User login
The Diagnosis: Glomangiomyoma
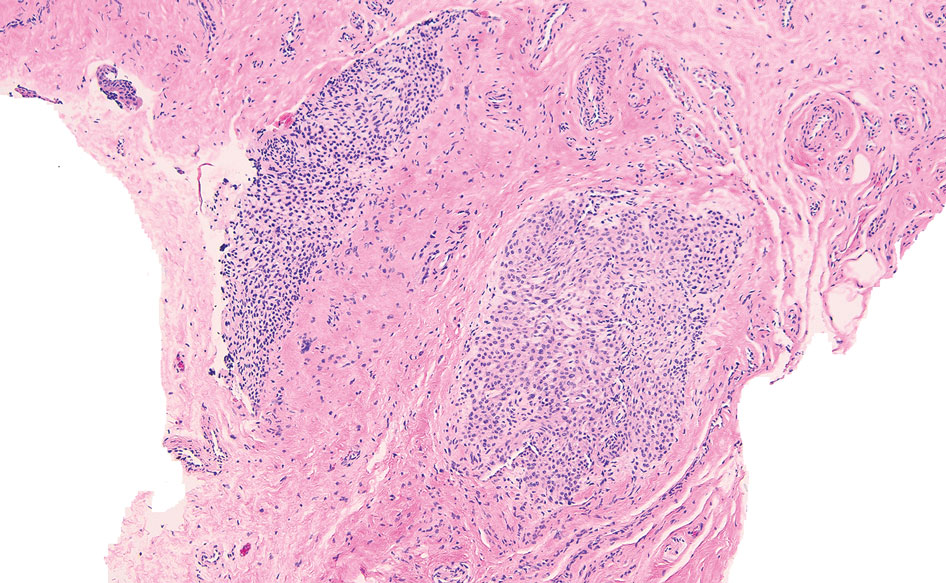
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7
Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
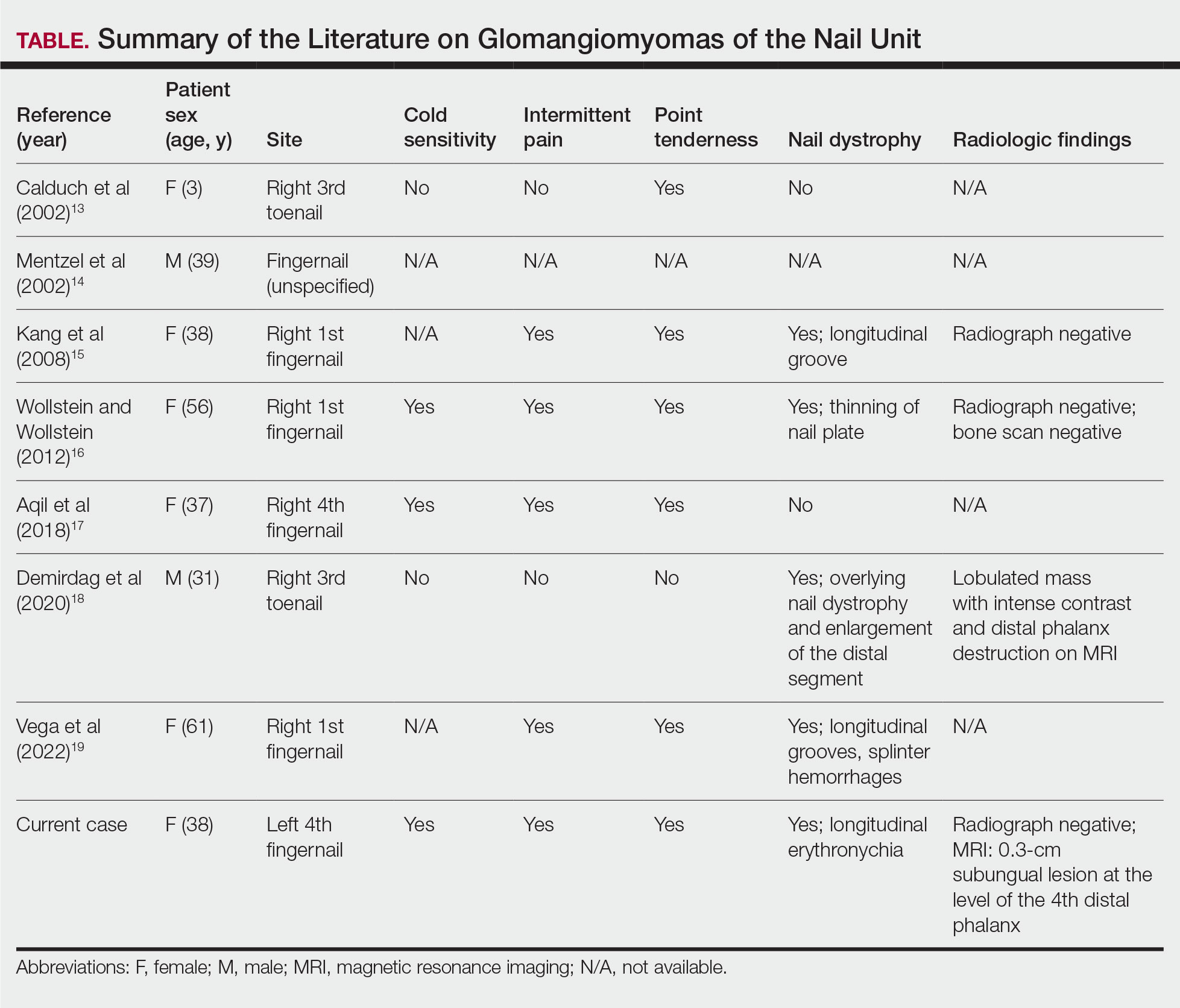
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20
A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
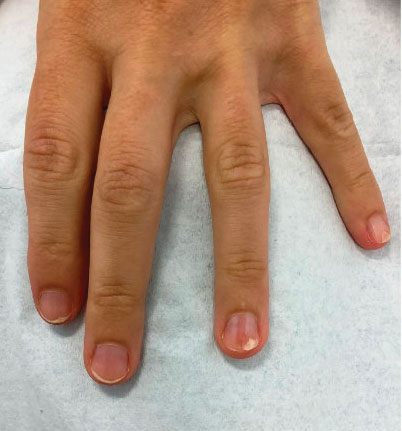
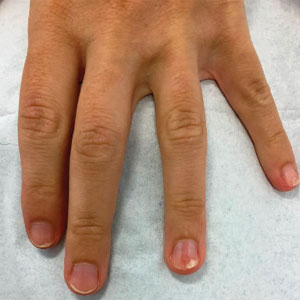
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.