressors.
Affective. One common assumption is that patients who engage in BFRBs are anxious. While it certainly may be the case, an array of affective states may accompany the repetitive behavior. Patients may describe feeling tense, bored, sad, anxious, excited, relieved, agitated, guilty, worried, or ashamed. It is typically helpful to inquire about affect before, during, and after. Knowing the emotional experiences during and outside of BFRBs can call attention to possible comorbidities that warrant treatment, such as a mood or anxiety disorder. Additionally, dysregulation in affective states during the BFRB may point to useful adjunctive skills, such as DBT.
Motor. Some patients describe being quite unaware of their BFRB (often called “automatic”), whereas for other patients pulling or picking may be directed and within awareness (often called “focused”). It is common for patients to have both automatic and focused behaviors. Questions to understand the motor experience include: Is the patient operating on autopilot when they are engaged in the behavior? Does the behavior occur more often in certain postures, such as when they are seated or lying in bed? Understanding the choreography of the BFRB can help in determining physical barriers to protect the skin or hair.
Place. Finally, ask the patient if they believe certain locations increase the occurrence of the BFRB. For instance, some patients may notice the behavior is more likely to occur in the bathroom or bedroom. Bathrooms often contain implements associated with these behaviors, including mirrors, tweezers, or bright lights. Knowing where the BFRB is most likely to occur can help the clinician develop planning strategies to minimize behavioral engagement. An example is a patient who is more likely to pull or pick on a long commute from work. Planning to have a hat and sweater in their vehicle for the drive home may serve as a deterrent and break the cycle. When considering the place, it may also be helpful to ask about the time of day and presence of others.
Gathering information from the SCAMP domains can lead to individualized approaches to care. Of course, nonsuicidal self-injury, delusional parasitosis, or body dysmorphic disorder are a few of the many differential diagnoses that should be considered during the assessment. After a detailed assessment, clinicians can proceed by collaboratively developing strategies with the patient, referring them to a clinician who specializes in treating BFRBs using a resource such as the TLC Foundation’s Find a Therapist directory (https://www.bfrb.org/find-help-support/find-a-therapist), or recommending a self-guided resource such as StopPulling.com or StopPicking.com.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
1. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. The TLC Foundation for Body-Focused Repetitive Behaviors (2016). Expert consensus treatment guidelines. Accessed November 30, 2021. https://www.bfrb.org/storage/documents/Expert_Consensus_Treatment_Guidelines_2016w.pdf
3. Mansueto CS, Vavricheck SM, Golomb RG. Overcoming Body-Focused Repetitive Behaviors: A Comprehensive Behavioral Treatment for Hair Pulling and Skin Picking. New Harbinger Publications; 2019.
Are we failing to diagnose and treat the many faces of catatonia?
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.








